Adalimumab biosimilar MSB11022 (Idacio ®) has been approved for the same indications as its originator (Humira ®), based on findings from clinical trials in plaque psoriasis. Data on its efficacy and safety in inflammatory bowel disease, however, are scarce.
MethodsRetrospective, observational study of 44 patients with inflammatory bowel disease: 30 were treated with originator adalimumab, 5 were directly started on MSB11022, and 9 switched from originator to biosimilar adalimumab. To evaluate the effectiveness of the use of adalimumab in inflammatory bowel disease, both laboratory markers (fecal calprotectin and C-reactive protein) and scales that measure the activity of inflammatory bowel disease using specific scales (Harvey-Bradshaw Index (HBI) have been usEd.) for Crohn's disease and Mayo Score for Ulcerative Colitis. Efficacy was evaluated by recording the adverse effects that could occur with the administration of adalimumab (original or biosimilar). The success of the switch was determined by analyzing meaningful differences in effectiveness and safety criteria. Concomitant therapy and the need for dose intensification were also analyzed. Objective of this study was to assess the effectiveness and safety of biosimilar adalimumab in adalimumab-naïve patients and patients switched from originator adalimumab.
ResultsNo significant differences were observed in clinical disease activity (P=.317) or biochemical parameters [fecal calprotectin (P=.445) and C-reactive protein P=.661)] after the switch from the originator adalimumab to MSB11022. There was not a significant reduction in the concomitant use of corticosteroids and thiopurines (P=.157). No emergency room visits or hospitalizations were observed during the study period and none of the patients experienced serious adverse effects.
ConclusionsBetween originator adalimumab and biosimilar-start cohorts, no differences were observed, between originator adalimumab and switch cohorts, no significant differences were found either, and with the pre- and post-switch to biosimilar comparison, 2 of the 9 patients experienced AEs after the switch.
The biosimilar showed a favorable safety profile (one patient with a serious adverse effect (rash) with biosimilar discontinued treatment) and no significant changes to clinical or biochemical parameters were observed after the switch.
Adalimumab biosimilar MSB11022 (Idacio ®) ha sido aprobado para las mismas indicaciones que el fármaco original (Humira ®), basado en los resultados de los ensayos clínicos en psoriasis en placa. Los resultados de eficacia y seguridad en enfermedad inflamatoria intestinal son escasos.
Material y MetodosSe realizó un estudio observacional retrospectivo incluyendo 44 pacientes con enfermedad inflamatoria intestinal: 30 fueron tratados con adalimumab original, cinco pacientes empezaron directamente con MSB11022 y nueve se cambiaron del adalimumab original al biosimilar. Para la evaluación de la eficacia del uso de adalimumab en enfermedad inflamatoria intestinal se han empleado tanto marcadores de laboratorio (calprotectina fecal y proteína C reactiva) como escalas que miden la actividad de la enfermedad inflamatoria intestinal empelando escalas específicas (Harvey-Bradshaw Index (HBI) para enfermedad de Crohn y Mayo Score para Colitis Ulcerosa). La eficacia se evaluó registrando los efectos adversos que se pudiesen presentar la administración de adalimumab (original o biosimilar). El éxito del cambio se determinó comparando diferencias relevantes en criterios de eficacia y seguridad. Así mismo, se analizó la medicación concomitante y la necesidad de intensificaciones de dosis. El objetivo del presente estudio fue evaluar la efectividad y seguridad del biosimilar de adalimumab en pacientes naïve y en pacientes que había realizado el cambio desde el adalimumab original.
ResultadosNo se detectaron diferencias significativas en los criterios de progresión clínica (p = 0,317) o en los parámetros bioquímicos (calprotectina fecal [p = 0,445] y proteína C reactiva [p = 0,661]) después de realizar el cambio del adalimumab original al MSB11022. Se apreció una reducción no significativa en el uso de corticoides y tiopurinas (p = 0,157). Durante el periodo de estudio, no se registraron visitas al servicio de urgencias ni hospitalizaciones de los pacientes analizados, ningún paciente presento ningún efecto adverso grave.
ConclusionesNo se presentaron diferencias entre los grupos que recibieron adalimumab original y los que empezaron con el biosimilar, tampoco se apreciaron diferencias entre los que recibieron adalimumab original y los que realizaron el cambio al biosimilar y en el análisis pre y post de los pacientes que cambiaron a adalimumab biosimilar, se registraron dos pacientes que presentaron efectos adversos después del cambio.
El biosimilar ha demostrado un perfil favorable (solo un paciente presentó un rash cutáneo que implicó la interrupción de su tratamiento) no apreciándose cambios en los parámetros clínicos o biológicos tras cambiar de adalimumab original a adalimumab biosimilar.
