Nuevos roles y retos del farmacéutico de hospital. New roles and challenges of the hospital pharmacist
Más datosArtificial intelligence is a broad concept that includes the study of the ability of computers to perform tasks that would normally require the intervention of human intelligence. By exploiting large volumes of healthcare data, Artificial intelligence algorithms can identify patterns and predict outcomes, which can help healthcare organizations and their professionals make better decisions and achieve better results. Machine learning, deep learning, neural networks, or natural language processing are among the most important methods, allowing systems to learn and improve from data without the need for explicit programming. Artificial intelligence has been introduced in biomedicine, accelerating processes, improving accuracy and efficiency, and improving patient care.
By using Artificial intelligence algorithms and machine learning, hospital pharmacists can analyze a large volume of patient data, including medical records, laboratory results, and medication profiles, aiding them in identifying potential drug–drug interactions, assessing the safety and efficacy of medicines, and making informed recommendations. Artificial intelligence integration will improve the quality of pharmaceutical care, optimize processes, promote research, deploy open innovation, and facilitate education. Hospital pharmacists who master Artificial intelligence will play a crucial role in this transformation.
La Inteligencia artificial es un concepto amplio que comprende el estudio de la capacidad de los ordenadores para llevar a cabo tareas que normalmente requerirían la intervención de la inteligencia humana. Mediante la explotación de grandes volúmenes de datos sanitarios los algoritmos de inteligencia artificial pueden identificar patrones y predecir resultados, lo que puede ayudar a las organizaciones sanitarias y sus profesionales, a tomar mejores decisiones y alcanzar mejorar resultados. Los métodos de aprendizaje automático, aprendizaje profundo, redes neuronales o el procesamiento natural del lenguaje son de los más importantes, permitiendo a los sistemas aprender y mejorar a partir de datos sin necesidad de programación explícita. La Inteligencia artificial se ha introducido en la biomedicina, acelerando procesos, mejorando la precisión y eficiencia, y mejorando la atención al paciente.
Mediante el uso de algoritmos de iIA y aprendizaje automático, los farmacéuticos de hospital pueden analizar un gran volumen de datos de pacientes, incluidos registros médicos, resultados de laboratorio y perfiles de medicamentos, ayudándolos a identificar posibles interacciones entre medicamentos, evaluar su seguridad y eficacia, así como tomar decisiones mejor informadas. La integración de la Inteligencia artificial mejorará la calidad de la atención farmacéutica, optimizará los procesos, promoverá la investigación, implementará la innovación abierta y facilitará la formación. Los farmacéuticos hospitalarios que dominen la Inteligencia artificial desempeñarán un papel crucial en esta transformación.
Artificial intelligence (AI) has in the past few years become the technology with the greatest transformative effect the world over. AI refers to a vast body of knowledge that brings together computer science with mathematical science and is dedicated to increasing the power of computers to perform tasks that would usually require human intelligence, such as learning, reasoning, critical thinking, decision making, and natural language processing (NLP).1
Although the term AI was first coined in 1956, it is the recent digital transformation that has brought AI to the limelight, given its potential impact on society.
These developments have been accelerated by the increasing processing power of computers and the greater accessibility to, and availability of, information in digital format. Moreover, great scientific and technological advancements have been made in areas such as machine learning (ML) and text, image, voice, and sound recognition.
In the realm of healthcare, AI has emerged as a technological strategy with multiple use cases, ranging from the discovery of new drugs, through the implementation of diagnostic tools, all the way to therapeutics, clinical follow-up, and the enhancement of the operational and logistic efficiency of drug-related processes, among others.
The processing of large volumes of clinical data allows AI algorithms to identify patterns and predict results, which may assist healthcare institutions and their professionals, including hospital pharmacists, in making more informed decisions and improving the outcomes of the different clinical, management and administrative processes involved in healthcare.2
With a view to providing further details on the concept of AI, below is a brief description of the most relevant types of AI for the health sector.
