The main objective is to describe the project and evaluate the impact of replacing repackaged medications with unit dose presentations in 15 public hospitals within a regional health system. Secondary objectives include identifying differences in the changes implemented across the 15 audited hospitals and conducting an exploratory analysis of the potential impact in other non-audited centers that requested participation in the project.
MethodA database containing over 2,000 medications available in unit dose format was developed and is updated monthly. In parallel, an automated system based on decision-making algorithms was implemented to identify improvement opportunities in medication procurement. The system was adopted either individually by hospitals or through centralized structures at the regional or private level. The analysis included data from 15 public hospitals, where the reduction in repackaging and its environmental, economic, and operational impact were assessed by measuring material, time, and cost savings. The results were subsequently extrapolated to the 172 hospitals that applied.
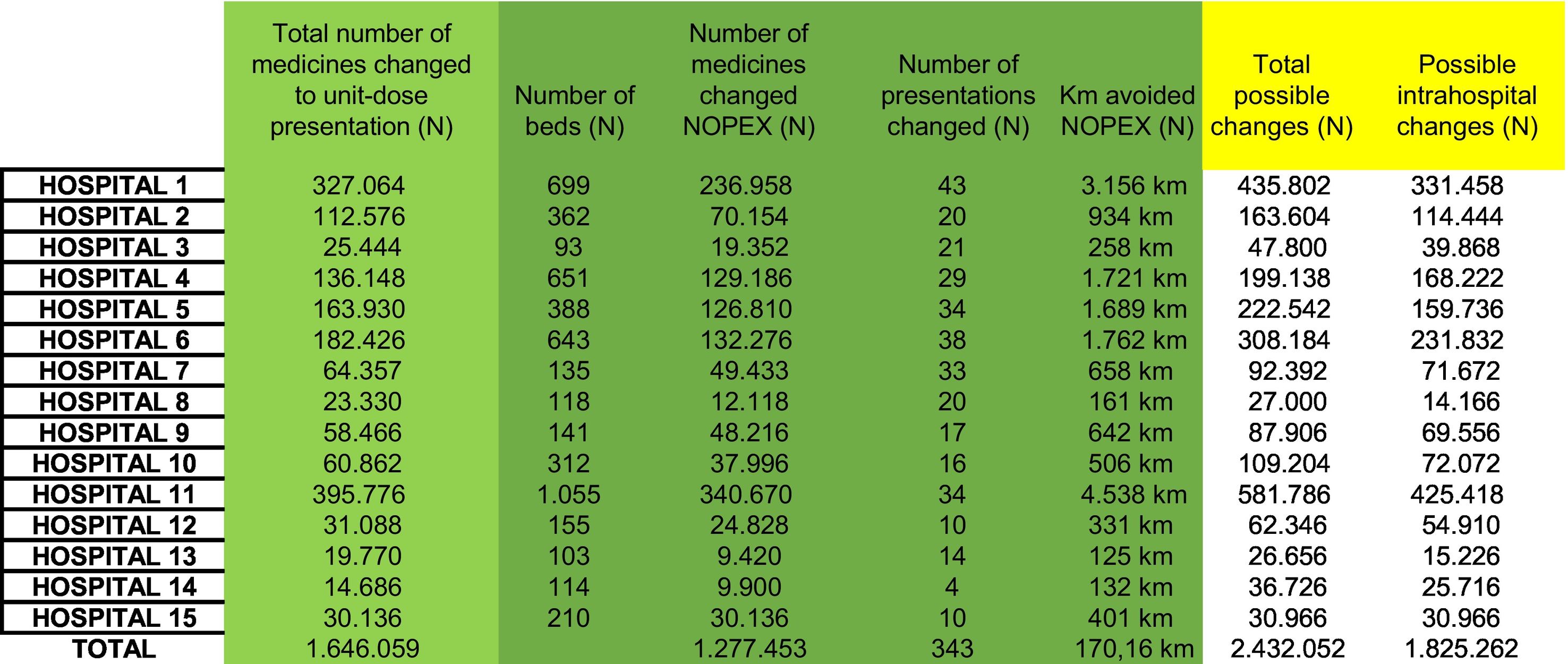
ResultsIn the 15 monitored hospitals, repackaging of approximately 1.27 million tablets per year was avoided, resulting in estimated savings of 17,016 km of packaging material, 866 kg in weight, and 113,693 min of labor. The avoided costs in materials and machinery amounted to 36,274€ annually. No statistically significant differences were observed in project adoption across the hospitals (p = 0.234). The extrapolation to 172 hospitals suggests a potential impact of 16.67 million tablets no longer requiring repackaging per year, with an estimated savings of 2220.13 km of material, 24,723 h of labor, and 451,768€ annually.