Inflammatory bowel disease (IBD) comprises different immune-mediated and auto-immune diseases that cause inflammation inside or outside the gut (extraintestinal manifestations). The 2 main types of IBD are Crohn disease (CD) and ulcerative colitis (UC), both chronic, progressive diseases with irregular periods of remission and relapse.1
Early diagnosis and treatment of IBD are essential for enhancing patient quality of life, preventing complications, and ensuring effective response. Current treatment strategies are based on different factors including disease severity, site and behavior, and response to treatment.2,3 The common pharmacological drugs used for IBD are corticosteroids, immunosuppressants [thiopurines (azathioprine, mercaptopurine) or methotrexate], and biologicals.4
Biologic drugs are large, complex, 3-dimensional structures produced using living organisms that are highly sensitive to environmental changes and external conditions. Because their production is a complex, multi-stage process, no 2 drugs are identical. Hence, biosimilars are biological products, similar, but not identical, to an already approved biological drug, named “originator.”5,6
Prognosis of IBD has been greatly improved by the introduction of biologics, in particular, monoclonal antibodies against tumor necrosis factor-alpha (anti-TNF-α).2 The originator adalimumab Humira ®, a fully human IgG1 antibody, was approved for medical use in 2002 and rapidly became the backbone therapy for moderate to severe IBD. Biologic therapy in IBD, places a significant financial burden on healthcare systems, limiting patient access to appropriate therapy.3 With many biotechnologically derived products reaching the end of their primary patent, more affordable treatment options of ADA biosimilars have been approved in recent years.7,8
Biosimilars approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) are considered equivalent to their biologic reference products, or originators. Switches from originators to biosimilars are therefore permitted. Once biosimilarity has been demonstrated in one indication, extrapolation to other indications of the originator is acceptable.9 The interchangeability of the ADA originator and biosimilars in the setting of IBD, however, is not without controversy.5,10
The FDA and EMA have approved several ADA biosimilars for use in IBD, but efficacy and safety are scarce. Biosimilar adalimumab (Idacio ®) was approved based on findings from the phase III AURIEL-PsO trial demonstrating equivalence to originator adalimumab (Humira ®), in moderate to severe chronic plaque-type psoriasis.11
Randomized controlled trials comparing biosimilars with the originator adalimumab are unavailable, and few real-world studies have analyzed outcomes in IBD patients switched to a biosimilar.12 No studies to date have analyzed efficacy and safety outcomes in IBD biologic-naïve patients started on adalimumab MSB11022 or in IBD patients switched from originator adalimumab to adalimumab MSB11022.
The primary aim of this study was to analyze effectiveness and safety after switching from originator adalimumab (Humira ®) to the biosimilar adalimumab MSB11022 (Idacio ®) in patients with IBD. A secondary aim was to compare outcomes in patients directly started on biosimilar and those treated with the originator adalimumab.
Materials and methodsA retrospective, observational study in a real-life cohort of adult patients with IBD was performed. Inclusion criteria were IBD diagnosed according to the European Crohn's and Colitis Organization (ECCO) criteria8,9; an age ≥18 years; ongoing treatment with the ADA originator and/or adalimumab biosimilar for at least 3 months in the non-switch cohorts and more than 6 months in the switch cohort. Exclusion criteria were patients who did not meet the above criteria, patients treated with a biosimilar adalimumab other than Idacio ®, and pregnant patients.
To calculate the sample size, we selected all patients who had started directly with the biosimilar adalimumab, all those who made the switch from the original to the biosimilar, and as a control group to be able to compare with the previous groups, all the patients who were receiving the original adalimumab.
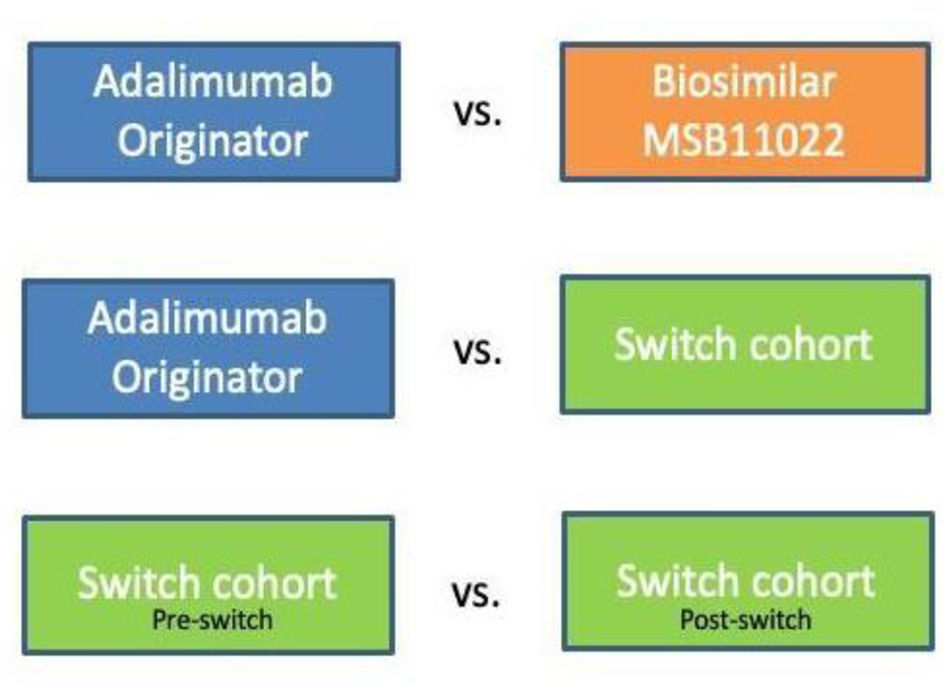
To analyze the effectiveness and safety of biosimilar adalimumab MSB11022 following the originator-to-biosimilar switch, we checked that there were no significant baseline differences between patients (Fig. 1):
- a)
Originator adalimumab cohort vs. patients who were directly started on biosimilar adalimumab MSB11022 cohort.
- b)
Switched from originator adalimumab to biosimilar adalimumab MSB11022 vs. patients from the originator adalimumab cohort.
- c)
Before and after the switch from originator adalimumab to biosimilar adalimumab MSB11022 in the switch cohort.
The data were collected from the Cerner Millennium electronic health record platform. Access to medical records for judicial, epidemiological, public health, research, or teaching purposes is governed by the provisions of current legislation on the protection of personal data, and by Law 14/1986, of 25 April, General Health Law.13 The Research Committee at our hospital approved this study and the analysis of data on all patients treated with ADA and subsequent correlations with clinical data.
The study was considered a review of clinical practice without patient intervention. Thus, no written consent or formal ethical approval. Appropriate measures were taken to guarantee full confidentiality of the patients analyzed in accordance with the Spanish Organic Law 3/2018, of December 5, on the Protection of Personal Data and Guarantee of Digital Rights.14 All data were duly anonymized to protect patient identities.
The following information was collected:
- •
Demographic information. Sex, age at IBD diagnosis, and smoking history.