Most relevant types of artificial intelligence for hospital pharmacyAI systems may be classified according to various criteria, the most commonly used of which is whether they can learn autonomously or whether, on the contrary, they require to be programmed to be able to learn.
Automatic learning-enabled AI systems can be classified into 2 distinct categories: those that are based on traditional automatic learning algorithms, also known as ML systems, and those made up of multi-layered neural networks, known as deep learning (DL) systems. Both are capable of learning from data or from examples, in such a way that the final result (known as output) does not depend on someone programming the systems with a series of pre-configured rules, but on the system learning the rules by itself.3
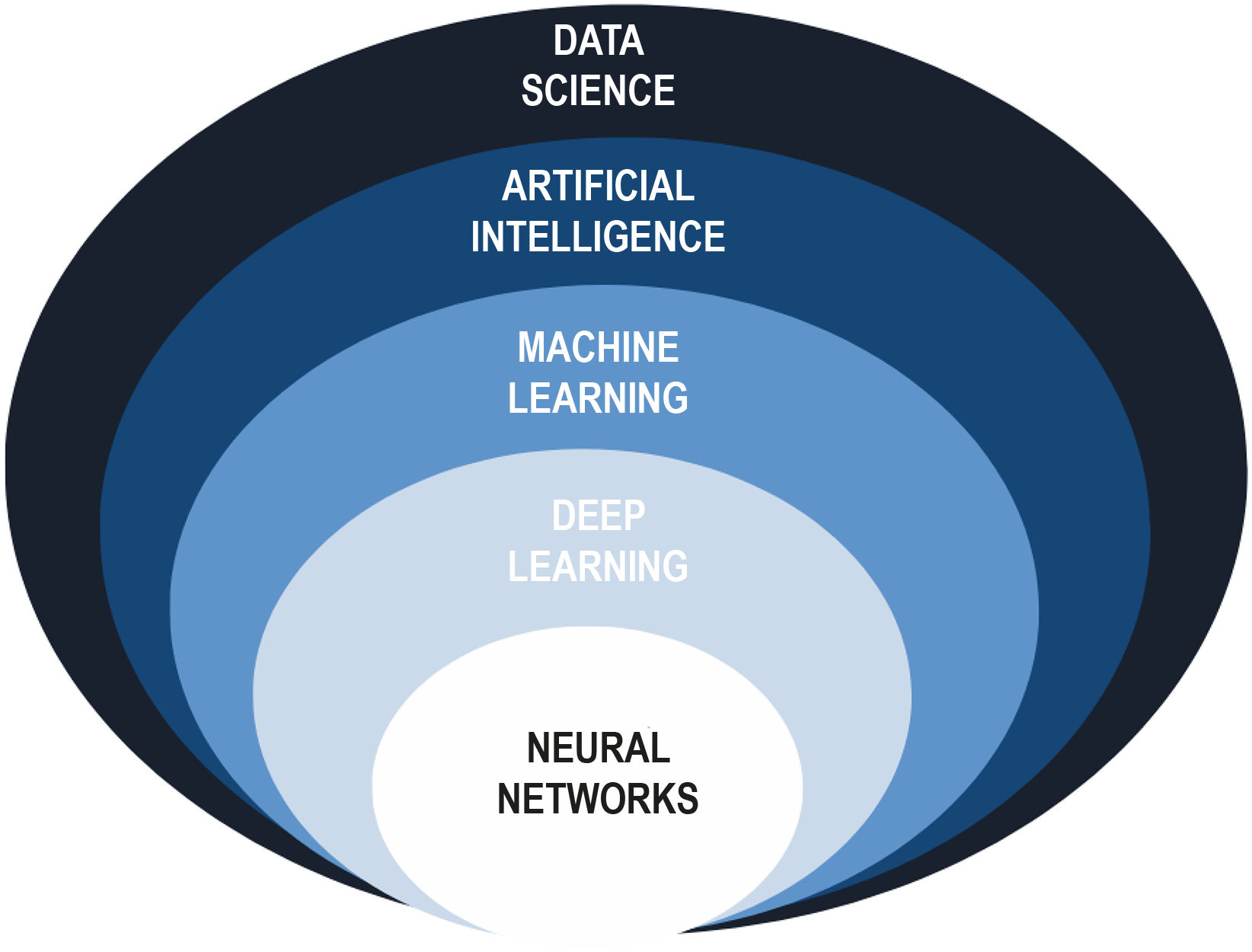
It can therefore be said that AI comprises various sets of techniques, including automatic learning, which encompasses a subset of deep leaning techniques Fig. 1.
