Rheumatoid arthritis (RA) is the most common chronic inflammatory rheumatic disease, its management and morbidity impose a great burden to healthcare systems. Development and rollout of biological disease modifying anti-rheumatic drugs has contributed to improvements for patients, however, high costs have prevented them to be widely used. This is being addressed with biosimilars, with equal benefit–risk profile and reduced costs. The objective is to analyze the cost-effectiveness of subcutaneous biosimilar tocilizumab (bsTCZ) for patients with moderate–severe RA in Spain from a healthcare system perspective.
MethodsA Markov model was developed with a lifetime horizon including 5 health states: remission of the disease; low, moderate, or high activity; and death. A PICO-S-T search retrieved efficacy of treatments in meta-analysis and network meta-analysis, and was further complemented with published clinical trials. Pharmacological costs were obtained from the BotPlus database, and medical resources costs from regional tariffs. Deterministic and probabilistic sensitivity analysis were performed to validate the robustness of results. Incremental cost-effectiveness ratio (ICER) for cost/percentage of remission and cost/quality-adjusted life year (QALY) gain were calculated.
ResultsLifetime cost of bsTCZ was 183 741€ (lowest) versus comparative costs ranging from 184 317€ for infliximab to 201 972€ (highest) for certolizumab. QALYs were 13.74 for upadacitinib and 13.73 for sarilumab and tocilizumab with values between 13.53 and 13.72 for the comparators. ICERs as €/remission and €/QALY showed that bsTCZ was either dominant in most of the comparisons or the most cost-effective alternative. The sensitivity analysis showed that bsTCZ long term cost, and transition from low to moderate disease activity health status were the most influential factors. Moreover, bsTCZ was either dominant or cost-effective in all the comparisons.
ConclusionsbsTCZ demonstrated to be a cost-effective and cost-saving alternative for the treatment of patients with RA in Spain when compared to all the available therapeutic alternatives.
La artritis reumatoide (AR) es la enfermedad reumática inflamatoria crónica más común, y su manejo y morbilidad suponen una gran carga para los sistemas de salud. El desarrollo y uso de fármacos antirreumáticos modificadores de la enfermedad han contribuido a mejoras para los pacientes, sin embargo, los altos costes han impedido su uso generalizado. La aparición de los biosimilares está cambiando este paradigma al ofrecer el mismo perfil de beneficio-riesgo a un menor coste. El objetivo es analizar el coste-efectividad de tocilizumab biosimilar (bsTCZ) subcutáneo en pacientes con AR moderada-severa en España desde la perspectiva del sistema de salud.
MétodosSe desarrolló un modelo de Markov con un horizonte temporal de toda la vida incluyendo cinco estados de salud: remisión de la enfermedad; actividad baja, moderada o alta; y muerte. Mediante una búsqueda PICO-S-T se identificó la eficacia de los tratamientos en metanálisis y metanálisis en red, y se complementó con evidencia de ensayos clínicos publicados. Los costes farmacológicos se obtuvieron de la base de datos BotPlus, y los de recursos de las tarifas regionales. Se realizaron análisis de sensibilidad determinísticos y probabilísticos para validar la robustez de los resultados. Se calculó la ratio coste-efectividad incremental (RCEI) para el coste/porcentaje de remisión y el coste/años de vida ganados ajustados por calidad (AVAC).
ResultadosEl coste durante toda la vida de bsTCZ fue 183.741€ (más bajo) frente a los comparativos que oscilaban entre 184.317€ para infliximab y 201.972€ (más alto) para certolizumab. Los AVAC fueron 13,74 para upadacitinib y 13,73 para sarilumab y tocilizumab, con valores entre 13,53 y 13,72 para los comparadores. El RCEI en €/remisión y €/AVAC mostraron que bsTCZ fue dominante o la alternativa más coste-efectiva en la mayoría de las comparaciones. Los análisis de sensibilidad mostraron que el coste a largo plazo de bsTCZ y la transición de baja a moderada fueron los factores más influyentes. Además, bsTCZ fue dominante o coste-efectivo en todas las comparaciones.
ConclusionesbsTCZ demostró ser una alternativa coste-efectiva y que genera ahorros en el tratamiento de pacientes con AR en España en comparación con las alternativas terapéuticas disponibles.