- •
Clinical information. Diagnosis, history of IBD-related surgery before ADA treatment (yes/no, type), extraintestinal manifestations, use of another biologic before ADA, switch to a biologic other than ADA (yes/no, reason).
- •
Montreal classification status. For CD: Age at diagnosis, location, behavior, and history of perianal disease. For UC: Disease extent and Mayo score (activity index).
- •
Treatment. Start and stop dates; total duration (months); dose intensification; IBD-related emergency room (ER) visits during treatment in the study period (yes/no, number of visits); IBD-related hospitalization during treatment in the study period (yes/no, number of days); IBD-related surgery during treatment in the study period (yes/no, type).
- •
Concomitant or sporadic use of immunosuppressants (thiopurines) or corticosteroids.
- •
Laboratory biomarkers. Fecal calprotectin (FC) (mg/kg) and C-reactive protein (CRP) (mg/L). FC is a stool inflammatory marker that correlates with neutrophil infiltrates in the gut and is used to detect intestinal inflammation. CRP is an interleukin-6-dependent acute-phase reactant that correlates with the inflammatory burden. Although it is not very specific, it is used to stratify IBD severity. Correlations have been observed between CRP and FC levels and endoscopic disease activity.
- •
IBD activity. Harvey-Bradshaw Index (HBI) for CD and partial Mayo Score for UC. HBI categorizes disease depending on 5 criteria while partial Mayo Score considers 3 clinical parameters. In both cases, final scores are obtained by adding up the scores of the individual items.15
There is no gold-standard approach for assessing clinical response to anti-TNF agents in IBD.16,17 In addition, clinical manifestations do not always correlate with objective assessments of disease activity, such as endoscopic activity or biomarker levels. Treatment effectiveness is therefore assessed using a combination of clinical data (e.g., activity scores) and biochemical data (e.g., biomarker levels).
Biochemical remission has been defined as an FC level ≤250 mg/kg and a CRP level ≤5 mg/L.18 According to the literature, remission in CD is defined as an HBI of 5 or less. Scores of 5–7 indicate mild activity; 8–16, moderate activity; and >16, severe activity. In the case of UC, partial Mayo scores of <2 indicate remission; 2–4, mild activity; 5–7, moderate activity; and >7, severe activity.15,17,19
Biosimilar adalimumab MSB11022 effectiveness was analyzed by comparing biochemical markers (FC and CRP) at baseline (first pre-switch levels recorded during the study period) and after the switch (last levels recorded during the study period). Both parameters were analyzed as continuous variables. Clinical remission was established as an HBI <4 for CD and a partial Mayo score ≤1 for UC. These scores were analyzed as categorical variables.18,20,21 Treatment failure was defined as meaningful clinical and biochemical differences compared with the ADA originator, need for dose intensification, need for concomitant treatment with corticosteroids or thiopurines, switch to another biologic, and IBD-related hospitalization, surgery,10,21,22 and/or ER visits. Safety was assessed by analyzing treatment interruptions and AEs.10,20,21 All AEs reported during follow-up, regardless of a potential link to biosimilar adalimumab MSB11022, were analyzed.19
Descriptive statistics were used to describe demographic and clinical characteristics. Categorical variables are expressed as numbers and percentages. The D'Agostino-Pearson test was used to assess the distribution of continuous variables, which were expressed as mean±standard deviation when normally distributed and median and interquartile range (IQR) otherwise.
Categorical variables were compared using the Fisher exact or chi-square test, as appropriate. Independent normally and non-normally distributed data were compared using the independent t-test and the Mann–Whitney U test, respectively. The non-parametric McNemar test was used to compare pre-and post-switch continuous variables by checking for significant differences between binary paired categorical variables.
A power calculation was not necessary, as this was an observational cohort study of all patients from our hospital who met the inclusion criteria. Statistical analysis was performed in Intercooled Stata 9.1 and Epidat 3.1. Statistical significance was set at a P-value of less than .05. A value of .05–.10 was considered to indicate a trend towards significance.
ResultsForty-four patients with CD or UC treated with originator adalimumab or biosimilar adalimumab MSB11022 at our hospital, between January 1, 2019 and February 28, 2022 were included. Thirty were assigned to the originator adalimumab cohort, 5 to the biosimilar adalimumab MSB11022 -start cohort, and 9 to the switch cohort.
