Cranial cerebrospinal fluid (CSF) fistulas involve CSF leakage from the subarachnoid space into an extracranial compartment, either acquired or spontaneous in origin.1
Nasal fistulas are the most common type of CSF leak and are characterised by the onset of headache and rhinorrhoea, which is the discharge of clear, watery fluid from one or both nostrils.2,3 They occur after rupture of the protective barrier separating the nasal cavities from the subarachnoid space, providing a pathway for the development of intracranial infection.4,5
Treatment consists of fistula closure, with the most common intervention being endonasal endoscopy. Intrathecal administration of fluorescein is used to localise the exact area of the fistula, as the bony defect is often not visible on imaging tests.4
By describing our experience with the use of intrathecal fluorescein, we aim to assess its efficacy and safety in the localisation of CSF fistulas. This contribution adds to the published knowledge, as there is no consensus on safe dosage, mode of administration, and off-label use. This study was approved by the Ethics Committee of the Hospital General Universitario de Elche (Spain). Patients were informed of the study aims by the neurosurgeon and their informed consent was obtained and appropriately recorded in the electronic medical record.
Clinical casesA 78-year-old woman presented with an intense headache of 3 weeks' duration and symptoms of rhinorrhoea following a catarrhal process. Her symptoms worsened after Valsalva manoeuvres and cephalic flexion.
Examination revealed no fractures at the base of the skull or fistulas at the level of the lamina cribrosa.
It was decided that the neurosurgery department would intervene to localise and close the CSF fistula of spontaneous aetiology using endonasal endoscopy.
To determine the exact location of the fistula, 5% diluted fluorescein was injected into the L4–L5 space via lumbar puncture. After administration, a high-flow CSF fistula was visualised at the ethmoid level, at the border of the left lamina cribrosa, and subsequently closed. There were no adverse events during surgery and no postoperative complications.
A 52-year-old woman presented with a recurrent CSF fistula that had been surgically treated twice and had remained asymptomatic for 3 years. After contracting COVID-19, she experienced recurrent symptoms of rhinorrhoea. Imaging tests revealed a small collection of fluid at the site of the bone fracture.
The neurosurgery department decided on a new operation. To determine the exact location of the fistula, 5% diluted fluorescein was injected into the L4–L5 space via lumbar puncture in the operating theatre. The outflow of the fistula was visualised at the ethmoid level, in the right lamina cribrosa, and subsequently sealed and occluded. There were no adverse events during surgery or the post-operative period.
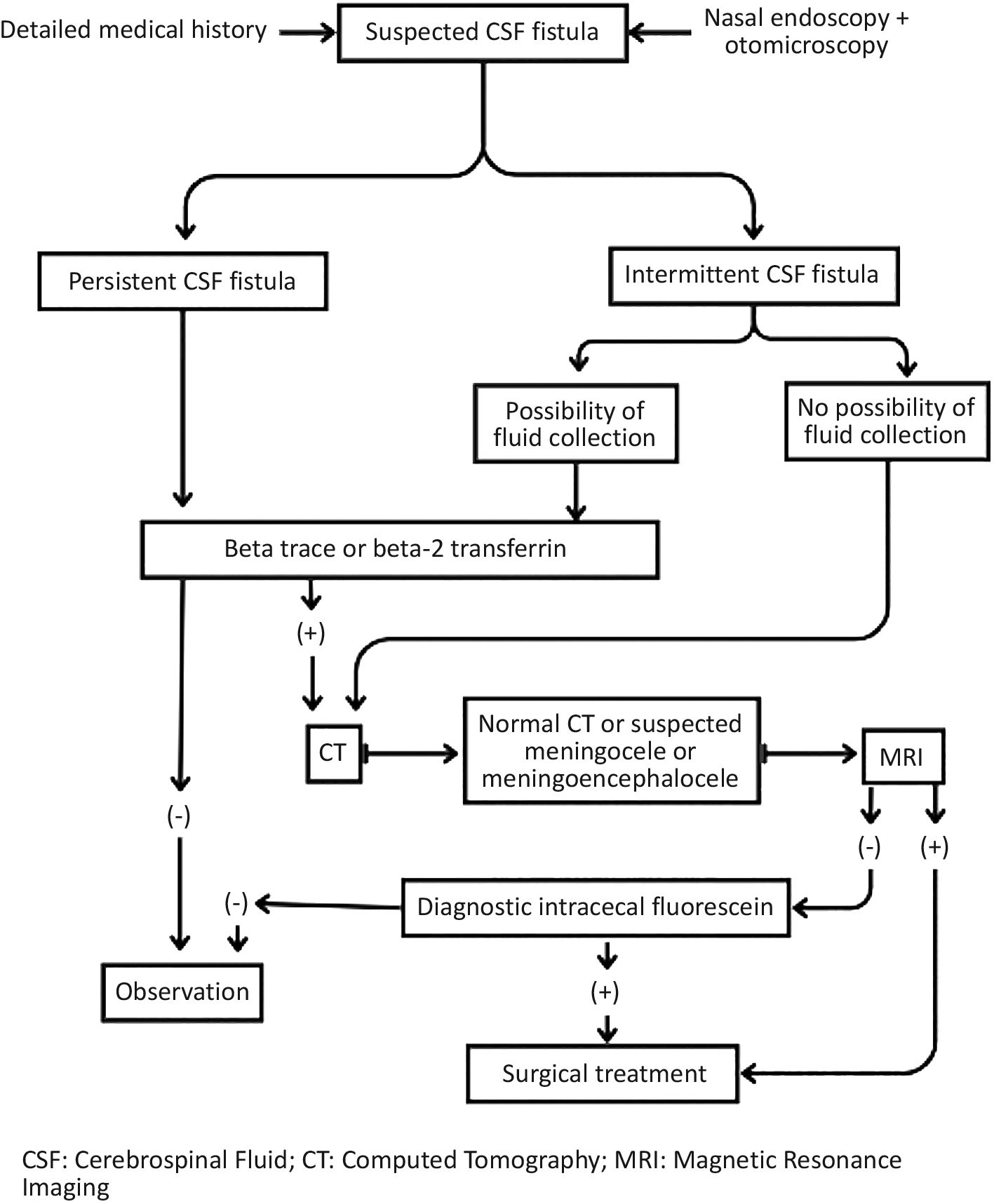
DiscussionCranial CSF fistulas occur when CSF leaks into an extracranial space. Their diagnosis is primarily clinical, with the main feature being rhinorrhoea. Diagnosis and management can be performed according to the algorithm shown in Fig. 1.3
Fluorescein is a water-soluble organic dye that, in solutions with pH greater than 7, produces an intense green fluorescent colour in daylight. In its sodium form, it is used as a biodegradable, non-contaminating, and non-toxic marker.6 It is used as a diagnostic agent, typically by oral, ophthalmic, or intravenous administration.7 After intrathecal administration into the CSF, it leaks through the fistula into the bone defect and can be easily visualised on physical examination or endoscopy.4,8
Complications following intrathecal fluorescein administration are mainly neurological (epileptic seizures, opisthotonos, meningitis) and related to high doses or rates of administration, due to fluorescein's irritant effect.3
The systemic review by Jolly et al. showed that most of the studies analysed used doses of fluorescein <50 mg, with higher toxicity in the group using doses ≥50 mg.9,10 The case published by Rodríguez-Navarro et al. used 5% fluorescein (50 mg) and reported self-limited occipital headache controlled with analgesia without further complications.4
