There is uncertainty about how age affects the efficacy of immunotherapy due to the natural process of immunosenescence. The aim of this systematic review and meta-analysis is to assess whether age over 65 years affects the efficacy, in terms of overall survival, of immunotherapy treatments in combination with chemotherapy or double immunotherapy, used in first-line metastatic non-small cell lung cancer without molecular alterations.
MethodsA systematic review and meta-analysis were performed. A systematic search of PubMed and Cochrane Library until April 30, 2024 was conducted to identify randomized clinical trials comparing an experimental treatment with immune checkpoint inhibitors plus chemotherapy versus a platinum-based chemotherapy doublet in patients with locally advanced or metastatic non-small cell lung cancer, without molecular mutations and with any level of programmed death ligand 1 expression. The primary endpoint was the difference in efficacy between those older and younger than 65 years, measured in terms of difference in overall survival hazard ratio. We calculated the hazard ratio for overall survival with its 95% confidence interval in both age groups and assessed heterogeneity using an interaction test.
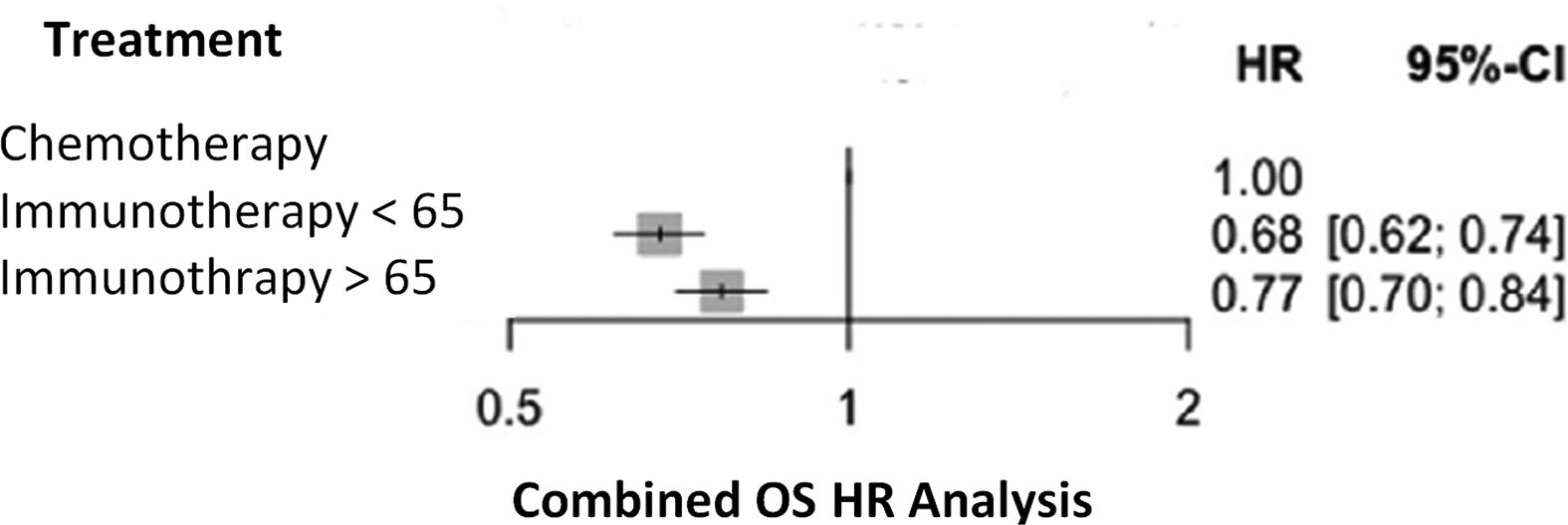
ResultsA total of 1,505 publications were identified, of which 7 clinical trials were included. In addition, the European public report evaluating pembrolizumab in combination with platinum and nab-paclitaxel was incorporated. In total, the analysis included 5,572 patients: 2,893 under 65 years of age and 2,679 aged 65 years or older. The pooled Hazard Ratio for overall survival for patients in the first group was 0.68 (95% CI: 0.62–0.74), and for the second 0.77 (95% CI: 0.70–0.84). The p-interaction between the pooled Hazard Ratio of both groups was 0.0551.
ConclusionsBoth those younger and older than 65 years benefit from immunotherapy combined with chemotherapy in the treatment of non-small cell lung cancer. Although there appears to be greater efficacy in those younger than 65 years, the influence of age is not entirely clear.
existe incertidumbre sobre cómo afecta la edad a la eficacia de la inmunoterapia debido al proceso natural de inmunosenescencia. El objetivo de esta revisión sistemática y metaanálisis es evaluar si la edad superior a 65 años afecta a la eficacia, en términos de supervivencia global, de los tratamientos de inmunoterapia en combinación con quimioterapia o doble inmunoterapia, utilizados en primera línea del cáncer de pulmón no microcítico metastásico sin alteraciones moleculares.
Métodose realizó una revisión sistemática y un metaanálisis. Se llevó a cabo una búsqueda sistemática en PubMed y Cochrane Library hasta el 30 de abril de 2024, para identificar ensayos clínicos aleatorizados que compararan un tratamiento experimental con inhibidores de puntos de control inmunitario más quimioterapia, frente a un doblete de quimioterapia basado en platino, en pacientes con cáncer de pulmón no microcítico localmente avanzado o metastásico, sin mutaciones moleculares y con cualquier nivel de expresión de ligando 1 de muerte programada. La variable principal fue la diferencia en eficacia entre mayores y menores de 65 años, medida en términos de diferencia en el hazard ratio de supervivencia global. Se calculó el hazard ratio de supervivencia global agrupado con su intervalo de confianza del 95%, en ambos grupos etarios, y se evaluó la heterogeneidad mediante una prueba de interacción.