Organization of artificial intelligence systems. Adapted from Pettit et al2.
Automatic learning, also known as ML, allows computer systems to learn and improve their ability to resolve tasks automatically drawing on a set of data, without the need of being explicitly programmed. ML uses algorithms mainly to analyze data and find patterns, trends, and relationships, which are subsequently used to adopt decisions, make predictions and classify data.
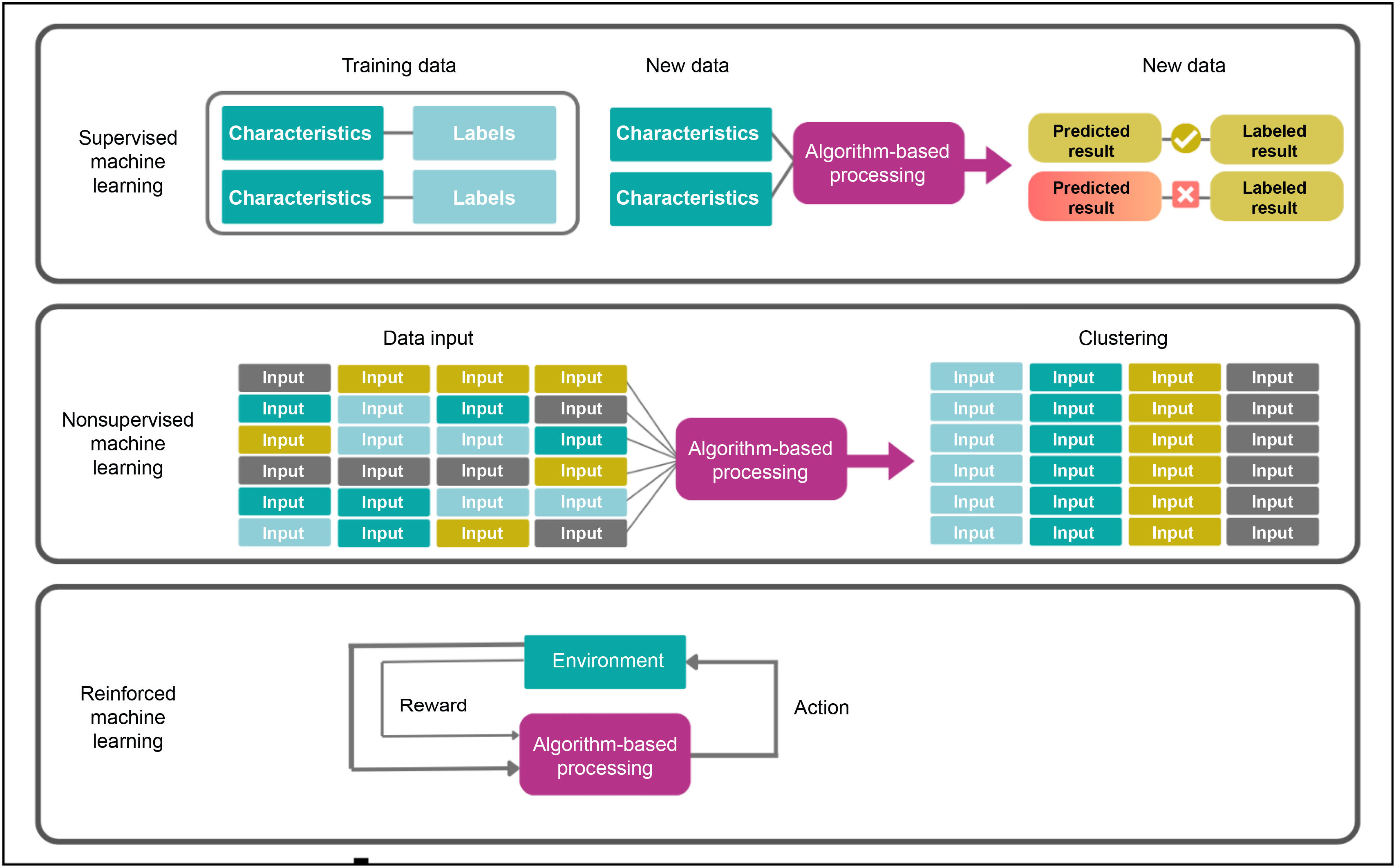
Fig. 2 shows the most common kinds of ML systems.