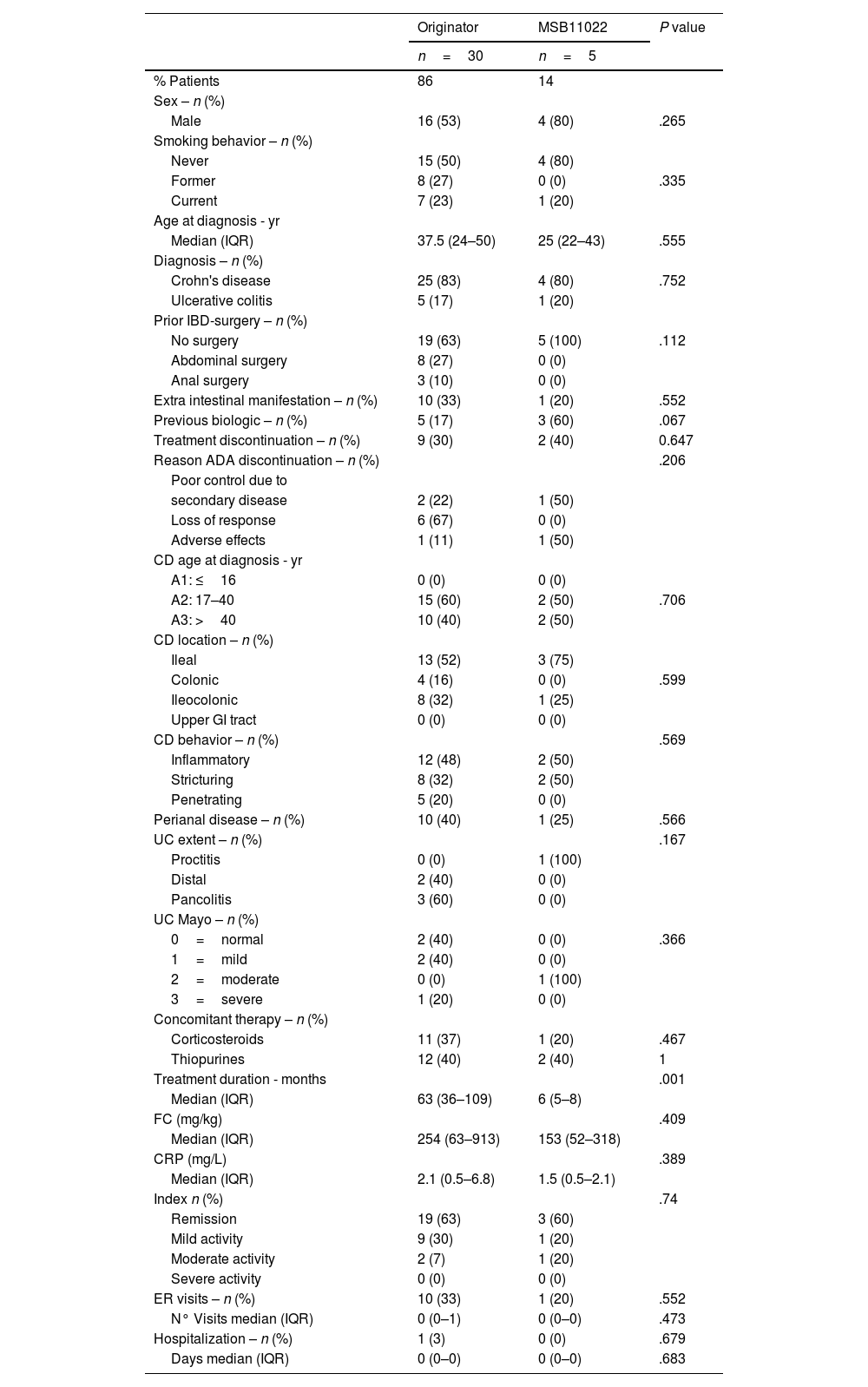
Baseline comparison of ADA originator (n=30) and MSB11022-start cohorts (n=5)The baseline comparison of the originator adalimumab and biosimilar adalimumab MSB11022 start cohorts involved 35 patients: 30 from the originator cohort and 5 from the biosimilar-start cohort. Their baseline demographic and clinical characteristics are shown in Table 1. No statistically significant differences between the 2 groups (originator adalimumab and biosimilar adalimumab MSB11022 start cohorts) were noted concerning age, gender, disease duration, disease behavior, tobacco smoking, concomitant therapy, and clinical characteristics. The only significant difference observed was a longer treatment duration in the originator adalimumab cohort (63 months vs. 6 months, P=.001)). There was a trend towards significance (P=.067) for the use of another biologic prior to adalimumab treatment, with a higher proportion of previous users in the biosimilar adalimumab MSB11022 start cohort. No differences were observed for treatment discontinuation (P=.647). One patient in the originator adalimumab cohort required hospitalization.
Baseline demographic data and clinical characteristics of the comparison: patients treated with originator vs. patients who started with MSB11022.
| Originator | MSB11022 | P value | |
|---|---|---|---|
| n=30 | n=5 | ||
| % Patients | 86 | 14 | |
| Sex – n (%) | |||
| Male | 16 (53) | 4 (80) | .265 |
| Smoking behavior – n (%) | |||
| Never | 15 (50) | 4 (80) | |
| Former | 8 (27) | 0 (0) | .335 |
| Current | 7 (23) | 1 (20) | |
| Age at diagnosis - yr | |||
| Median (IQR) | 37.5 (24–50) | 25 (22–43) | .555 |
| Diagnosis – n (%) | |||
| Crohn's disease | 25 (83) | 4 (80) | .752 |
| Ulcerative colitis | 5 (17) | 1 (20) | |
| Prior IBD-surgery – n (%) | |||
| No surgery | 19 (63) | 5 (100) | .112 |
| Abdominal surgery | 8 (27) | 0 (0) | |
| Anal surgery | 3 (10) | 0 (0) | |
| Extra intestinal manifestation – n (%) | 10 (33) | 1 (20) | .552 |
| Previous biologic – n (%) | 5 (17) | 3 (60) | .067 |
| Treatment discontinuation – n (%) | 9 (30) | 2 (40) | 0.647 |
| Reason ADA discontinuation – n (%) | .206 | ||
| Poor control due to | |||
| secondary disease | 2 (22) | 1 (50) | |
| Loss of response | 6 (67) | 0 (0) | |
| Adverse effects | 1 (11) | 1 (50) | |
| CD age at diagnosis - yr | |||
| A1: ≤16 | 0 (0) | 0 (0) | |
| A2: 17–40 | 15 (60) | 2 (50) | .706 |
| A3: >40 | 10 (40) | 2 (50) | |
| CD location – n (%) | |||