Resultadosse identificaron 1.505 publicaciones, de las cuales se incluyeron 7 ensayos clínicos. Además, se incorporó el informe público europeo de evaluación de pembrolizumab en combinación con platino y nab-paclitaxel. En total, el análisis incluyó 5.572 pacientes: 2.893 menores de 65 años y 2.679 de 65 años o más. El hazard ratio agrupado de supervivencia global para los pacientes del primer grupo fue de 0,68 (IC 95%: 0,62–0,74), y para el segundo de 0,77 (IC 95%: 0,70–0,84). La p de interacción entre los hazard ratio combinados de ambos grupos fue de 0,0551.
Conclusionestanto los menores como los mayores de 65 años se benefician de la inmunoterapia combinada con quimioterapia en el tratamiento del cáncer de pulmón no microcítico. Aunque parece haber una mayor eficacia en los menores de 65 años, la influencia de la edad no es completamente clara.
Lung cancer is the third most commonly diagnosed malignancy worldwide.1 In 2023, as much as 238,340 cases of lung cancer were newly diagnosed and 127,070 people died from the disease. Lung cancer is the leading cause of mortality worldwide (20.8% of all cancer-related deaths).2
This malignancy is classified into two groups as a function of cell morphology: small cell –or microcytic– cancer and non-small cell lung cancer (NSCLC), with the later accounting for 80–85% of all cases of lung cancer. In turn, NSCLC is classified into three histological subtypes, namely: adenocarcinoma, squamous cell carcinoma, and large cell carcinoma.3
The management of NSCLC is determined by tumor histology, disease stage, and the presence of molecular mutations, as well as age, performance status, comorbidities and patient preferences. In the case of metastatic disease, the standard first-line treatment for carriers of molecular mutations is targeted therapy.4 In carriers of mutation-negative tumors, the therapeutic approach depends on tumor histology and genotype, levels of programmed death-ligand (PD-L1) expression, performance status, comorbidities and patient preferences. When immunotherapy is not contraindicated and the patient has a good performance status (0–1) according to the Eastern Cooperative Oncology Group (ECOG), therapeutic options include immunotherapy alone, in combination with chemotherapy, or combinations of different immunotherapies depending on the level of PD-L1 expression, among other factors.5
The immune checkpoint proteins programmed cell death protein 1 (PD-1)/PD-L1 and cytotoxic T-lymphocyte antigen 4 (CTLA-4) play a major role in tumor development. Interaction of ligands with their receptors negatively regulates and inhibits T cell activity, thereby impairing the immune system and favoring tumor cell proliferation. Immune checkpoint inhibitors (ICIs), targeted drugs against these receptors and ligands, anti-PD-1/PD-L1 and anti-CTLA-4, prevent T lymphocyte interaction with tumor cells, leading to a positive regulation of T cell activity.6
These agents form part of the standard of care for metastatic mutation-negative NSCLC.
Immunosenescence refers to the progressive deterioration of the immune system as a result of the aging process. This phenomenon influences tumor progression and affects the effectiveness of anti-tumor immune response due to the reduced ability of the body to induce an effective response.7 Although the incidence of cancer in patients with an advanced age is growing, the inclusion of this patient subgroup in cancer-related clinical trials (CTs) is limited. In the case of lung cancer, in clinical practice, the median age at diagnosis is 70 years, and approximately half of the patients belong to the geriatric population. However, lung cancer-related CTs most frequently include samples of younger adults.8 This may be explained by the high incidence of comorbidities in this population, which prevents patients from fulfilling clinical trial inclusion criteria.9
There is inconsistent evidence on the potential clinical benefits of ICI therapy in the elderly population, both when administered alone10–12 or in combination therapies.10,12 The evidence available is derived from subgroup analyses performed in CTs with low statistical power and real-world studies.13–15
The objective of this systematic review and meta-analysis is to determine whether age over 65 years impacts the efficacy –measured by overall survival–, of first-line combination therapy with immunotherapy plus chemotherapy or dual immunotherapy in patients with metastatic NSCLC without molecular alterations.
MethodsThe protocol for this review was registered in the PROSPERO database (CRD420251032819).16
Search strategyThe systematic review and meta-analysis were conducted in accordance with PRISMA guidelines. A systematic search for phase III clinical trials was conducted on PubMed and the Cochrane Library up to 30 April 2024. Other documents were reviewed, including relevant European Public Assessment Reports (EPARs) published by the European Medicines Agency in relation to the agents considered in this study. Literature search was carried out by two reviewers (A.A. and L.M.). Search terms included “pembrolizumab”, “atezolizumab”, “nivolumab”, “ipilimumab”, “durvalumab”, “tremelimumab”, “cemiplimab”, “non-small cell lung cancer” and “randomized controlled trial”. The following search strategies were applied: ([pembrolizumab] AND [non-small cell lung cancer] AND [randomized controlled trial]), ([atezolizumab] AND [non-small cell lung cancer] AND [randomized controlled trial]), ([nivolumab] AND [ipilimumab] AND [non-small cell lung cancer] AND [randomized controlled trial]), ([durvalumab] AND [tremelimumab] AND [non-small cell lung cancer] AND [randomized controlled trial]), ([cemiplimab] AND [non-small cell lung cancer] AND [randomized controlled trial]).