ConclusionsReplacing repackaged medications with commercially available unit dose formats significantly reduced material consumption, labor time, and repackaging-related costs in the evaluated hospitals. The implementation of the project was consistent across the 15 monitored hospitals. Furthermore, the model proved to be scalable. The main limitation identified was the limited availability of unit dose medications on the market; therefore, it is recommended to prioritize their inclusion in procurement processes and to promote their development by the pharmaceutical industry.
el objetivo principal es describir el proyecto y evaluar el impacto de sustituir los medicamentos reenvasados por presentaciones en dosis unitaria en 15 hospitales públicos de una comunidad autónoma. Los objetivos secundarios consisten en identificar diferencias en los cambios implementados entre los 15 hospitales en los que se ha auditado y realizar un análisis exploratorio sobre el posible impacto en el resto de centros no auditados que solicitaron el proyecto.
Métodose desarrolló una base de datos, actualizada mensualmente, que recopila más de 2.000 medicamentos disponibles en dosis unitaria. Paralelamente, se implantó un sistema automatizado con algoritmos de decisión, orientado a identificar oportunidades de mejora en la adquisición. El sistema fue implementado de forma individual en hospitales o a través de estructuras centralizadas, autónomas o privadas. El análisis incluyó datos de 15 hospitales públicos, en los que se evaluó la reducción del reenvasado y su impacto ambiental, económico y operativo, midiendo el ahorro de material, tiempo y costes. Los resultados se extrapolaron posteriormente a los 172 hospitales solicitantes.
Resultadosen los 15 hospitales monitorizados se evitó el reenvasado de 1,27 millones de comprimidos al año, con un ahorro estimado de 17,016 km de material, 866 kg de peso y 113.693 minutos de trabajo. Los costes evitados en materiales y maquinaria ascendieron a 36.274 euros anuales. No se observaron diferencias estadísticamente significativas entre hospitales en cuanto a la adopción del proyecto (p = 0,234). La extrapolación a 172 centros sugiere un impacto estimado de 16,67 millones de comprimidos no reenvasados al año, con un ahorro de 2.220,13 km de material, 24.723 horas de trabajo y 451.768 euros anuales.
Conclusionesla sustitución de medicamentos reenvasados por presentaciones en dosis unitaria ha permitido reducir el consumo de materiales, el tiempo de trabajo y los costes asociados al reenvasado en los hospitales evaluados. La implementación del proyecto en los 15 hospitales monitorizados fue homogénea. Además, el modelo ha demostrado ser escalable. La principal limitación detectada es la disponibilidad limitada de medicamentos en dosis unitaria, por lo que se recomienda priorizar su inclusión en los concursos de adquisición y fomentar su desarrollo por parte de la industria.
In a world in which environmental sustainability is a global priority, the healthcare sector produces large quantities of waste that negatively impact the environment.1,2 Hospitals face the challenge of mitigating this impact without compromising the quality of care, while also dealing with the consequences of delayed action that worsens these problems.3 Within this framework, this project forms part of the SEFH 2023 + Sustainable Project,4 and is aligned with the Sustainable Development Goals.5
As the central hub for managing medicines and healthcare resources, hospital pharmacies play a key role in this environmental commitment.6 Hospital pharmacists can implement various strategies to transform medicine procurement, production, and distribution processes, promoting more efficient and sustainable management.7 Among these processes, medicine repackaging has a marked environmental impact and incurs significant costs in terms of materials, machinery, and human resources.8
This problem is exacerbated by the lack of medicines in unit doses and the absence of an updated database indicating which medicines are available in this form. These aspects, combined with frequent shortages and the manual analysis of offers, hinder the adoption of more sustainable and safer alternatives.