| Ileal | 13 (52) | 3 (75) | |
| Colonic | 4 (16) | 0 (0) | .599 |
| Ileocolonic | 8 (32) | 1 (25) | |
| Upper GI tract | 0 (0) | 0 (0) | |
| CD behavior – n (%) | .569 | ||
| Inflammatory | 12 (48) | 2 (50) | |
| Stricturing | 8 (32) | 2 (50) | |
| Penetrating | 5 (20) | 0 (0) | |
| Perianal disease – n (%) | 10 (40) | 1 (25) | .566 |
| UC extent – n (%) | .167 | ||
| Proctitis | 0 (0) | 1 (100) | |
| Distal | 2 (40) | 0 (0) | |
| Pancolitis | 3 (60) | 0 (0) | |
| UC Mayo – n (%) | |||
| 0=normal | 2 (40) | 0 (0) | .366 |
| 1=mild | 2 (40) | 0 (0) | |
| 2=moderate | 0 (0) | 1 (100) | |
| 3=severe | 1 (20) | 0 (0) | |
| Concomitant therapy – n (%) | |||
| Corticosteroids | 11 (37) | 1 (20) | .467 |
| Thiopurines | 12 (40) | 2 (40) | 1 |
| Treatment duration - months | .001 | ||
| Median (IQR) | 63 (36–109) | 6 (5–8) | |
| FC (mg/kg) | .409 | ||
| Median (IQR) | 254 (63–913) | 153 (52–318) | |
| CRP (mg/L) | .389 | ||
| Median (IQR) | 2.1 (0.5–6.8) | 1.5 (0.5–2.1) | |
| Index n (%) | .74 | ||
| Remission | 19 (63) | 3 (60) | |
| Mild activity | 9 (30) | 1 (20) | |
| Moderate activity | 2 (7) | 1 (20) | |
| Severe activity | 0 (0) | 0 (0) | |
| ER visits – n (%) | 10 (33) | 1 (20) | .552 |
| N° Visits median (IQR) | 0 (0–1) | 0 (0–0) | .473 |
| Hospitalization – n (%) | 1 (3) | 0 (0) | .679 |
| Days median (IQR) | 0 (0–0) | 0 (0–0) | .683 |
IBD: inflammatory bowel disease; ADA: adalimumab; CD: Crohn's disease; GI: gastrointestinal; UC: ulcerative colitis; FC: fecal calprotectin; CRP: C-reactive protein; ER: emergency room.
Three of the five patients in the biosimilar adalimumab MSB11022 start cohort experienced AEs (2 cases of musculoskeletal pain and 1 case of hair loss and skin rash). The patient who experienced hair loss and the skin rash discontinued treatment.
Eight of the 30 patients in the originator adalimumab group experienced AEs (3 cases of hypertransaminasemia and 1 case each of periocular rash, bone fracture, musculoskeletal pain, herpes zoster, blurry vision, headache, cataract formation, and body tremors). One patient discontinued treatment because of cataract formation.
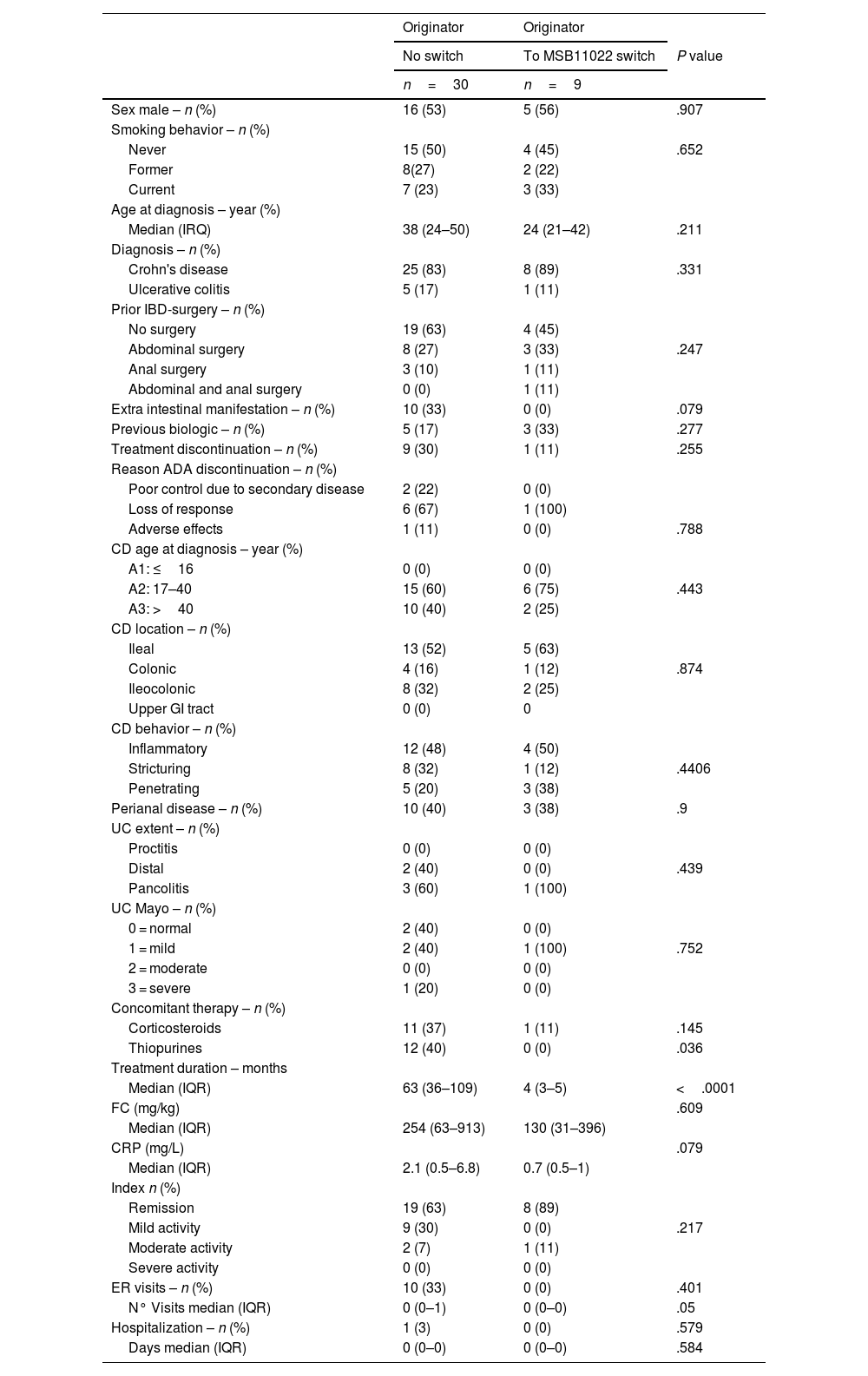
Baseline comparison of ADA originator (n=30) and switch cohorts (n=9)Thirty-nine patients were included in the baseline comparison of patients treated with the originator adalimumab only (n=30) and those switched from originator adalimumab to biosimilar adalimumab MSB11022 (n=9). Their demographic and clinical characteristics are summarized in Table 2.