Machine learning categories. Extracted from Nelson SD.4
Supervised ML consists in labeling and training datasets so that they can predict expected outcomes. The term supervised refers to the fact that the model is provided with the outcome of the variable to be predicted (target variable) once the dataset has been trained. The essence of the process lies in the mapping of a dataset where the input variables (characteristics) and the outcomes (labels) are known, which makes it possible for the computer to learn and generate patterns following an appropriate training process. The labelling of a variable depends on whether it is a categorical variable (e.g., “adverse event” or “non-adverse event”) or a continuous variable (e.g., plasma hemoglobin levels). This allows systems to make accurate predictions about a target variable in new cases.
Supervised ML systems can be used in hospital pharmacy (HP) departments to predict the efficacy or the toxicity of a drug in a given patient, which makes it possible to make informed decisions when the goal is to personalize a treatment or minimize potential side effects. Hospital pharmacists can play an essential role in supervised learning programs, contributing to the identification of outcome variables it could be beneficial to predict, determining what input characteristics may provide useful information to make such predictions, and identifying datasets that contain these characteristics, labels, or outcomes.
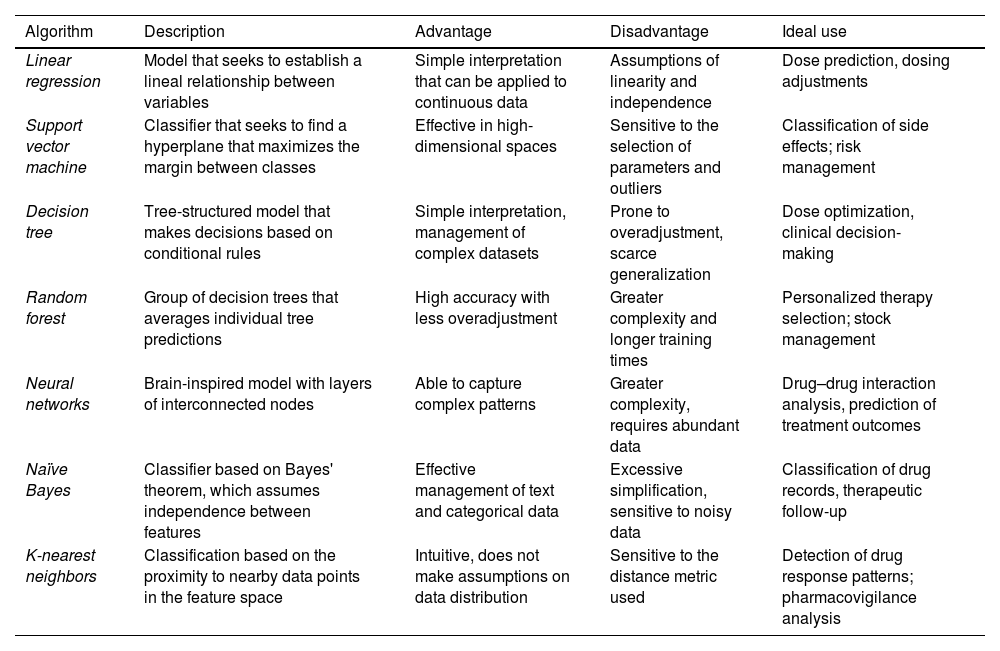
Table 1 describes the most common algorithm-based supervised ML techniques used in healthcare.5
Most common algorithm-based supervised ML techniques used in healthcare.