Inclusion criteriaThe initial sample was composed of all placebo-controlled, randomized, phase-3 clinical trials (CTs) including adult patients with locally-advanced/metastatic mutation-negative NSCLC with squamous and non-squamous histology, PS 0–1, and any level of PD-L1 expression. The final sample included CTs comparing a first-line experimental treatment with pembrolizumab, atezolizumab, nivolumab, ipilimumab, durvalumab, tremelimumab or cemiplimab in combination with chemotherapy, or without chemotherapy in the case of nivolumab-ipilimumab, versus a control group receiving platinum-based doublet chemotherapy. We included the nivolumab-ipilimumab combination without chemotherapy, as this treatment is positioned at the same level as the other immunotherapy + chemotherapy combinations in patients with PD-L1 expression ≥ 1%.5 For inclusion, CTs were required to assess efficacy in terms of hazard ratio (HR) for overall survival (OS) according to age.
Exclusion criteriaProspective and retrospective studies, along with real-world case series, case reports and phase II CTs were excluded. If multiple publications from the same CT providing results for different years were identified, we selected those with similar follow-up durations to ensure data comparability. The other reports corresponding to the same CT were excluded from analysis.
Full-text papers published in languages other than English or Spanish were excluded. Real-world cohort studies and phase II CTs were excluded to ensure the internal validity of the results of our study.
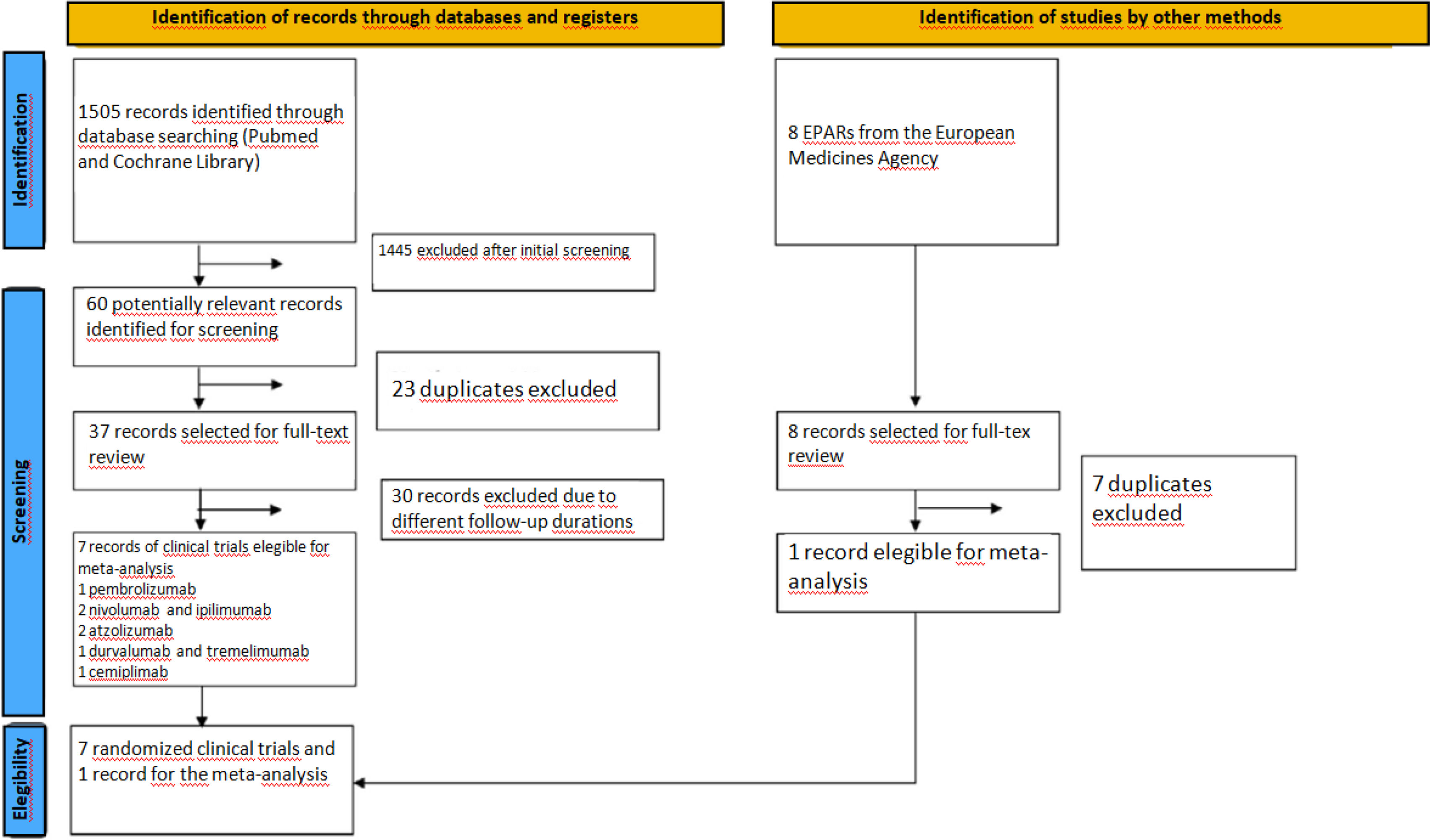
The list of selected articles was independently evaluated by two reviewers (A.A. and L.M.) to identify those that met the inclusion criteria (Fig. 1). Discrepancies were discussed and solved by consensus with a third reviewer (J.C.).