Results baseline demographic and clinical characteristics of the comparison: patients treated with originator vs. patients who switch to MSB11022.
| Originator | Originator | ||
|---|---|---|---|
| No switch | To MSB11022 switch | P value | |
| n=30 | n=9 | ||
| Sex male – n (%) | 16 (53) | 5 (56) | .907 |
| Smoking behavior – n (%) | |||
| Never | 15 (50) | 4 (45) | .652 |
| Former | 8(27) | 2 (22) | |
| Current | 7 (23) | 3 (33) | |
| Age at diagnosis – year (%) | |||
| Median (IRQ) | 38 (24–50) | 24 (21–42) | .211 |
| Diagnosis – n (%) | |||
| Crohn's disease | 25 (83) | 8 (89) | .331 |
| Ulcerative colitis | 5 (17) | 1 (11) | |
| Prior IBD-surgery – n (%) | |||
| No surgery | 19 (63) | 4 (45) | |
| Abdominal surgery | 8 (27) | 3 (33) | .247 |
| Anal surgery | 3 (10) | 1 (11) | |
| Abdominal and anal surgery | 0 (0) | 1 (11) | |
| Extra intestinal manifestation – n (%) | 10 (33) | 0 (0) | .079 |
| Previous biologic – n (%) | 5 (17) | 3 (33) | .277 |
| Treatment discontinuation – n (%) | 9 (30) | 1 (11) | .255 |
| Reason ADA discontinuation – n (%) | |||
| Poor control due to secondary disease | 2 (22) | 0 (0) | |
| Loss of response | 6 (67) | 1 (100) | |
| Adverse effects | 1 (11) | 0 (0) | .788 |
| CD age at diagnosis – year (%) | |||
| A1: ≤16 | 0 (0) | 0 (0) | |
| A2: 17–40 | 15 (60) | 6 (75) | .443 |
| A3: >40 | 10 (40) | 2 (25) | |
| CD location – n (%) | |||
| Ileal | 13 (52) | 5 (63) | |
| Colonic | 4 (16) | 1 (12) | .874 |
| Ileocolonic | 8 (32) | 2 (25) | |
| Upper GI tract | 0 (0) | 0 | |
| CD behavior – n (%) | |||
| Inflammatory | 12 (48) | 4 (50) | |
| Stricturing | 8 (32) | 1 (12) | .4406 |
| Penetrating | 5 (20) | 3 (38) | |
| Perianal disease – n (%) | 10 (40) | 3 (38) | .9 |
| UC extent – n (%) | |||
| Proctitis | 0 (0) | 0 (0) | |
| Distal | 2 (40) | 0 (0) | .439 |
| Pancolitis | 3 (60) | 1 (100) | |
| UC Mayo – n (%) | |||
| 0 = normal | 2 (40) | 0 (0) | |
| 1 = mild | 2 (40) | 1 (100) | .752 |
| 2 = moderate | 0 (0) | 0 (0) | |
| 3 = severe | 1 (20) | 0 (0) | |
| Concomitant therapy – n (%) | |||
| Corticosteroids | 11 (37) | 1 (11) | .145 |
| Thiopurines | 12 (40) | 0 (0) | .036 |
| Treatment duration – months | |||
| Median (IQR) | 63 (36–109) | 4 (3–5) | <.0001 |
| FC (mg/kg) | .609 | ||
| Median (IQR) | 254 (63–913) | 130 (31–396) | |
| CRP (mg/L) | .079 | ||
| Median (IQR) | 2.1 (0.5–6.8) | 0.7 (0.5–1) | |
| Index n (%) | |||
| Remission | 19 (63) | 8 (89) | |
| Mild activity | 9 (30) | 0 (0) | .217 |
| Moderate activity | 2 (7) | 1 (11) | |
| Severe activity | 0 (0) | 0 (0) | |
| ER visits – n (%) | 10 (33) | 0 (0) | .401 |
| N° Visits median (IQR) | 0 (0–1) | 0 (0–0) | .05 |
| Hospitalization – n (%) | 1 (3) | 0 (0) | .579 |
| Days median (IQR) | 0 (0–0) | 0 (0–0) | .584 |
IBD: inflammatory bowel disease; ADA: adalimumab; CD: Crohn's Disease; GI: Gastrointestinal; UC: Ulcerative Colitis; FC: fecal calprotectin; CRP: C-reactive protein; ER: Emergency room.
No statistically significant differences between the 2 groups (originator adalimumab and switch cohorts) were noted concerning age, gender, disease duration, disease behavior, tobacco smoking, and clinical findings regarding FC, CRP, and disease index. Significant differences were observed between the originator cohort and the switch cohort for use of thiopurines (12 vs. 0, P=.036, respectively) and treatment duration (63 vs. 4 months, P<.001 respectively). There was a trend towards higher CRP levels (2.1 vs. 0.7, P=.079) and more IBD-related ER visits in the originator adalimumab group (P=.050). The patients in the switch cohort had a lower median age at diagnosis of IBD, but the difference was not significant (P=.211).