Rheumatoid arthritis (RA) is an inflammatory-mediated immune disease (IMID), and its clinical manifestations represent a humanistic burden1,2 as well as a high economic burden on healthcare systems worldwide, due to the high resource consumption generated in the appropriate management of affected patients, both for the disease itself and the associated morbidity.3 The burden of the disease in Spain is much higher than in Europe and other parts of the world, and it also has a greater affectation for women.4 RA accounts for 5% of the total burden of rheumatic diseases in Spain (4.4% of the total burden of disease in Western Europe and 4% globally).4 The prevalence of RA and multimorbidity are increasing exponentially, as well as hospitalization rates (ranging between 31.6/100 000 inhabitants in 2002 and 56.3/100 000 inhabitants in 2017),5 lead in part by a reduction in mortality due to RA. This reduction was 43.8% in high-income countries, due to a better management of patients by disease control and treat-to-target treatments.2
RA is the most common chronic inflammatory rheumatic disease and its estimated prevalence in Spain has been reported to be 0.82% (95%CI 0.59–1.15) affecting predominantly women.6 In this study by Silva-Fernández et al, they also estimate that there are between 220 000 and 430 000 RA patients older than 20 years old in Spain. The majority of the patients were aged 40–59 years old, an age when patients have an active working life,1,6 which combined with its debilitating effect in those patients who suffer the disease, entails a high humanistic and economic burden.3
The treatment paradigm for these patients changed drastically 20 years ago with the development of biological disease modifying anti-rheumatic drugs (bDMARDs), which have supposed a great improvement for patient's lives by achieving a better control of the disease, a fewer consumption of steroids,7 and the attainment of a better overall quality of life.8 These bDMARDs have a significant impact over the disease effect and its progression.9 However, the costs associated with these therapies have been a constraint for their widespread use, an obstacle that has been addressed by the appearance of biosimilars, which maintain the benefit–risk profile at a lower cost.9,10
Currently, there are numerous bDMARDs, targeted synthetic, and biosimilar therapeutic alternatives for the management of RA, such as: abatacept, adalimumab, baricitinib, certolizumab, etanercept, golimumab, infliximab, rituximab, sarilumab, tocilizumab, tofacitinib, and upadacitinib,.11,12 Among them, tocilizumab (TCZ) has been approved for RA in the EU since 2009 for the treatment of methotrexate-naïve patients, as well as those patients with moderate to severe RA without response or intolerance to disease modifying anti-rheumatic drugs (DMARDs) or anti-tumor necrosis factor α drugs (anti-TNF-α).13 In the past years, it has been noted of paramount importance to follow closely the activity of the disease after diagnosis, to achieve “Treat to Target” guidance, which proposes that the therapeutic target in RA should be a state of remission, or alternatively a low disease activity.14 In Spain, the most commonly used index for evaluation of disease activity is the Disease Activity Score 28 (DAS28) which examines 28 joints and either ESR (erythrocyte sedimentation rate) or CRP (C reactive protein) and classifies the results into 4 categories.15–17
To make a well-informed decision for both the patient and the healthcare system according to efficacy–safety profile as well as costs associated with each treatment, it is mandatory to generate evidence that allows an appropriate decision-making process. The latest biosimilar approved and currently on the market in Spain aimed for the treatment of RA is MSB-11456, a tocilizumab biosimilar (bsTCZ), which prompted a refreshed search and updated evidence generation with its integration into the current market dynamics. Thus, the aim of this study is to develop a cost-effectiveness analysis of subcutaneous (SC) bsTCZ for the treatment of RA compared to the most common alternatives in Spain from the healthcare perspective.