Data extractionA.A. and L.M. independently extracted relevant information from the studies included through a data extraction form. The collected data included study title; lead author; year of publication; treatment assigned to the control group; patient characteristics; length of follow-up; hazard ratio (HR) for overall survival (OS) according to age; sample size of each group; and median OS in the control group. The characteristics and results of the studies included were summarized in tables.
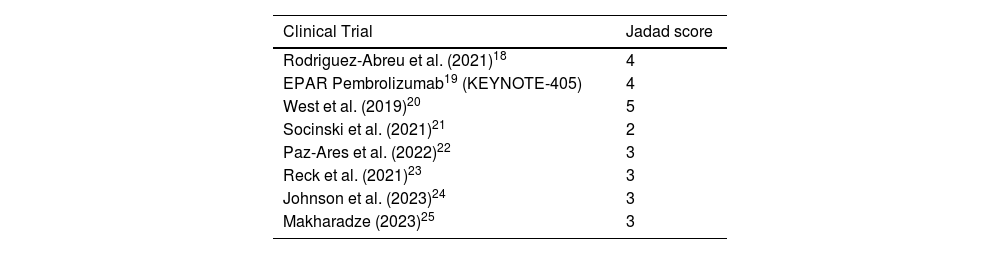
The methodological quality of CTs was assessed using the Jadad classification. The primary endpoint assessed the efficacy of immunotherapy + chemotherapy combination therapies by comparing hazard ratios (HRs) for overall survival (OS) between patients younger and older than 65 years.
A random-effects model was applied to calculate the combined HRs and their 95% confidence intervals (95%CI) for patients above and below 65 years of age. Heterogeneity between these estimates was assessed using an interaction test, while heterogeneity across studies was evaluated with Cochran's Q test. In addition, the I2 statistic was calculated to quantify the proportion of total variability attributable to between-study heterogeneity.
The meta-analysis was performed using the RStudio V4.01, netmeta library.
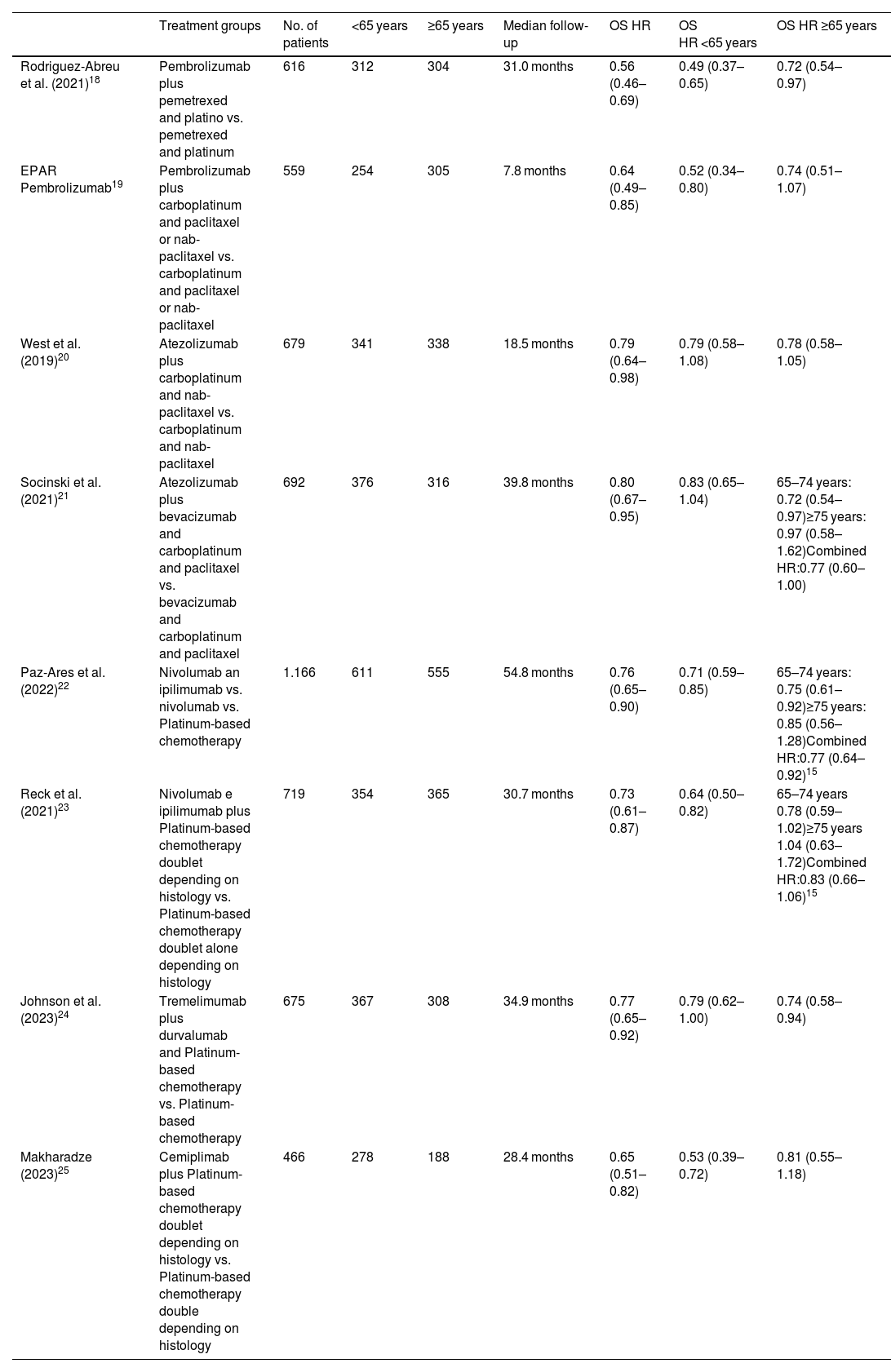
ResultsThrough the use of the selected search terms, a total of 1505 articles were retrieved from MEDLINE/PubMed and Cochrane Library, of which 60 potentially relevant articles were selected. Duplicates and reports for the same CT reporting results for different follow-up periods were eliminated. Seven placebo-controlled CTs were identified to fulfill the inclusion criteria (Table 1) and constituted the final sample. The publications selected included a CT on pembrolizumab; two on atezolizumab; two on nivolumab and ipilimumab; one on durvalumab and tremelimumab; and one on cemiplimab. All CTs were phase III and involved treatment-naïve patients with locally advanced or metastatic NSCLC. The sample also included the EPAR for the pembrolizumab plus platinum combination and nab-paclitaxel, since it provided age-disaggregated data from the second interim analysis of the KEYNOTE-40517 clinical trial. The other articles retrieved did not provide data stratified by age. A total of 5572 patients were included, of whom 2893 were <65 years and 2679 were ≥65 years. A total of 1175 patients received pembrolizumab; 1371 were administered atezolizumab; 1885 received nivolumab and ipilimumab; 675 were administered durvalumab and tremelimumab, and 466 received cemiplimab. The median duration of follow-up was 30.8 months (range 7.8–54.8 months).