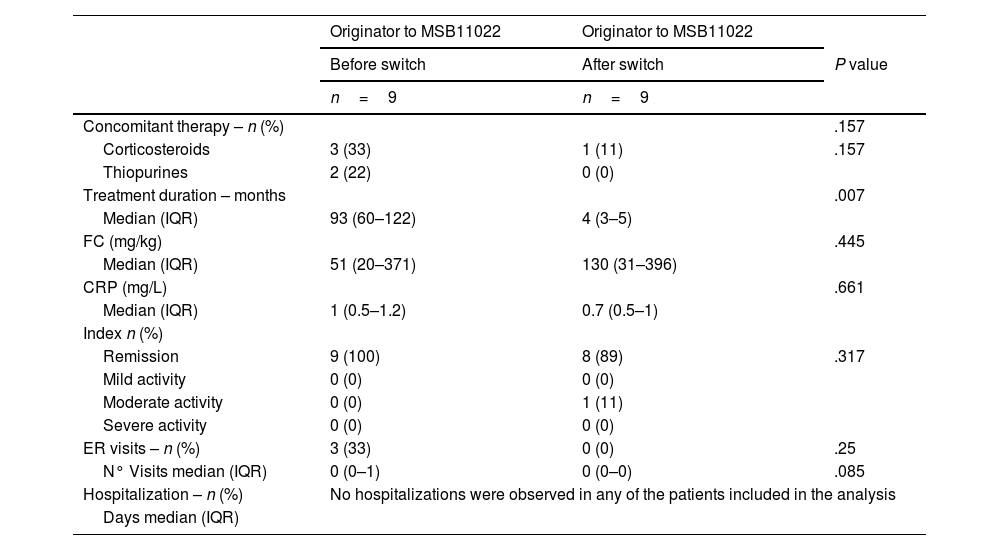
Pre- and post-switch comparison (n=9)The 9 patients from the switch cohort were included in the comparison of data from before and after the switch from originator adalimumab to biosimilar adalimumab MSB11022 cohort. Their clinical and biochemical characteristics are shown in Table 3.
Clinical and biochemical characteristics of the analysis before and after the switch.
| Originator to MSB11022 | Originator to MSB11022 | ||
|---|---|---|---|
| Before switch | After switch | P value | |
| n=9 | n=9 | ||
| Concomitant therapy – n (%) | .157 | ||
| Corticosteroids | 3 (33) | 1 (11) | .157 |
| Thiopurines | 2 (22) | 0 (0) | |
| Treatment duration – months | .007 | ||
| Median (IQR) | 93 (60–122) | 4 (3–5) | |
| FC (mg/kg) | .445 | ||
| Median (IQR) | 51 (20–371) | 130 (31–396) | |
| CRP (mg/L) | .661 | ||
| Median (IQR) | 1 (0.5–1.2) | 0.7 (0.5–1) | |
| Index n (%) | |||
| Remission | 9 (100) | 8 (89) | .317 |
| Mild activity | 0 (0) | 0 (0) | |
| Moderate activity | 0 (0) | 1 (11) | |
| Severe activity | 0 (0) | 0 (0) | |
| ER visits – n (%) | 3 (33) | 0 (0) | .25 |
| N° Visits median (IQR) | 0 (0–1) | 0 (0–0) | .085 |
| Hospitalization – n (%) | No hospitalizations were observed in any of the patients included in the analysis | ||
| Days median (IQR) | |||
FC: fecal calprotectin; CRP: C-reactive protein; ER: Emergency room.
Significant differences were found in treatment duration, it was longer in the pre-switch period (93 vs. 4 months P=.008). Concomitant and sporadic use of corticosteroids and thiopurines decreased significantly after the switch, but it was not significant (P=.157). A trend towards significance was observed for the number of ER visits (P=.085), with fewer visits occurring after the switch.
Two of the nine patients experienced AEs after the switch (hypertransaminasemia in one case and musculoskeletal pain in the other). Neither of them discontinued treatment as a result. None of the patients required hospitalization or surgery.
Just one patient discontinued adalimumab treatment due to a loss of response after the switch. The patient had moderate disease activity at the time of the switch and had been previously treated with several biologics, including other anti-TNFα agents.
DiscussionThe emergence of biosimilars changed the daily clinical practice, however, the extrapolation of efficacy and safety data to other indications of the originator drug has sparked some controversy in relation to potential increases in immunogenicity (presence of anti-drug antibodies)23 linked to the use of batches with possible variations in epitopes. This effect, however, has not been detected in clinical studies.24 Neither Barbier et al25 nor McKinnon,26 in their systematic reviews of more than 170 studies, found that originator-to-biosimilar switching resulted in significant differences in efficacy, safety, or immunogenicity.
This is the first Spanish study to analyze the effectiveness and safety of biosimilar adalimumab MSB11022 (Idacio ®) in IBD patients started on this biosimilar as a new treatment strategy and switched from originator adalimumab.
No meaningful changes were observed in clinical or biochemical markers of disease activity following the originator-to-biosimilar switch. In addition, the effectiveness analysis showed no significant differences between the originator adalimumab cohort and the biosimilar adalimumab MSB11022 start cohort. Of note, there was a trend towards higher CRP levels in the originator cohort compared with the switch cohort (2.1 vs. 0.7, P=.079).
Patients in the biosimilar adalimumab MSB11022 (Idacio ®) start and switch cohorts were younger than those in the originator adalimumab cohort, indicating an earlier diagnosis of IBD and earlier introduction of biologic therapy, maybe relationship use of biosimilars. No sex-based differences were observed in the distribution of IBD among this adult population.
Nine patients from the originator adalimumab cohort, 2 from the biosimilar adalimumab MSB11022 start cohort, and 1 from the switch cohort discontinued adalimumab treatment (Table 2). The reasons for discontinuation were:
- a)
non-serious AEs (one patient from the originator adalimumab cohort),
- b)
poor control of a secondary disease (2 patients from the originator adalimumab cohort),
- c)
loss of response (6 patients from the originator adalimumab cohort and 1 from the switch cohort).