MethodsTo contrast bsTCZ administered subcutaneously to its comparators (SC abatacept, biosimilar SC adalimumab, oral -O- baricitinib, SC certolizumab, SC etanercept, O filgotinib, SC golimumab, biosimilar intravenous -IV- infliximab, IV rituximab, SC sarilumab, biologic SC tocilizumab -refTCZ-, O tofacitinib, and O upadacitinib), a cost-effectiveness model was constructed from the Spanish healthcare perspective. Studies including patients being treated with any of the comparators were included in the model.
Literature reviewFollowing Cochrane guidelines, a systematic literature review was carried out in: MEDLINE; Database of Abstracts of Reviews of Effects, National Health Service Economic Evaluation Database, and Health Technology Assessment. The search was designed and performed following a PICO-S-T structure (Supplementary table 1) to retrieve efficacy data.
- -
P (Patient): Rheumatoid arthritis.
- -
I (Intervention): Tocilizumab.
- -
C (Comparator): Etanercept, infliximab, certolizumab, golimumab, adalimumab, tofacitinib, upadacitinib, baricitinib, abatacept, sarilumab, rituximab, filgotinib.
- -
O (Outcome): Disease Activity Index (DAS28), cost per responder, cost per response, number needed to treat, NNT, responder.
- -
S (Study type): Meta-analyses (MA), network Meta-Analysis (NMA).
- -
T (Time Frame): From 2011 to August 2023.
Searches were limited to articles published in English and Spanish. Titles and abstracts were reviewed independently by 2 investigators (Supplementary tables 2, 3, and 4), and the quality of those articles that were selected was assessed using the checklist of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) extension for network meta-analysis guidelines as the percentage of accordance.18
For those comparators that were not represented in the MA or NMA retrieved, or data retrieved was insufficient, ad hoc searches were performed to find those clinical trials (CTs) that allowed to include them in the model. Additionally, comparators (as defined in the PICO-S-T search) included in arms in these CTs were also incorporated in the analysis.
For the studies that were finally included, information regarding comparators (as monotherapy or methotrexate add-on), patient population (naïve or with prior treatment), follow-up, outcome, and meta-analysis method used were extracted.
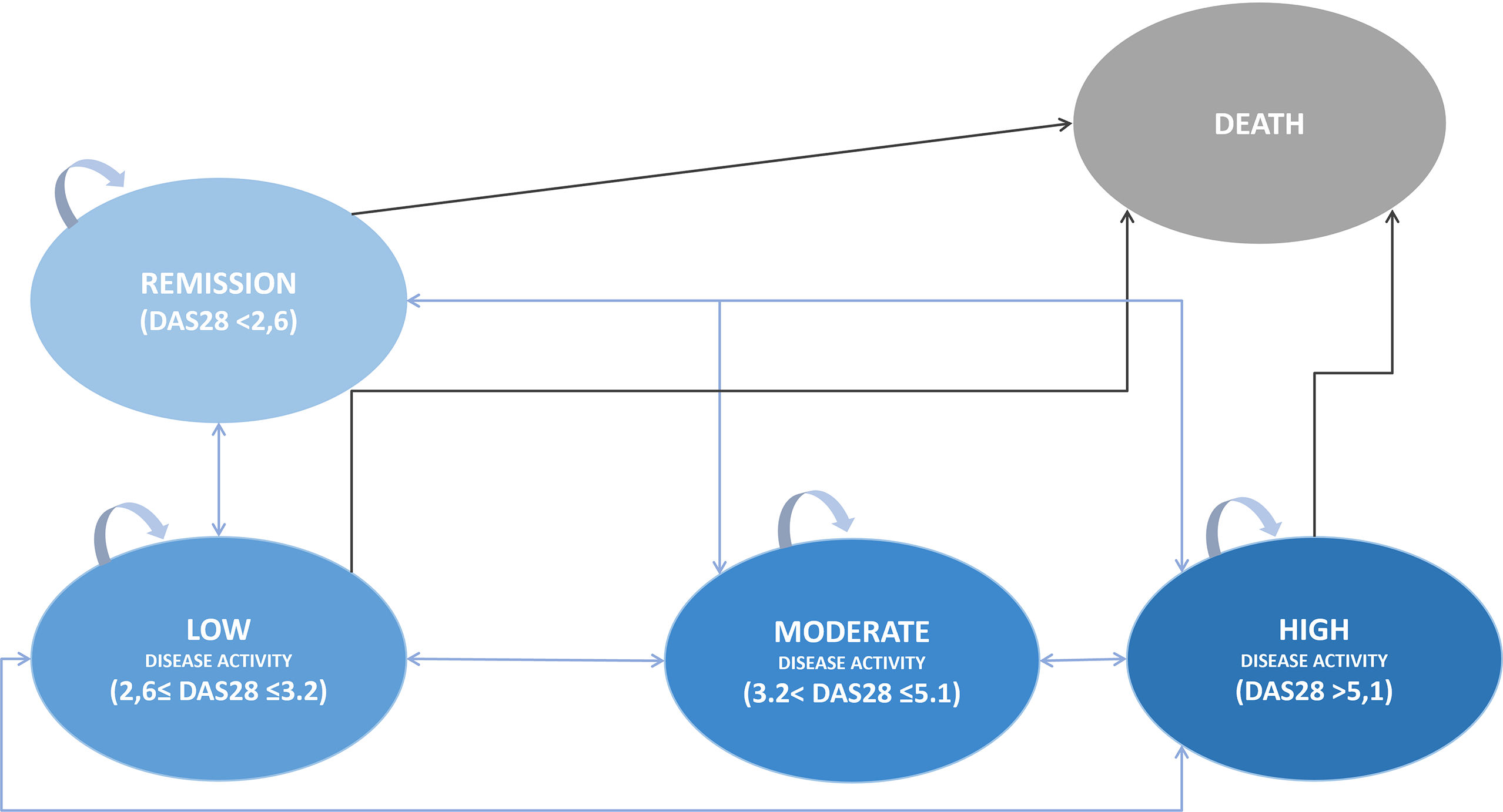
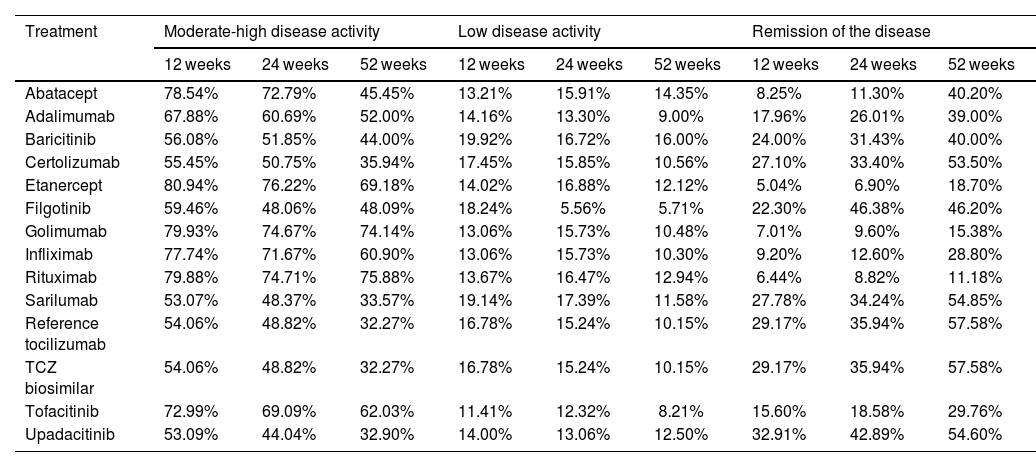
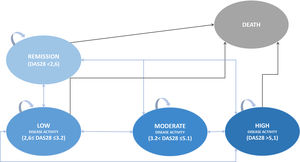
Cost-effectiveness analysisA Markov model 3-months cycle was built considering the Spanish clinical practice in the management of RA patients using 5 health states considering DAS28, the most used clinical parameter in Spain19: high disease activity, moderate disease activity, low disease activity, remission of the disease, and death as an absorbing health status (Fig. 1); and simulating a lifetime horizon to compare bsTCZ to all the available therapeutic alternatives. Patients initially present in a moderate-to-severe state, with the possibility of transitioning to any other states or to stay the same (High, Moderate, Low, Remision, or Death), according to the specific efficacy proportions of each treatment (Table 1). These efficacy estimates derive from literature retrieved and, in cases where data were insufficient, approximations have been made based on the closest comparable product within the same generation. Data for the first year were taken as the starting point and every 52 weeks the change of treatment was assessed depending on the treatment, the health state of the patient and whether the patient had changed the health state (improved/worsened) or stayed the same. Patients changing treatment after 52 weeks were in the high disease activity status and transitioned to a hypothetical pool of treatment alternatives with estimated efficacy based on corresponding clinical trials and market share of each treatment according to clinical experts For these estimations, the treatments in which the patient reached the high disease activity health status was disregarded in the pool. After the week 52, the transition probabilities were equal for all the treatments (Supplementary table 5).20 A discount of 3% was applied to both costs and effects.
Estimated transition probabilities for a patient for the different treatments and health states.