Description of the CTs included in the meta-analysis.
| Treatment groups | No. of patients | <65 years | ≥65 years | Median follow-up | OS HR | OS HR <65 years | OS HR ≥65 years | |
|---|---|---|---|---|---|---|---|---|
| Rodriguez-Abreu et al. (2021)18 | Pembrolizumab plus pemetrexed and platino vs. pemetrexed and platinum | 616 | 312 | 304 | 31.0 months | 0.56 (0.46–0.69) | 0.49 (0.37–0.65) | 0.72 (0.54–0.97) |
| EPAR Pembrolizumab19 | Pembrolizumab plus carboplatinum and paclitaxel or nab-paclitaxel vs. carboplatinum and paclitaxel or nab-paclitaxel | 559 | 254 | 305 | 7.8 months | 0.64 (0.49–0.85) | 0.52 (0.34–0.80) | 0.74 (0.51–1.07) |
| West et al. (2019)20 | Atezolizumab plus carboplatinum and nab-paclitaxel vs. carboplatinum and nab-paclitaxel | 679 | 341 | 338 | 18.5 months | 0.79 (0.64–0.98) | 0.79 (0.58–1.08) | 0.78 (0.58–1.05) |
| Socinski et al. (2021)21 | Atezolizumab plus bevacizumab and carboplatinum and paclitaxel vs. bevacizumab and carboplatinum and paclitaxel | 692 | 376 | 316 | 39.8 months | 0.80 (0.67–0.95) | 0.83 (0.65–1.04) | 65–74 years: 0.72 (0.54–0.97)≥75 years: 0.97 (0.58–1.62)Combined HR:0.77 (0.60–1.00) |
| Paz-Ares et al. (2022)22 | Nivolumab an ipilimumab vs. nivolumab vs. Platinum-based chemotherapy | 1.166 | 611 | 555 | 54.8 months | 0.76 (0.65–0.90) | 0.71 (0.59–0.85) | 65–74 years: 0.75 (0.61–0.92)≥75 years: 0.85 (0.56–1.28)Combined HR:0.77 (0.64–0.92)15 |
| Reck et al. (2021)23 | Nivolumab e ipilimumab plus Platinum-based chemotherapy doublet depending on histology vs. Platinum-based chemotherapy doublet alone depending on histology | 719 | 354 | 365 | 30.7 months | 0.73 (0.61–0.87) | 0.64 (0.50–0.82) | 65–74 years 0.78 (0.59–1.02)≥75 years 1.04 (0.63–1.72)Combined HR:0.83 (0.66–1.06)15 |
| Johnson et al. (2023)24 | Tremelimumab plus durvalumab and Platinum-based chemotherapy vs. Platinum-based chemotherapy | 675 | 367 | 308 | 34.9 months | 0.77 (0.65–0.92) | 0.79 (0.62–1.00) | 0.74 (0.58–0.94) |
| Makharadze (2023)25 | Cemiplimab plus Platinum-based chemotherapy doublet depending on histology vs. Platinum-based chemotherapy double depending on histology | 466 | 278 | 188 | 28.4 months | 0.65 (0.51–0.82) | 0.53 (0.39–0.72) | 0.81 (0.55–1.18) |
HR: hazard ratio; OS: overall survival.
Two CTs, conducted by West et al.11 and Socinski et al.,12 included carriers of molecular mutations; however, for the populations of our meta-analyses to be more homogeneous, only patients with mutation-negative tumors were included. The characteristics and results of the CTs included in the meta-analysis are detailed in Table 1.