| Algorithm | Description | Advantage | Disadvantage | Ideal use |
|---|---|---|---|---|
| Linear regression | Model that seeks to establish a lineal relationship between variables | Simple interpretation that can be applied to continuous data | Assumptions of linearity and independence | Dose prediction, dosing adjustments |
| Support vector machine | Classifier that seeks to find a hyperplane that maximizes the margin between classes | Effective in high-dimensional spaces | Sensitive to the selection of parameters and outliers | Classification of side effects; risk management |
| Decision tree | Tree-structured model that makes decisions based on conditional rules | Simple interpretation, management of complex datasets | Prone to overadjustment, scarce generalization | Dose optimization, clinical decision-making |
| Random forest | Group of decision trees that averages individual tree predictions | High accuracy with less overadjustment | Greater complexity and longer training times | Personalized therapy selection; stock management |
| Neural networks | Brain-inspired model with layers of interconnected nodes | Able to capture complex patterns | Greater complexity, requires abundant data | Drug–drug interaction analysis, prediction of treatment outcomes |
| Naïve Bayes | Classifier based on Bayes' theorem, which assumes independence between features | Effective management of text and categorical data | Excessive simplification, sensitive to noisy data | Classification of drug records, therapeutic follow-up |
| K-nearest neighbors | Classification based on the proximity to nearby data points in the feature space | Intuitive, does not make assumptions on data distribution | Sensitive to the distance metric used | Detection of drug response patterns; pharmacovigilance analysis |
Unsupervised ML systems use training data to identify patterns and trends, or to automatically generate classifications into different groups (clustering) based on various unlabeled variables. These kinds of models provide an understanding of input variables, making it possible to classify, though not predict, them. At the same time, the most recent ML techniques allow the use of data-based discoveries, ranging from unknown drug-related adverse events to emerging diseases.4 A specific example of the use of unsupervised ML in HP departments in the clustering of patients according to certain variables (e.g., genetic or clinical) with a view to permitting a personalized choice of the best drug for every new case.
In addition to the discovery of new clinical–pharmacological knowledge, these models may facilitate the adoption of clinical decisions regarding patients' drug therapy as well as promote further development of personalized medicine.
The need of expert clinical and pharmacological knowledge for identifying patterns and evaluate them, and for validating the results obtained, means that hospital pharmacists are ideally placed to participate in, or even lead, unsupervised ML projects.
Reinforced learningReinforced learning systems are trained with data obtained from interactions with the environment or with simulations. While they execute different actions, models receive feedback in the form of rewards or penalties, which facilitates the learning process and the continuous refinement of their parameters.
Unlike supervised ML, previous labeling of the data is not required here as the model learns through continuous exploration and adaptation, depending on the feedback received. This means that the model learns and makes decisions by itself during the learning process.
Reinforced learning algorithms can be used by HP departments to optimize dosing schedules, particularly in patients exhibiting continuous changes in their clinical status. In these cases, the rewards of the system may encompass various effectiveness indicators, such as the reduction of symptoms or changes in some analytical or clinical variable. As systems accumulates more information on the patient and adapt the doses they receive, they develop the ability to administer drugs in an increasingly efficient and personalized manner, accurately catering for the needs of each individual patient.
Artificial neural networks and deep learningArtificial neural networks (ANNs), inspired by the functioning of the human nervous system, are ML models whose structure is formed by layers of interconnected nodes, known as artificial neurons. Each neuron corresponds to a computational unit responsible for processing and transforming information and conveying the output to the nodes in the next layers.