| Treatment | Moderate-high disease activity | Low disease activity | Remission of the disease | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 12 weeks | 24 weeks | 52 weeks | 12 weeks | 24 weeks | 52 weeks | 12 weeks | 24 weeks | 52 weeks | |
| Abatacept | 78.54% | 72.79% | 45.45% | 13.21% | 15.91% | 14.35% | 8.25% | 11.30% | 40.20% |
| Adalimumab | 67.88% | 60.69% | 52.00% | 14.16% | 13.30% | 9.00% | 17.96% | 26.01% | 39.00% |
| Baricitinib | 56.08% | 51.85% | 44.00% | 19.92% | 16.72% | 16.00% | 24.00% | 31.43% | 40.00% |
| Certolizumab | 55.45% | 50.75% | 35.94% | 17.45% | 15.85% | 10.56% | 27.10% | 33.40% | 53.50% |
| Etanercept | 80.94% | 76.22% | 69.18% | 14.02% | 16.88% | 12.12% | 5.04% | 6.90% | 18.70% |
| Filgotinib | 59.46% | 48.06% | 48.09% | 18.24% | 5.56% | 5.71% | 22.30% | 46.38% | 46.20% |
| Golimumab | 79.93% | 74.67% | 74.14% | 13.06% | 15.73% | 10.48% | 7.01% | 9.60% | 15.38% |
| Infliximab | 77.74% | 71.67% | 60.90% | 13.06% | 15.73% | 10.30% | 9.20% | 12.60% | 28.80% |
| Rituximab | 79.88% | 74.71% | 75.88% | 13.67% | 16.47% | 12.94% | 6.44% | 8.82% | 11.18% |
| Sarilumab | 53.07% | 48.37% | 33.57% | 19.14% | 17.39% | 11.58% | 27.78% | 34.24% | 54.85% |
| Reference tocilizumab | 54.06% | 48.82% | 32.27% | 16.78% | 15.24% | 10.15% | 29.17% | 35.94% | 57.58% |
| TCZ biosimilar | 54.06% | 48.82% | 32.27% | 16.78% | 15.24% | 10.15% | 29.17% | 35.94% | 57.58% |
| Tofacitinib | 72.99% | 69.09% | 62.03% | 11.41% | 12.32% | 8.21% | 15.60% | 18.58% | 29.76% |
| Upadacitinib | 53.09% | 44.04% | 32.90% | 14.00% | 13.06% | 12.50% | 32.91% | 42.89% | 54.60% |
Population for the model was obtained from Leil et al. 202121 and was set as patients suffering RA with moderate to high disease activity (mean DAS28 5.7 (SE 0.52)), 80.6% women, mean age 52.7 (SE 1.0)) and mean disease duration 7.45 years (SE 2.72). The assessment of the quality of life for this baseline case varies depending on the progression of the disease using the values 0.839, 0.8, 0.679, and 0.492 for remission, low, moderate, and high disease activity states, respectively, as adapted from Chiou et al. (2004).22 These utilities were used to estimate the quality-adjusted life years (QALY) for the healthcare outcomes. According to a recent research, mortality was considered equal to the general population during the first 10 years of the disease23 and from then on, an increment on mortality was applied (standardized mortality rate 1.49 (95%CI 1.30–1.71).24 The Incremental Cost-Effectiveness Ratio scores cost per percentage of remission (€/remission) and cost per QALY (€/QALY) were calculated.
Patient management and use of resourcesData on patient management in Spain were accounted for in terms of resource use and costs depending on treatment according to expert opinion (Table 2). As common management for all drugs, rheumatology specialist visit was considered every 3 months for those patients in the high disease activity health status, every 6 months for those in the moderate disease activity health status, and once a year for the low disease activity and remission health status. Yearly tests included a chest X-ray, 3 blood tests (including liver and renal function tests, ESR, C-reactive protein, and hemogram). Additionally, in the first visit a Mantoux tuberculin skin test was considered as well (Supplementary table 6). When necessary, costs were updated to EUR2023.
Use of resources depending on treatment.
| Treatment (% patients) | Background methotrexate, % patients | Route of administrationa | Other treatment-associated drugs |
|---|---|---|---|
| Abatacept (6.9%) | 0% | SC | No |
| Adalimumab (44.3%) | 100% [43% oral, 57% SC] | SC | No |
| Baricitinib (11.8%) | 0% | Oral | Herpes-Zoster vaccine (first year) |
| Certolizumab (0.5%) | 100% [43% oral, 57% SC] | SC | No |
| Etanercept (15.8%) | 100% [43% oral, 57% SC] | SC | No |
| Filgotinib (0.5%) | 0% | Oral | Herpes-Zoster vaccine (first year) |
| Golimumab (0.5%) | 100% [43% oral, 57% SC] | SC | No |
| Infliximab (0.0%) | 100% [43% oral, 57% SC] | Perfusion at Daycare hospital (4 h) | No |
| Rituximab (1.0%) | 0% | Perfusion at Daycare hospital (8 h) | Paracetamol, difenhidramine, metilprednisolone |
| Sarilumab (3.0%) | 0% | SC | |
| Reference tocilizumab (9.9%) | 0% | SC | |
| Tofacitinib (2.0%) | 0% | Oral | Herpes-Zoster vaccine (first year) |
| Upadacitinib (3.9%) | 0% | Oral | Herpes-Zoster vaccine (first year) |
To estimate the pharmacological cost of the treatments, “BOT PLUS” database from the general pharmaceutical council of Spain was used at ex-factory price in 2023. From this price, the discount from Royal Decree-Law 08/201025 was applied (Supplementary table 7) and the price for the annual treatment was estimated using the appropriate dosage or necessary combination treatment (such as the case for anti-TNF-α, bDMARDS and the addition of MTX in a 57% SC–47% PO distribution with their respective prices) for each comparator. Regarding bsTCZ price, it is assumed that, based on the pricing trends of biosimilars in Spain, it will be launched at a price reflecting a 30% discount compared to refTCZ.
Sensitivity analysisA univariant sensitivity analysis was performed for every variable by changing their value in the model according to their probability range to assess the influence of each of the variables in the final results and to detect the way it ensured the credibility of the results. Univariant sensitivity analysis was represented in the tornado diagram based on Net Monetary Benefit. In addition, scenario analysis was performed modifying the probability of switching treatment in patients with high disease activity (0%–100%) or moderate disease activity (0%–100%); adding a range of discount to comparators (0%–10%); and using different probability distributions (normal, gamma, log-normal, and Poisson) to initially allocate patients according to disease severity. The robustness was assessed by a probabilistic sensitivity analysis with a Monte Carlo simulation of second order. Dirichlet/Beta distributions were used for the probabilities, Gamma distribution for the utilities, and log-normal distributions for the costs and hazard ratios.