Table 2 contains the Jadad scores for the CTs selected. Patients <65 years treated with a combination of immunotherapy plus chemotherapy, or without chemotherapy in the case of nivolumab and ipilimumab, had a significantly lower risk of mortality, as compared to the patients in the control group (combined HR for OS 0.68 (95% CI): 0.62–0.74; p < 0.000001). As compared to the control group, the clinical benefits obtained with the experimental treatment in patients older than 65 years yielded a combined OS HR of 0.77 (95%CI: 0.70–0.84), p < 0.000001) (Fig. 2).
Heterogeneity estimates were as follows: Cochran's Q 14.84, p = 0.03812, I2 53% (95%CI: 0–79%) in the group of patients <65 years, and Cochran's Q 0.81, p = 0.99.733, I2 0% (95%CI 0–0%) in patients >65 years.
Interaction p-value calculated for the combined hazard ratios for the study groups was 0.0551.
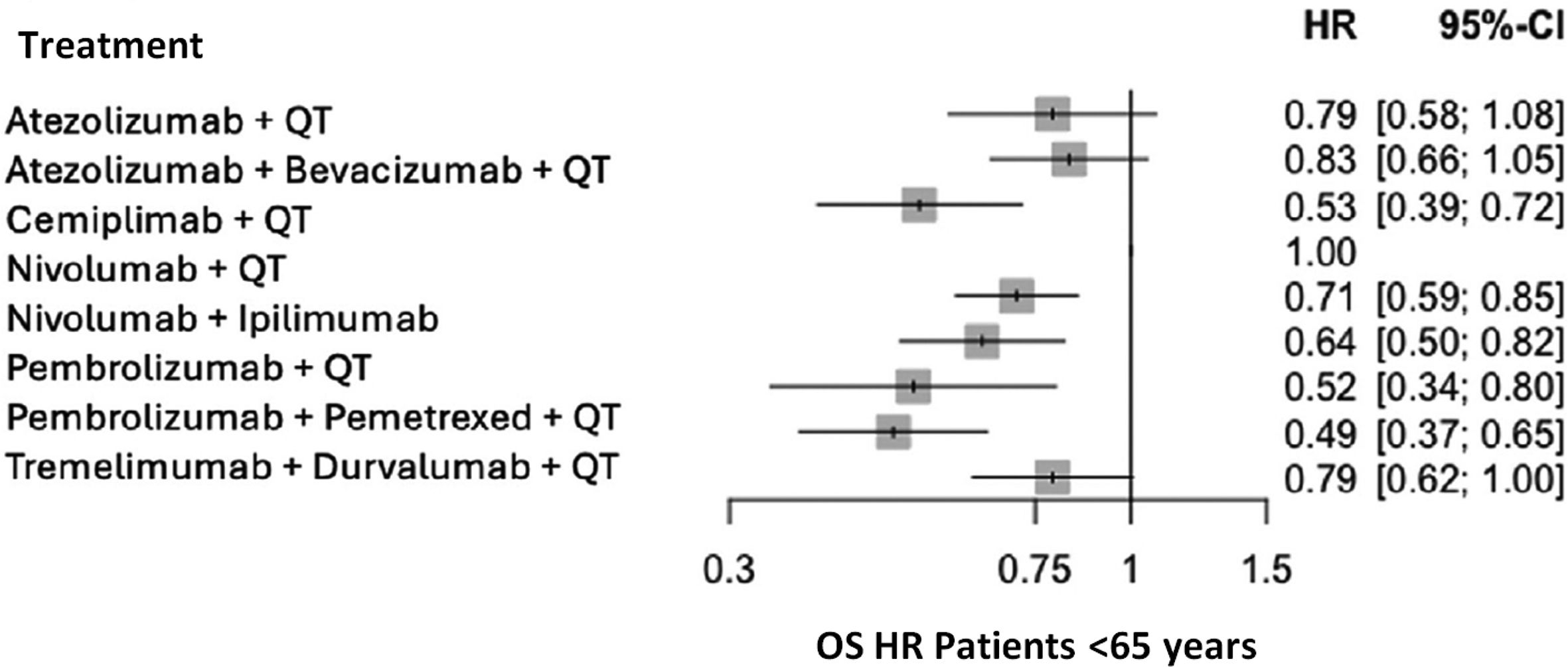
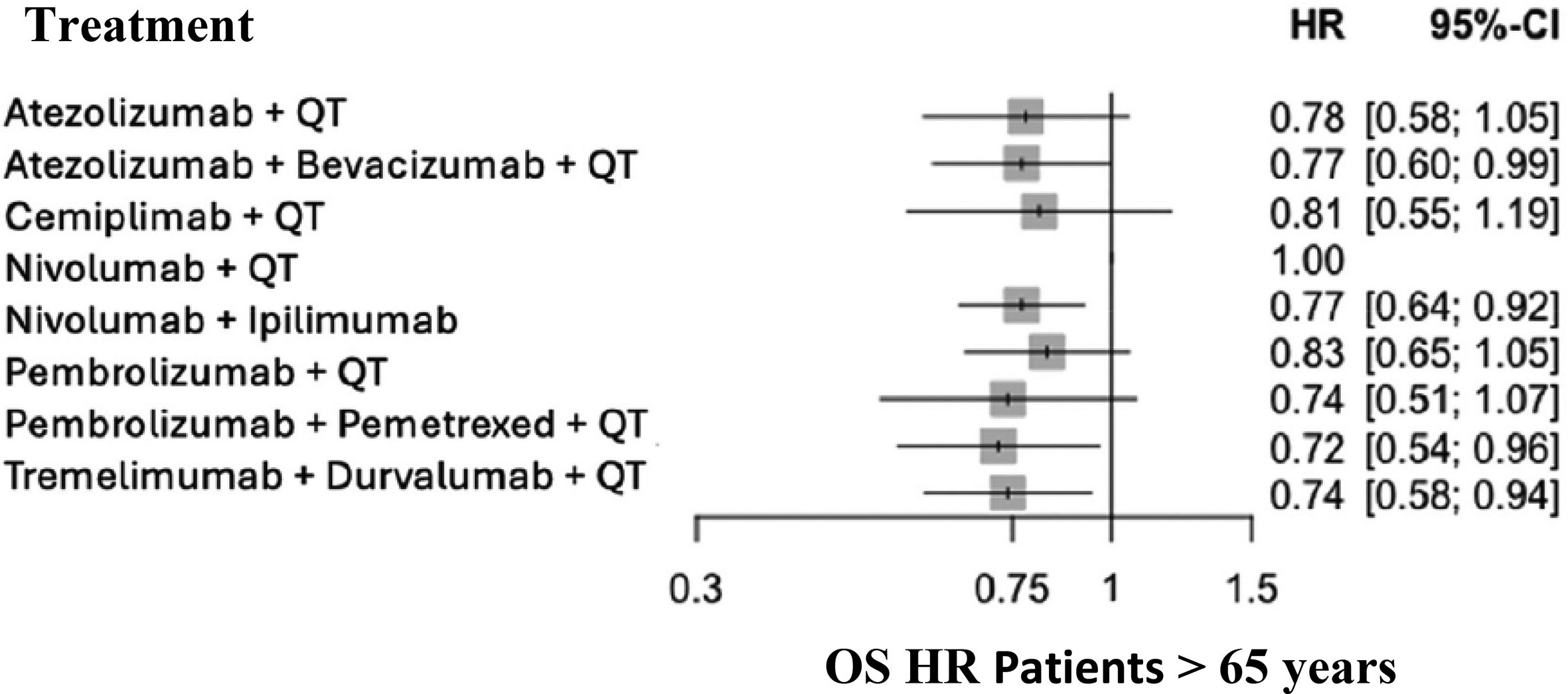
The OS HR for all treatment groups is presented in Fig. 4 by age group: <65 years (Fig. 3) and >65 years.
Significant differences were observed between the immunotherapy + chemotherapy combination and placebo + chemotherapy in the two age groups, in favor of immunotherapy + chemotherapy. The results obtained suggest a weaker effect in patients >65 years, although the immunotherapy + chemotherapy combination was effective in the two age groups.
The results obtained support the hypothesis that immunosenescence could affect ICI efficacy in patients with an advanced age.26
The heterogeneity analysis revealed consistent results in patients aged over 65 (I2 = 0%), but not in those younger than 65 (I2 = 53%). The heterogeneity observed in the younger group can be explained by the two pembrolizumab CTs, which reported better HRs. Nonetheless, this finding alone does not allow conclusions regarding differential drug efficacy in this age group.
These results are not consistent with those of the meta-analysis conducted by Wu et al,27 revealing a trend in patients >65 years to benefit more from immunotherapy, as compared to younger patients (OS HR: 0.64 vs. 0.73; p = 0.025). However, differences in study design and composition should be considered. Wu et al.27 included a more heterogeneous population in terms of disease histology. In addition, age differences have a greater impact on OS in patients with melanoma as compared to patients with NSCLC. In addition, the study treatments were administered in monotherapy, whereas our study was primarily focused on immunotherapy + chemotherapy combinations. These methodological differences could explain the disparity across results.