Loss of response rates are consistent with previous reports describing rates in the range of 10%–20% and 13%–30% depending on the study. AE rates are also in line with previous reports.22 None of the patients included in this study needed ADA dose intensification or surgery during treatment in the study period.
Our findings show no changes in clinical or biochemical parameters in patients switched from originator adalimumab to biosimilar adalimumab MSB11022 (Idacio ®). They also show that biosimilar adalimumab MSB11022 is both safe and effective in ADA-naïve patients with IBD. Stable pharmacokinetics and biochemical disease activity had been found in a significant cohort of patients switching from originator to biosimilar adalimumab as part of a real-world switching program.27 Clinical benefit of originator adalimumab was sustained after a switch to an adalimumab biosimilar MSB11022 where there was no risk of relapse, emergency visit, or hospital admission seen in this study. This Canadian study established the safety and efficacy of switch to an adalimumab biosimilar agent.28
Our study has several strengths, including our dual analysis of IBD patients directly started on adalimumab biosimilar MSB11022 and patients who switched to this drug after treatment with the originator adalimumab. Selection bias was minimized by using a protocol-driven collection procedure to gather clinical, biochemical, and disease severity parameters from the hospital's integrated electronic health record platform.
In keeping with this study also has limitations, including the different sizes of the study cohorts (adalimumab biosimilar MSB11022 (Idacio ®) was introduced to our hospital in 2021, whereas originator adalimumab (Humira ®) has been in use since 2008). This situation is reflective of routine clinical practice, as the longer a person is on biological therapy, the more likely is to lose response.22 Another limitation that may affect our findings on the effectiveness of adalimumab biosimilar MSB11022 is the lack of endoscopic monitoring, but just a few of the patients on maintenance adalimumab therapy underwent endoscopic examination during follow-up. Also a limitation of the present study is the few patients treated with the adalimumab biosimilar MSB11022. Finally, data regarding therapeutic drug monitoring, like anti-drug antibody and adalimumab levels were missing since these parameters were only collected in patients with suspected treatment failure in our hospital.
Despite the above limitations, this study provides valuable data on short-term responses and reflects real-world practice with adalimumab biosimilar MSB11022 treatment, enabling the direct translation of results into routine clinical practice at our hospital.
While biosimilars offer several socioeconomic benefits over originator products, effectiveness and safety should remain the ultimate goal of any treatment. The potential cost savings associated with biosimilars, however, present an excellent opportunity for expanding access to biologic therapies and improving the efficiency of healthcare systems.
ConclusionsBetween originator adalimumab and adalimumab biosimilar MSB11022 start cohorts, no differences were observed, between originator adalimumab and switch cohorts, no significant differences were found either, and with the pre- and post-switch to adalimumab biosimilar MSB11022 comparison, 2 of the 9 patients experienced AEs after the switch.
The adalimumab biosimilar MSB11022 (Idacio ®) showed a favorable safety profile (one patient with a serious adverse effect (rash) with biosimilar discontinued treatment) and no significant changes to clinical or biochemical parameters were observed after the switch.
Financial disclosures and conflicts of interestThis study did not incur any financial costs.
None of the researchers received any financial remuneration.
FundingNone sources of support.
Ethical considerationsOur submitted manuscript named: “Eficacia and safety of adalimumab biosimilar in patients with inflammatory bowel disease” is an original contribution not previously published, and has not been mailed to be under consideration for publication elsewhere.
Following authors (IIS, JEPJ, AMD, AVP, FJCH) follow ICMJE recommendations so all of them made substantial contributions to the conception or design of the work; for the acquisition, analysis, or interpretation of data for the work; all authors, reviewed it critically for important intellectual content, and drafted final approval of the version to be published. All of them ensured that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved. TGP just passed away few months ago but she meet the criteria for inclusion as coauthor, so should be included and we will note indicating the date of death. We never used artificial intelligence (AI)-assisted technologies in the production of submitted work.
Planning, conduct, and reporting of this research has been made are in accordance with the Helsinki Declaration, revised in 2013. This is a retrospective investigation without any intervention over patients, so Investigational Committee of our hospital considered que, we were exonerated for collecting patient consent. All authors confirm avoiding any potential identifiable material.
Biosimilars approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) are considered equivalent to their biologic reference products, or originators. Switches from originators to biosimilars are therefore permitted. Once biosimilarity has been demonstrated in one indication, extrapolation to other indications of the originator is acceptable. Biologic therapy in IBD, places a significant financial burden on healthcare systems (ADA originator (Humira), has ranked among the world's top-selling drugs in the past decade), limiting patient access to appropriate therapy.
The ADA biosimilar MSB11022 (brand name Idacio) was approved for the same indications as its originator. This first-ever comparison of MSB11022 and the adalimumab originator in the setting of IBD found no meaningful differences in effectiveness or safety in patients who started on MSB11022 or switched to this biosimilar from the originator.
In our opinion, our research has provided results that can significantly improve health care for our patients. The lower costs of biosimilars present an excellent opportunity for expanding patient access to biological therapies and improving healthcare system efficiencies.
CRediT authorship contribution statementJaime E. Poquet-Jornet: Writing – review & editing, Writing – original draft, Validation, Supervision, Methodology, Formal analysis, Data curation, Conceptualization. Inés Ibáñez-Sala: Writing – review & editing, Writing – original draft, Visualization, Methodology, Investigation, Formal analysis, Data curation. Teresa Garrigues-Pelufo: Conceptualization. Adrián Munilla-Das: Writing – review & editing, Writing – original draft, Validation, Supervision, Formal analysis, Data curation. Antonio Valdivia-Pérez: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Methodology, Formal analysis, Data curation. Francisco Javier Carrera-Hueso: Writing – review & editing, Writing – original draft, Validation, Methodology, Formal analysis, Data curation.