ResultsLiterature reviewThe PICO-S-T search resulted in 31 studies, that were peer reviewed for inclusion. 18 were excluded by title or abstract and 13 of them were reviewed in full text. Reasons of exclusion included lack of comparators, other outcomes, different type of study, or full-text not available. No NMA that included upadacitinib, baricitinib, sarilumab, and filgotinib were found, and thus ad hoc searches of appropriate clinical trials were carried out to include these comparators in the model.
Finally, regarding efficacy, meta-analyses and a series of clinical trials were included for the different treatments: abatacept, Guyot et al. (2011)26; adalimumab, Burmester et al. (2016); baricitinib, Dougados et al. (2016) and Genovese et al. (2016); certolizumab, Guyot et al. (2011); etanercept, Guyot et al. (2011); filgotinib, Westhovens et al. (2020) and Genovese et al. (2019); golimumab, Guyot et al. (2011); infliximab, Guyot et al. (2011); rituximab, Guyot et al. (2011); sarilumab, Burmester et al. (2016); refTCZ and bsTCZ, Leil et al. (2021)27; tofacitinib, Kremer et al. (2013) and Vollenhoven et al. (2020); and upadacitinib, Genovese et al. (2018) and Vollenhoven et al. (2020).
EffectivenessThe thresholds of DAS28 considered for each health status were as follows: DAS28 < 2.6, remission; 2.6 ≤ DAS28 ≤ 3.2, low disease activity; 3.2 < DAS28 ≤ 5.1, moderate disease activity, and DAS28 > 5.1, high disease activity. Estimated transition probabilities for a patient for the different treatments can be seen in Table 1, with figures between 11% for rituximab and 57% for tocilizumab (refTCZ or bsTCZ) in the first year. In the long term (52 weeks), those treatments with a better performance (higher percentage of patients in the remission status) were tocilizumab, upadacitinib, certolizumab, and filgotinib. Regarding those in which patients were mostly in the moderate-high disease activity status at 52 weeks, rituximab, golimumab, and etanercept can be found. In the Low Disease Activity Status, all treatments showed a similar performance.
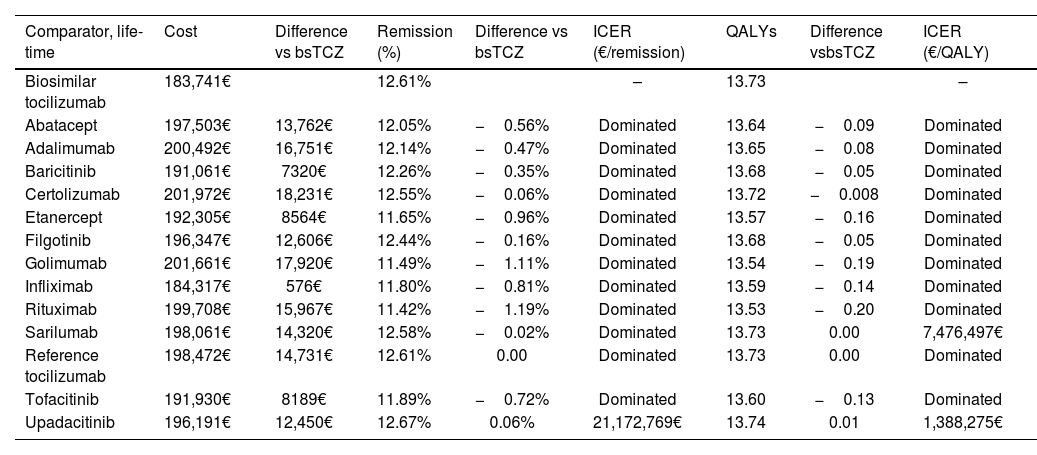
Base caseIn the lifetime horizon, those treatments with a higher percentage of patients on the remission health status were upadacitinib (12.67%), tocilizumab (12.61%), and sarilumab (12.58%) (Table 3). Regarding QALYs, values of 13.74 were obtained for upadacitinib, and 13.73 for sarilumab and tocilizumab. bsTCZ showed the lowest total cost per patient with a figure of 183 741€, followed by infliximab (184 317€) compared to certolizumab, which showed the highest cost per patient (201 972€).
Costs and effectiveness for each treatment in a lifetime horizon.
| Comparator, life-time | Cost | Difference vs bsTCZ | Remission (%) | Difference vs bsTCZ | ICER (€/remission) | QALYs | Difference vsbsTCZ | ICER (€/QALY) |
|---|---|---|---|---|---|---|---|---|
| Biosimilar tocilizumab | 183,741€ | 12.61% | – | 13.73 | – | |||
| Abatacept | 197,503€ | 13,762€ | 12.05% | −0.56% | Dominated | 13.64 | −0.09 | Dominated |
| Adalimumab | 200,492€ | 16,751€ | 12.14% | −0.47% | Dominated | 13.65 | −0.08 | Dominated |
| Baricitinib | 191,061€ | 7320€ | 12.26% | −0.35% | Dominated | 13.68 | −0.05 | Dominated |
| Certolizumab | 201,972€ | 18,231€ | 12.55% | −0.06% | Dominated | 13.72 | −0.008 | Dominated |
| Etanercept | 192,305€ | 8564€ | 11.65% | −0.96% | Dominated | 13.57 | −0.16 | Dominated |
| Filgotinib | 196,347€ | 12,606€ | 12.44% | −0.16% | Dominated | 13.68 | −0.05 | Dominated |
| Golimumab | 201,661€ | 17,920€ | 11.49% | −1.11% | Dominated | 13.54 | −0.19 | Dominated |
| Infliximab | 184,317€ | 576€ | 11.80% | −0.81% | Dominated | 13.59 | −0.14 | Dominated |
| Rituximab | 199,708€ | 15,967€ | 11.42% | −1.19% | Dominated | 13.53 | −0.20 | Dominated |
| Sarilumab | 198,061€ | 14,320€ | 12.58% | −0.02% | Dominated | 13.73 | 0.00 | 7,476,497€ |
| Reference tocilizumab | 198,472€ | 14,731€ | 12.61% | 0.00 | Dominated | 13.73 | 0.00 | Dominated |
| Tofacitinib | 191,930€ | 8189€ | 11.89% | −0.72% | Dominated | 13.60 | −0.13 | Dominated |
| Upadacitinib | 196,191€ | 12,450€ | 12.67% | 0.06% | 21,172,769€ | 13.74 | 0.01 | 1,388,275€ |
bsTCZ: biosimilar tocilizumab; ICER: incremental cost-effectiveness ratio; QALY: quality-adjusted life year; TCZ Biosimilar: tocilizumab biosimilar. Note: A treatment is dominated when tocilizumab biosimilar is less costly and more effective than the alternative.