Our results are more consistent with those reported by Ferrara et al.,11 who described a significant benefit of anti-PD-1 and PD-L1 in older adults, except for patients >75 years. The lack of clinical benefit of ICIs in patients >75 years is consistent with CTs reporting disaggregated OS HRs for this specific subgroup of patients (table 121–23); however, this age group was excluded from analysis.
On another note, Ferrara et al.11 included larger real-world studies, which found no significant differences in the efficacy or toxicity of these therapies between young adults and adults older than 70 years.
Although nearly half of the patients diagnosed with lung cancer are older than 70 years, the efficacy and safety of anti-PD-1/PD-L1 agents have not yet been explored in the elderly population. Casaluce et al.28 reviewed subgroup efficacy results for elderly patients from the pivotal trials that supported FDA approval of anti-PD-1/PD-L1 agents for NSCLC. The authors concluded that data were limited by the underrepresentation of this population in CTs, and the results were inconclusive regarding the impact of age on treatment response and the magnitude of this effect.
Other recent studies and reviews provide complementary insights. For instance, the narrative review carried out by Zhang et al.29 revealed that the clinical benefit of pembrolizumab, nivolumab or atezolizumab alone in patients >65 years with NSCLC is comparable to that observed in younger patients. However, this finding is based on CTs involving patients with an excellent health and physical condition. This limits applicability to the general elderly population, which generally presents comorbidities, frailty and other characteristics that could influence response to treatment. This limitation was also remarked by Tagliamento et al.,10 who examined the efficacy of immunotherapy in combination with chemotherapy in patients with metastatic NSCLC. The authors emphasized that the underrepresentation of patients older than 75 years in CTs limits the generalizability of the findings to this population subgroup.