These models can be divided into 2 categories, depending on the number of layers in the network. Models with just a few layers of connected neurons are known as non-deep or shallow models, while those with a larger number of layers and greater complexity are called DL models.
In DL models, each layer in the neural network performs mathematical operations and non-linear transformations of the initial data it is provided with. As progress is made toward the more internal layers in the network, mathematical operations become increasingly complex and non-linear. This leads to the creation of more and more abstract representations of the information, which are less and less directly connected with the original data input. For that reason, DL algorithms require larger numbers of operations than traditional ML models, which significantly increases the magnitude of the datasets and the computational power required by the system.6
Unlike standard ML systems, where the models developed allow machines to learn from the data and make decisions based on patterns, DL systems are characterized by capturing complex patterns and highly abstract representations from the data.
Among its multiple applications in healthcare, DL has been proposed as a tool to improve the diagnostic process, enhance the prediction of the efficacy of clinical outcomes, optimize drug therapies, and facilitate treatment personalization on the basis of patients' individual (genetic, clinical, etc.) characteristics. Other potential uses of DL in healthcare are related with developing smart medical records, automating administrative tasks, improving the management and assignment of hospital resources, and even identifying disease patterns among the population.
Natural language processing and large language modelsNLP is a branch of computer science that brings together AI with human language processing. NLP aims to develop algorithms and models capable of enabling computers to effectively understand, interpret and generate human language.
One of the most noteworthy recent achievements of NLP has been the development of large language models (LLMs), which are a direct byproduct of supervised ML. These language models, driven by advanced DL techniques, have been shown to be able to process and generate human language with impressive coherence levels. Their operation relies on being exposed to massive amounts of previously labeled language data, which allows them to learn patterns and grammatical structures in an autonomous way.
The ability of LLMs to interpret complex questions and generate coherent answers has created new opportunities to enhance the automation of administrative and clinical tasks, optimize the preparation of medical reports, analyze massive amounts of scientific and clinical evidence, improve accessibility and communication between patients and physicians, etc.7
Artificial intelligence in the health sciencesTechnology and health sciences have followed parallel paths in recent years. Technological advancements have contributed to modifying people's concept of health. At the same time, people's needs and expectations with regard to the promotion and maintenance of health are influencing the development of technology.
As mentioned above, AI is formed by a series of sufficiently trained logical algorithms on the basis of which machines are able to make decisions for specific cases drawing on a set of general norms. Combining AI with robotics, it is possible to create smart machines able to make diagnostic proposals, which can boost the efficiency of clinical processes. This means that AI, in the form of machines or computer software, is set to become a key technology in healthcare in ways that will be more or less transparent for the user. Healthcare professionals will have to become acquainted with this technology, as well as its advantages and disadvantages, as it is set to become a natural part of their work.8
Although multiple retrospective clinical trials have demonstrated the sheer power of AI, the number of AI tools used in clinical practice remains scarce.9 Critics point out that AI systems could in practice prove less useful than demonstrated by retrospective studies. Indeed, some systems may be too slow or complex to be used in real medical practice,10 or unforeseen complications may arise in the way in which humans interact with AI.11
In addition, retrospective “in silico” datasets are constantly filtered and subjected to exhaustive data cleaning procedures, which could make them less representative of real-world medicine. Randomized clinical trials (RCTs) and prospective studies could be capable of bridging the gap between theory and practice, more rigorously showing that AI models can have a quantifiable effect and a positive impact when implemented in real-world clinical environments.