ICER for both percentage of patients in remission health status or QALY showed that bsTCZ was either dominant or cost-effective versus all the comparators. Apart from the base case, additional scenario analysis were simulated. In the first one, 100% of patients with moderate or high disease activity changed their treatment, in the second one, a 10% discount was applied to ex-factory price to all comparators and 0% to bsTCZ, and lastly, with different allocation of patients by disease at the beginning based on different distributions. Results for the aforementioned cases were maintained being bsTCZ dominant or cost-effective in all the comparisons.
Sensitivity analysisThe deterministic univariant sensitivity analysis showed probability of transition from remission to low disease activity health status, bsTCZ cost in the long term, and probability of transition from low to moderate disease activity health status were the most influential factors in the variability of the results (Supplementary Figs. 1–13). These factors introduced variability in the univariate analysis, but in all scenarios, bsTCZ was the most cost-effective treatment option.
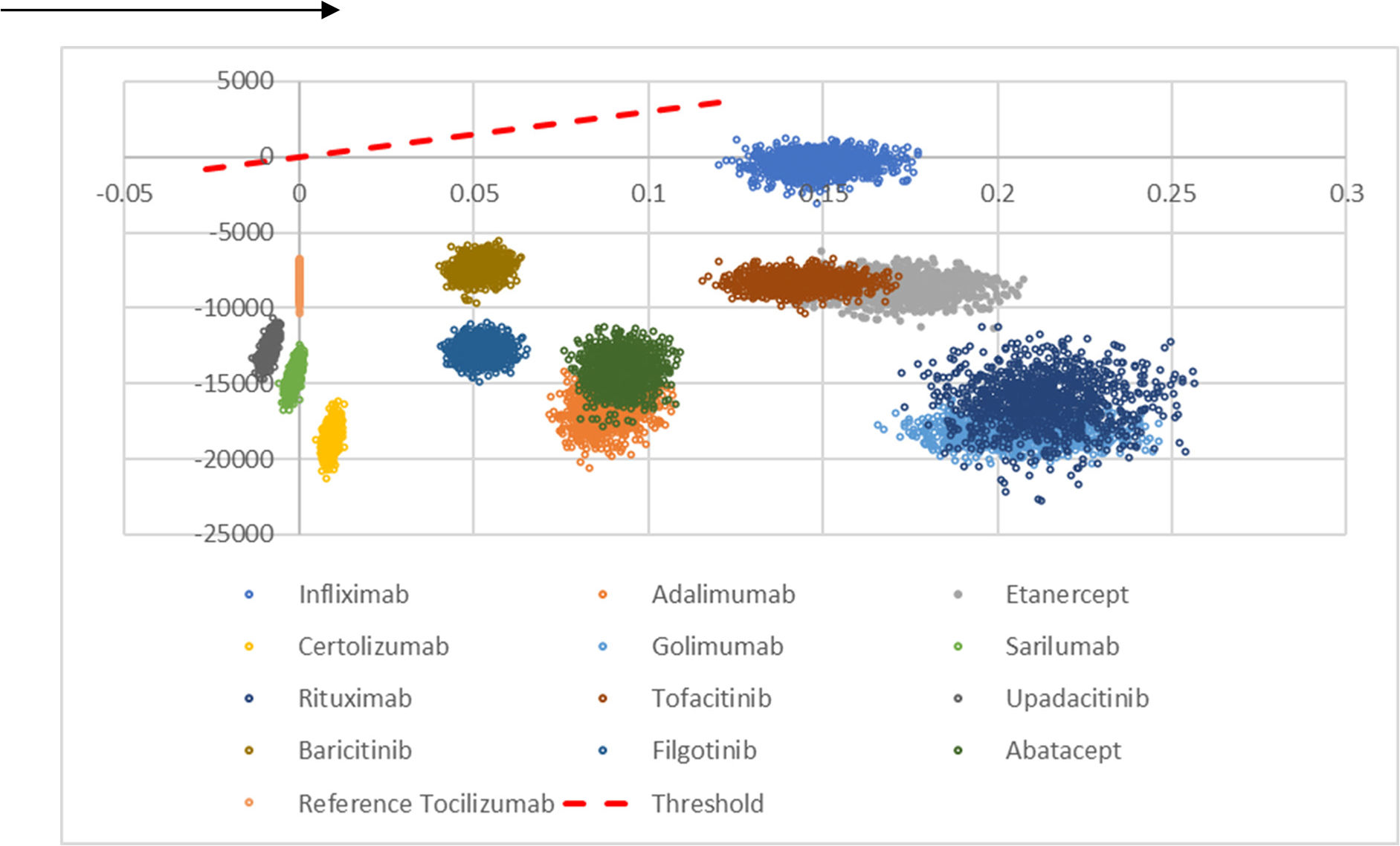
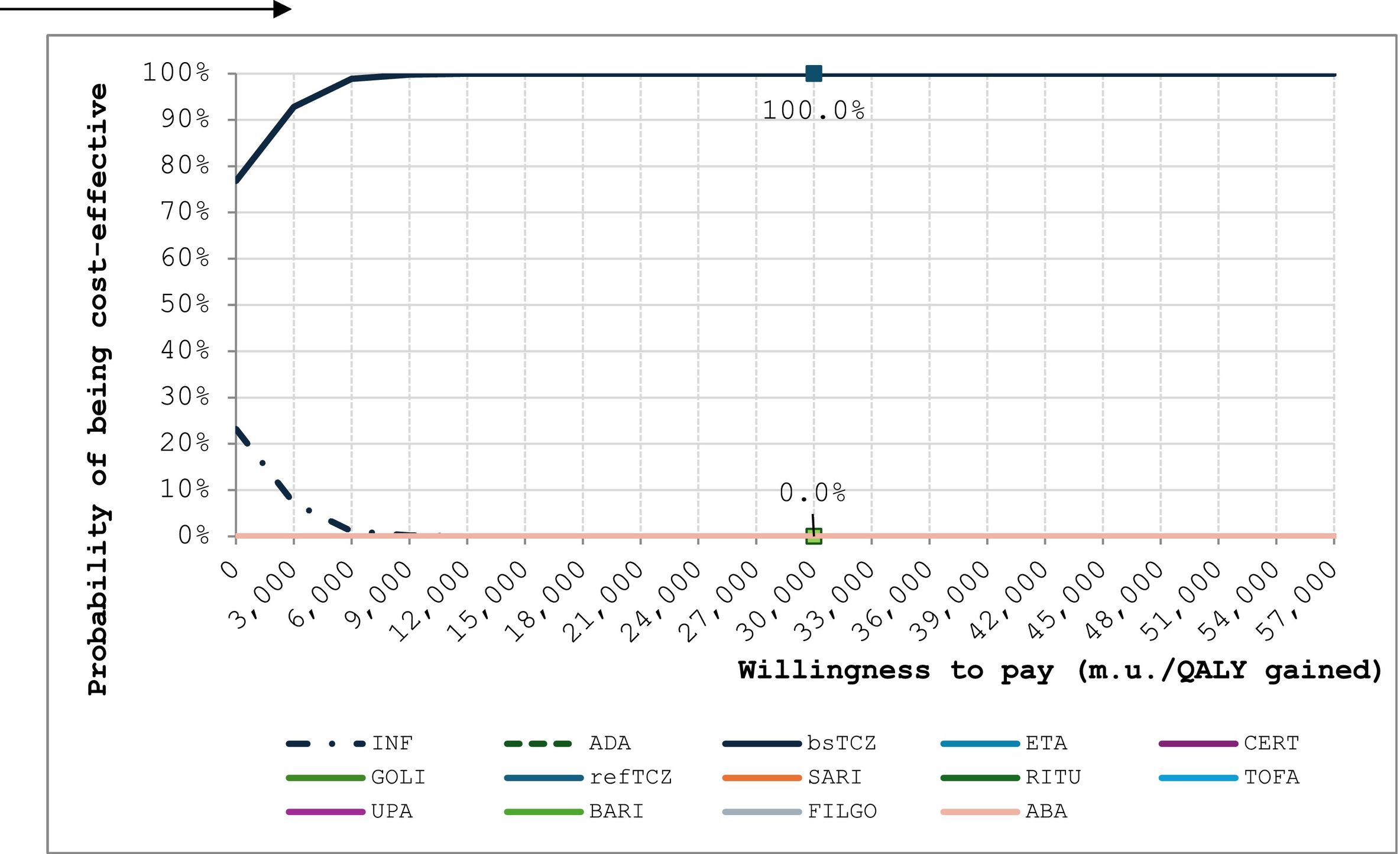
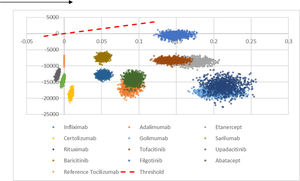
The probabilistic sensitivity analysis (n = 1000 simulations) showed that bsTCZ was either dominant or cost-effective in all the comparisons (Fig. 2). When analyzing the probability of being cost-effective, bsTCZ had a 100% chance of being cost-effective when compared to the other treatments for both the 30 000€ and the 22 000€ thresholds, which are the most used in Spain (Fig. 3).28
Cost-effectiveness plane of tocilizumab biosimilar vs. all the comparators. It represents the 1000 simulations run by the model. Comparisons located in the positive x-axis represent better QALYs for bsTCZ, those in 0 represent same QALYs, and those in negative x-axis represent worse QALYs; comparisons in costs are represented in the y-axis, and all of them are located in the negative y-axis meaning that bsTCZ mostly represents a lower cost (except for the simulations in the comparison vs infliximab).