Data from real-world studies provide an additional perspective on the efficacy of ICIs in the elderly population. Ramos et al.30 carried out a retrospective, real-world, cohort study to assess the effectiveness of immunotherapy in young and older adults with metastatic solid tumors. The administration of ICIs did not induce significant differences in OS and progression-free survival between age groups (p = 0.388). These results support the idea that chronological age alone cannot be used as the sole criterion in therapeutic decision-making. Additionally, these findings emphasize the relevance of individualized patient evaluation based on geriatric assessment tools.
Gomes et al.12 highlighted the importance of generating evidence about the use of immunotherapy in the elderly population through studies assessing variables such as frailty and patient-reported quality of life. The authors underline the relevance of assessing immunosenescence through the use of biological markers and specific tests to determine whether age-related changes in the immune system influence treatment efficacy and toxicity. Hence, Gomes et al. highlight the importance of developing CTs specifically targeting this patient population, integrating elderly subgroups into pivotal CTs, and fostering the publication of real-world data.
In the same vein, Montrone et al.31 recommend the administration of immunotherapy in elderly patients. However, they urge the scientific community to assess the risk–benefit balance of this therapy in patients with a very advanced age, since some combinations –such as anti-PD-1 and anti-CTLA-4– may cause high toxicity and be ineffective in this age group. Additionally, the authors urge the scientific community to perform a preliminary evaluation using frailty assessment tools and taking polypharmacy into consideration when elderly patients are involved.
Hu et al.32 conducted a meta-analysis comparable to ours assessing the impact of sex, age, performance status and smoking habits on OS in patients with advanced NSCLC treated with ICIs. The study revealed that ICIs significantly improve survival both in patients younger than 65 years (HR 0.74) and over 65 years (HR 0.80), irrespectively of age. Likewise, a meta-analysis conducted by Kim et al.33 disclosed that the efficacy of ICIs was comparable between patients younger and older than 65 years, with a OS HR of 0.77 for both groups. The results of these two meta-analyses are consistent with our findings.
In general terms, decision-making regarding the use of an ICI therapy should be performed considering such relevant factors as the systematic underrepresentation of elderly patients (especially of patients >75 years) in CTs10,28; the need for frailty and geriatric assessment scales to ensure the adequate selection of candidates to immunotherapy12,31; and the added value of real-world studies supporting CT findings.12
From a methodological point of view, a strength of this study is that it involves eight CTs reporting overall survival for 5572 patients, which supports the robustness and internal validity of our results. However, the analysis presents some limitations. Firstly, our study included CTs where the experimental group received ICIs with different immunological mechanisms of action (anti-PD-L1, anti-PD-1, anti-CTLA-4), and some used combinations of these agents. The meta-analysis included the nivolumab + ipilimumab combination without chemotherapy, since it forms part of the therapeutic armamentarium approved for this scenario. It is worth noting that the uncertainty as to the efficacy results obtained is comparable to that of other immunotherapy + chemotherapy combinations.
In addition, the study included treatment regimes approved for non-squamous, squamous cell lung cancer and both histological types, and analyzed efficacy aggregately. However, subgroup analysis of the combinations approved for the two histological types revealed no differences in terms of efficacy between squamous and non-squamous cell lung cancer. Therefore, a relevant bias was not expected to affect the meta-analysis.
A relevant limitation of this study lies in the cut-off age. It was decided to establish 65 years, since it was the most commonly used criterion in the studies reviewed. However, it would have been interesting that disaggregate data had been reported, especially for older adults, whose response to treatment could be influenced by immunosenescence. In addition, it seems reasonable to assume that the older adults included in studies on chemotherapy are mostly those in a better health condition.
Finally, this study only included randomized CTs to ensure the internal validity of the study results and control potential biases. However, this involved excluding data from real-world cohort studies, which limits the generalizability of findings to routine clinical practice.
In conclusion, patients younger and older than 65 years benefit from the combination of immunotherapy plus chemotherapy in the treatment of locally advanced or metastatic NSCLC. Although our results suggest a higher efficacy in patients younger than 65, there is uncertainty about potential age-related effects.
Contribution to the scientific literatureThis meta-analysis assesses the influence of age on the efficacy of immunotherapy + chemotherapy combination treatments or dual immunotherapy in patients with metastatic non-microcytic lung cancer. This approach is crucial, as it enables the provision of disaggregated data on treatment benefits, providing a more specific, personalized perspective, which is essential in evidence-based medicine.
The results of this meta-analysis are especially relevant in the clinical setting, as patients older than 65 years are most usually underrepresented in CTs, despite accounting for a significant proportion of patients in routine clinical practice. Generating specific evidence for this age group will contribute to adjusting therapeutic decision-making to their particular characteristics. This information offers healthcare professionals a valuable tool that guides therapeutic decisions by balancing the risk–benefit balance by age and ensuring a more appropriate, safer patient care relying on specific data for patients with an advanced age. This information provides healthcare professionals with a valuable tool to better guide treatment decisions, allowing risks and benefits to be balanced according to age and thereby ensuring more appropriate, safer, evidence-based patient care for patients for elderly patients.
CRediT authorship contribution statementAlicia Aguado-Paredes: Writing – original draft, Visualization, Validation, Supervision, Software, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Laura Moñino-Dominguez: Writing – original draft, Visualization, Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Jaime Cordero-Ramos: Writing – original draft, Validation, Supervision, Software, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Emilio Alegre-Del-Rey: Writing – original draft, Validation, Supervision, Software, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
FundingThis study did not receive any funding.
The authors declare no conflict of interest.