Furthermore, look-alike primary packaging contributes to medication errors.9 Although not exclusive to repackaging, standardising the appearance of packaging intensifies this problem. The Institute for the Safe Medication Practices (ISMP) has stressed the need for proactive strategies to prevent errors associated with similarities in names, packaging, and labelling.10 It is essential in daily hospital pharmacy practice to implement the use of unit-dose medicines that are clearly identified by their active ingredient, trade name, batch number, and expiry date.11 The “NO REPACKAGING unless necessary” (Spanish: NO REENVASES sin necesidad) project has been developed to address these issues. Our main objective was to describe the project and assess the impact of replacing repackaged medicines with unit-dose medicines in 15 public hospitals in an autonomous community in Spain. The secondary objectives were to identify the differences in changes implemented by the 15 audited hospitals and to conduct an exploratory analysis of their potential impact on other non-audited centres which had asked to participate in the project.
MethodTo reduce repackaging, we developed a database that compiles information on medicines available in unit doses. This database includes more than 2000 medicines that are clearly identified on each tablet or capsule and is updated monthly. In addition, we implemented an automated system that analyses the medicines used in the hospital, identifies available unit-dose alternatives, and suggests changes.
To assess the environmental impact and efficiency of the repackaging process, data from previous studies8 were used: the amount of packaging material avoided (paper, adhesive tape, opaline) per tablet in weight (0.000678 kg), surface area (0.00759 m2), and length (0.0001332 km); cost savings in packaging per tablet (€0.01774) and the annual depreciation cost of a repackaging machine (€907.5; using a DEXTROPAC 2018 packaging machine as a model, with an estimated average lifespan of 20 years) per hospital. The time taken to repackage a tablet was 5.35 s.
Information about the project was shared via the SEFH mailing list. Based on these communications, interested hospitals asked to participate in the project. They either adopted the automated system that had been developed, or simply downloaded the complete database and implemented it independently of the automatic system: https://www.scmfh.es/ver_datos.asp?id_sec=5.
The programme offers 2 implementation options: in individual hospitals, the automated system can be used independently to analyse purchases and identify improvements in the procurement of unit-dose medicines; in centralised models, regional systems or private groups manage the programme jointly, analysing overall purchases and implementing large-scale strategic changes.
Purchasing information from the 15 public hospitals in an autonomous community was processed to identify purchases of medicines without unit doses. A chi-squared test was used to identify any significant differences in the adoption of changes across the participating hospitals.
The calculations were based on the total number of tablets avoided, with outpatient settings excluded since the entire box is typically dispensed in these cases without the need for unit doses. The data were obtained from 15 monitored public hospitals. These were then extrapolated to the other participating centres, with the data adjusted in proportion to the size of each centre. This was achieved by using the ratio between the number of beds in the monitored hospitals and the number of beds in the other participating hospitals.
ResultsA total of 166 hospitals and 6 non-hospital centres (nursing homes and prisons) asked to participate in the project; however, since the database is available online, the actual impact may have been much greater. The project's impact was multiplied by implementing the system through centralised systems in hospitals across 3 autonomous communities and an entire group of private hospitals.
In the 15 monitored public hospitals (see Table 1), the repackaging of 1.277 million tablets/year was avoided, which were intended for hospitalised patients. This saving represents the elimination of 17.016 km/year of material used, with a cumulative surface area of 9698.93 m2, a total weight of 866.37 kg, and an estimated time saving of 1894.89 h per year. No statistically significant differences were found between hospitals in the percentage of changes made (p = 0.234).
Eliminating the repackaging process resulted in annual savings of €22,662 in material costs. In addition, the cost associated with machinery depreciation was reduced by €13,612.50 per year by avoiding the use of repackaging machines, whose annual depreciation cost per hospital was significant. The total costs avoided amounted to €36,274.50 per year.