Acceptability curves for tocilizumab biosimilar and the comparators.
ABA: abatacept; ADA: adalimumab; BARI; baricitinib; CERT: certolizumab; ETA; etanercept; FILGO: filgotinib; GOLI: golimumab; INF: infliximab; RITU: rituximab; SARI: sarilumab; bsTCZ: tocilizumab biosimilar; TOCI: tocilizumab; TOFA: tofacitinib; UPA: upadacitinib.
bDMARDs remain an essential pillar in the management of RA, however, due to their economic impact, their use has been limited in some cases29 and it was not until the introduction of biosimilars that they had a more widespread use. It is important to assess the efficiency of the updated therapeutic arsenal due to the increasing availability and use of biosimilars, to provide decision-makers and clinicians with all the tools to achieve an optimized patient management.
What is more important about the introduction of biosimilars is that efficacy of the treatment remains stable while costs are drastically reduced, entailing great benefits for the healthcare system, and improving its efficiency.30 In the findings of this study, it can be seen that bsTCZ is a good therapeutic alternative for patients suffering RA. Although the differences in the percentage of patients in the remission health status and in QALYs versus the other available treatments are minor, the lowest cost for bsTCZ among all the comparators makes bsTCZ dominate almost all the other alternatives according to their ICER. Even in cases where alternatives achieve higher QALYs, the small difference still positions bsTCZ as cost-effective. As bsTCZ has been recently approved in Europe,13 this is the first health-economic study with the aim to assess its cost-effectiveness in the treatment of patients with RA in Spain in comparison to all the available therapeutic alternatives from a healthcare system perspective.