The multicentre observational study by Felisati et al. monitored potential complications associated with intrathecal use of fluorescein. The preliminary results in 53 patients showed that lumbar intrathecal fluorescein administration is a safe procedure as long as the criteria of a maximum dose of 50 mg, additional dilution in CSF, and slow administration are met.9
In our hospital, we have Fluorescein Oculos 10% (100 mg/mL) in 5 mL injection solutions (Laboratorios SERB) at our disposal. Following a review of the literature, the pharmacy service recommends adding 0.5 mL of physiological saline solution to 0.5 mL of the 10% Fluorescein Oculos ampoule (final volume 1 mL) to give a final mixture of 5% fluorescein, which is diluted in 9 mL of the patient's own CSF. It must be administered immediately and slowly through the puncture site from which the 9 mL of CSF was obtained,4,8,9 and is therefore prepared in the operating theatre.
One of the limitations of our review is the number of cases. Case series are intended to increase the level of evidence, but there is a risk of bias due to the lack of a control group. Furthermore, the sensitivity and the specificity of the test were not evaluated; the same dose of fluorescein was used in both cases without establishing a correlation between the dose used and the sensitivity or specificity of the test.
In conclusion, our experience with the use of intrathecal fluorescein 5% has been favourable in terms of both efficacy and safety, as we have been able to accurately localise CSF fistulae with no adverse effects or incidents observed during or after administration.
Contribution to the scientific literatureCSF fistulas are problematic because of their potential impact on patients, such as intracranial infection, therefore localising and closing these fistulas is critical. The use of intrathecal fluorescein during endonasal endoscopy is necessary for the accurate localisation of CSF fistulas, as this is not always possible with diagnostic imaging techniques. Indeed, in our patients' case, we were able to localise the CSF fistulae using fluorescein accurately.
Several studies in the literature describe different doses of fluorescein, with low doses being the most common. However, in our study, the administration of 5% fluorescein was favourable in terms of efficacy and safety. It accurately localised the fistulas and caused no adverse effects as a result of its administration. Thus, we can conclude that the use of fluorescein 5% is safe and effective for the localisation of CSF fistulas.
Ethical responsibilitiesThis study was submitted to the Drug Research Ethics Committee of the Hospital General Universitario de Elche (Spain) which, after evaluation, approved the study on 24 May 2023.
Data confidentiality is regulated by Organic Law 3/2018, 5 December on the Protection of Personal Data and the guarantee of digital rights. In addition, since 25 May 2018, EU legislation on personal data, namely the Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of personal data (GDPR) is fully applicable.
A patient education document was designed to explain to patients the intention to publish a paper on the benefits of fluorescein administration. Patients understood the purpose of the study and gave their consent for it to be conducted.
FundingNone declared.
AuthorshipAll authors declare that they have substantially participated in the drafting, editing, and approval of the final version of this manuscript.
CRediT authorship contribution statementIsabel Carreño Dato: Writing – review & editing, Writing – original draft, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Carmen Matoses Chirivella: Writing – review & editing, Writing – original draft, Validation, Supervision, Methodology, Formal analysis, Conceptualization. Lara Peral Ballester: Writing – review & editing, Writing – original draft, Validation, Supervision. Daniel García Sánchez: Visualization, Validation. Sergio Maciá Soriano: Visualization. Andrés Navarro Ruiz: Visualization.