The impact of these savings was very significant when these results were extrapolated to the 172 applicant centres. The 15 public hospitals have a combined total of 5179 beds and the 172 centres have a combined total of 67,572. Repackaging 16.67 million tablets per year would be avoided (95% confidence interval [CI]: 6.26–27.01). Unused material would amount to 2220.13 km/year (95% CI: 844.55–3595.19). The surface area of the material avoided would be 126,544.9118 m2 and the total weight would be 11,303.79 kg.
An estimated 24,723 h per year would be freed up. In this scenario, the annual material costs savings could reach €295,678.04, while the savings due to avoiding machinery depreciation costs would be €156,090. In total, the annual savings would amount to €451,768.04.
DiscussionThe results of this study demonstrate that eliminating unnecessary repackaging by purchasing medicines in unit doses is an effective strategy to improve environmental sustainability. The repackaging of more than 1.27 million tablets per year was avoided in the 15 monitored hospitals, representing significant savings in terms of materials, machinery costs, and working time. This figure could be reduced even further, as it can be affected by many factors, such as public tenders, look-alike problems, and specific shortages.
The project was implemented uniformly across the 15 monitored hospitals (p = 0.234). The results show that the principles of sustainability and efficiency can be successfully integrated into different hospital settings. However, as the project was limited to a single autonomous community, its results may not be fully representative of other healthcare systems with different management models.
Although hospital repackaging is a common practice, there is little information on its impact on the quality and stability of medicines during storage.12 This can lead to premature expiry and increased waste, meaning that the environmental benefit of unit-dose purchasing could be even greater.13 Furthermore, eliminating unnecessary repackaging also eliminates the need for the associated quality control processes.14
The transition to unit doses may be challenging for suppliers, who could struggle to respond to growing demand and exacerbate existing shortages in Spain due to logistical and financial difficulties. However, it may also encourage the industry to prioritise their production, as they become recognised as a necessity within the healthcare system. An official list from the Ministry of Health would contribute to consolidating sustainability and standardisation criteria in pharmaceutical management.
The extension to 172 centres highlights not only the adaptability of the project but also the commitment of both the SEFH and hospital pharmacists themselves to advancing towards more sustainable pharmacy services.15 In addition, the availability of the online database could amplify its impact beyond the 172 applicant centres.
Manually comparing numerous offers is inefficient in the 21st century. In a context of budgetary pressure and sustainability, these results reinforce the importance of adopting more efficient models. Unit-dose purchasing optimises resources and minimises environmental impact. The time saved by not repackaging allows human resources to be redirected to tasks with greater added value.
Contribution to the scientific literatureThis study shows that automating unit-dose purchasing reduces the need for repackaging and optimises the use of resources. The results support changes to hospital procurement that promote sustainability and healthcare efficiency.
Use of artificial intelligenceThe authors used ChatGPT 4.0 and DeepSeek to improve their writing. All content was subsequently reviewed and edited, with the authors assuming final responsibility for the manuscript.
Conference presentationsWe declare that this manuscript is original, has not been previously published, and is not currently under review by any other journal. Some of the results were presented in the form of a poster at the 69th SEFH Congress, where the project was awarded the sustainability prize.
It was presented at the conference organised by the SEFH at 69th Congress, which took place between the 17th to the 19th October, 2024.
Responsibility and transfer of rightsAll authors accept the responsibility defined by the International Committee of Medical Journal Editors (available at http://www.icmje.org/).
In the event of publication, the authors exclusively transfer the rights of reproduction, distribution, translation, and public communication (by any means or sound, audiovisual, or electronic medium) of our work to Farmacia Hospitalaria and, by extension, to the SEFH. To this end, a letter of transfer of rights will be signed at the time of submission of the work through the online manuscript management system.
CRediT authorship contribution statementDavid García-Martínez: Writing – review & editing, Writing – original draft, Visualisation, Validation, Supervision, Software, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualisation. Manuela Martínez-Camacho: Writing – review & editing, Visualisation, Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualisation. Aida Rueda-Naharro: Writing – review & editing, Visualisation, Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualisation. David García-Marco: Writing –review & editing, Visualisation, Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
FundingNone declared.
None declared.