It is widely known that an early establishment of treatment improves patient outcomes due to the reduction in the time in which the disease is active (such as joint damage). Moreover, there must be a treat-to-target strategy with the purpose of achieving disease remission or low disease activity as early as possible.15 The availability of treatments such as bsTCZ, which has a proven good efficacy profile and an affordable price, could make a difference in the management and treatment access for these patients.
Some assumptions had to be made for this model, for example, the basal distribution of patients among the different health states (low, moderate, and high disease activity) was calculated from the mean basal DAS28 distribution in Leil et al. (2021)21 assuming a normal distribution, and when there was no data regarding patients distribution between moderate-high, 15.3% were calculated to belong to the moderate disease activity health status. It was seen that in the older CTs, DAS28--ESR was mainly used in the past, but has progressively shifted into using DAS28-CRP in the newest trials as endpoint. According to Leil et al. (2021),21 the values between both markers are very similar and moreover, there was no possibility to estimate the health status, so data were assumed to be equivalent. Regarding the lifetime horizon, 2 assumptions were made: the effectiveness of the pivotal studies is assumed up to 52 weeks, from then on, the same transition probabilities have been applied for all treatments from a study for patients on anti-TNF-α therapy.20 From week 52 onwards, patients in the high disease activity health status switched to a treatment pool defined according to the market share in Spain. All the assumptions were made in a conservative manner, always in detriment of bsTCZ results so that obtained results and extracted conclusions from this analysis were not overestimating bsTCZ or underestimating its comparators. Even though a sensitivity analysis using different ranges of discount for the comparators was carried out, it should be considered that ex-factory prices are used in this analysis as final reimbursed prices are not publicly available and this might have an impact in the final results. We believe that it will prove to be useful for all relevant stakeholders involved in the treatment of RA to have an updated and refreshed pharmacoeconomic exercise available, due to the increasingly expanding availability of therapeutic options for this condition.
Even though there are no specific cost-effectiveness studies of bsTCZ for the treatment of RA in other countries, studies of other biosimilars in RA31,32 or bsTCZ in the treatment of other pathologies have shown that biosimilars are a cost-effective and cost-saving alternative33,34 and thus biosimilars should be positioned early in the treatment scheme to leverage its benefits. Introduction of biosimilars allows for cost savings and in the case of Spain, it has been reported that increased biosimilar adoption would allow additional patients to be treated by redirecting these savings,34 improving healthcare resource allocation and a better patient care.
In conclusion, this study demonstrates through a proposed model that bsTCZ is either dominant or at least cost-effective versus all the therapeutic alternatives for the treatment of RA from the Spanish healthcare system perspective.
Sources of fundingThis work was funded by Fresenius Kabi Spain S.A.U.
Submissions for presentation at congresses:Organization 1: the European Alliance of Associations for Rheumatology
Venue: Messe Wien Congress Center, Vienna, Austria.
Date: June, 12th–15th, 2024
Organization 2: the Spanish Society of Rheumatology.
Venue: Palacio de Congresos Expomeloneras, Gran Canaria, Spain.
Date: May, 7th–10th, 2024
Ethical ConsiderationsAll authors accept the responsibilities defined by the International Committee of Medical Journal Editors (ICMJE). The study was performed using administrative and public data. No patient information was used.
Transparency DeclarationThe corresponding author, in the name of the rest of the signatories, declares that the data and information contained in the study are precise, transparent, and honest; that no relevant information has been omitted; and that all the discrepancies among authors have been adequately resolved and described”.
Liability and transfer of rightsThe co-authors accept the responsibilities defined by the International Committee of Medical Journal Editors (available at http://www.icmje.org/).
In the event of publication, the authors exclusively transfer to the Revista and, by extension, to SEFH their rights to reproducing, distributing, translating, and publicly communicating their work by any sound, audiovisual or electronic medium or format. To that effect, a letter transferring such rights shall be attached to the paper when submitting it through the online manuscript management system.
Contributions to scientific literatureThe study constitutes a cost-effectiveness comparison of biosimilar tocilizumab versus other therapeutic alternatives for the treatment of patients suffering moderate to severe rheumatoid arthritis in Spain.
The analysis provides an insight on the cost-effectiveness of different treatment alternatives thus, providing clinicians, hospital pharmacists, and decision makers with evidence to consider the most appropriate choice for the management of these patients from both a clinical and economic perspective.
CRediT authorship contribution statementFernando Pérez-Ruiz: Writing – review & editing, Validation, Supervision, Conceptualization. Carlos Crespo-Diz: Writing – review & editing, Validation, Supervision, Conceptualization. Joan Antoni Schoenenberger-Arnaiz: Writing – review & editing, Validation, Supervision, Conceptualization. Mónica Cerezales: Writing – review & editing, Writing – original draft, Visualization, Project administration, Methodology, Investigation, Formal analysis, Conceptualization. Carlos Crespo: Writing – review & editing, Writing – original draft, Validation, Project administration, Methodology, Investigation, Formal analysis, Conceptualization. Marcelo Alejandro Guigini: Writing – review & editing, Validation, Supervision, Conceptualization. José Ignacio Peinado-Fabregat: Writing – review & editing, Validation, Supervision, Conceptualization. Mónica Climente-Martí: Writing – review & editing, Validation, Supervision, Conceptualization.
FPR has earned advisory fees from protalix, horizon, arthrosis, and LG Pharma; speaker fees from Menarini, and Bioepis; and research grants from Asociación de Reumatólogos de Cruces. CCD has earned fees from: Abbvie, Almirall, Amgen, Biogen, Bristol Myers Squibb, Fresenius Kabi, Janssen-Cilag, Kern Pharma, Merck Sharp Done, Pfizer, Roche, UCB Pharma. JASA has earned fees from Fresenius Kabi and IQVIA. MC and CC are employees of Axentiva, a consulting firm that works for several pharmaceutical and medical devices companies. MAG and JIPF are employees of Fresenius Kabi Spain. MCM has earned fees from Abbvie, Fresenius Kabi, Janssen-Cilag, Kern Pharma, Leo-Pharma, Pfizer, and Roche.