Recently, various RCTs have demonstrated the usefulness of AI in the clinical setting. In addition to analyzing accuracy, a wide range of metrics have been used to evaluate the usefulness of AI, providing a holistic picture of its impact on health sciences.12,13
Recently adopted guidelines such as SPIRIT-AI and CONSORT-AI, as well as forthcoming ones, such as STARD-AI, could help standardize the available publications on the application of AI to healthcare, including protocols, guidelines, and clinical trial outcomes. This would make it easier for the scientific community to share findings and more rigorously investigate the usefulness of AI in clinical settings.14,15
More rigorous standards are needed for AI systems to earn the trust of healthcare professionals, with stricter rules to enhance the transparency of reports, reinforce validation processes, and demonstrate the impact of AI on clinical outcomes.16
Below are some specific examples of the practical application of AI to healthcare and to the development of biomedical science.
An RCT carried out to evaluate an insulin dosing AI system measured the length of time patients remained within the target glucose range.17 Another RCT analyzed an AI system designed to follow up intraoperative hypotension and tracked the mean duration of hypotension episodes.18 Finally, another study looked into an AI system for identifying intracranial bleeding capable of reducing response times.19
DL has resulted in a flourishing of research into the medical applications of AI, particularly in areas that are heavily dependent on the interpretation of images such as radiology, pathology, gastroenterology, and ophthalmology.
Moreover, the accuracy of several AI systems for applications such as radiology, breast scan interpretation,20 heart function assessment,21 and lung cancer detection22 has been significantly improved, as has their diagnosis, prediction, and risk management potential.23
In recent times, AI has also made great strides in the realm of biochemistry, improving our understanding of the structure and the behavior of biomolecules.24,25 The work of Senior et al. in AlphaFold constituted a great advancement in protein folding, a fundamental task that consists in predicting the 3D structure of a protein from its chemical sequence. A greater ability to predict the structure of proteins will contribute to a better understanding of various phenomena, such as the relationship between drugs and proteins, as well as their interactions and the effects of their mutations.
AI has also achieved significant improvements in the realm of genomics, despite the complexities inherent in modeling 3D genomic interactions. When applied to data on the free DNA of circulating cells, AI has allowed the detection, prognosis, and identification of the origin of tumors.26,27
DL has enhanced CRISPR-based gene editing efforts, helping predict the behavior of guide RNA and identify anti-CRISPR protein families.28 Transcriptomic and genomic data have been used to rapidly detect antibiotic resistance in various pathogens. These advancements have made it possible for physicians to quickly select the most effective treatments, potentially reducing mortality and preventing the unnecessary use of broad-spectrum antibiotics.29
On the other hand, AI is starting to accelerate the discovery of new drugs as it reduces the need to perform slow and costly physics experiments. Such models have proved useful to predict important physical properties such as the bioactivity or the toxicity of potential drugs. One study used AI to identify a drug subsequently shown to be effective against antibiotic-resistant bacteria in experimental models.30
Finally, recent research has exploited the large amounts of medical data available for processing natural language. An AI system called BioBERT, trained with a varied typology of medical texts, has been shown to answer biomedical questions with greater accuracy than classical natural language interpreting systems.31 Various AI models have been used to improve our understanding of problems such as what drugs can interact with one another,32 or how to automatically label radiological reports.33
Applying artificial intelligence to hospital pharmacyAs one may well imagine, HP has not been immune to the transformation taking place in the health system. AI could play a key role in this process as an accelerator of change. Integrating and combining AI with the different processes carried out in HP departments would improve and optimize many of their services and internal operations. This integration would comprise 2 dimensions,34 a clinical one and an operational one.
- •
Clinical dimension: providing more precise pharmaceutical care with a particular focus on prevention, minimization of errors, and promoting the adoption of decisions based on data and the personalization of therapies and care.
- •
Operational dimension: automating administrative tasks and optimizing management processes so as to be able to focus on working on high-value activities and gear pharmacists' cognitive processes toward areas where their skills are required.
The potential benefits of AI for HP have to do with identifying patterns and/or relationships in large datasets. This makes it possible to extract valuable and relevant information and to incorporate new knowledge that may help predict the outcomes of drug therapies,35 make decisions in real time, and optimize pharmaceutical care.36–39 This should contribute to reducing red tape and ensure that care is centered on the individual and on the more complex problems.40
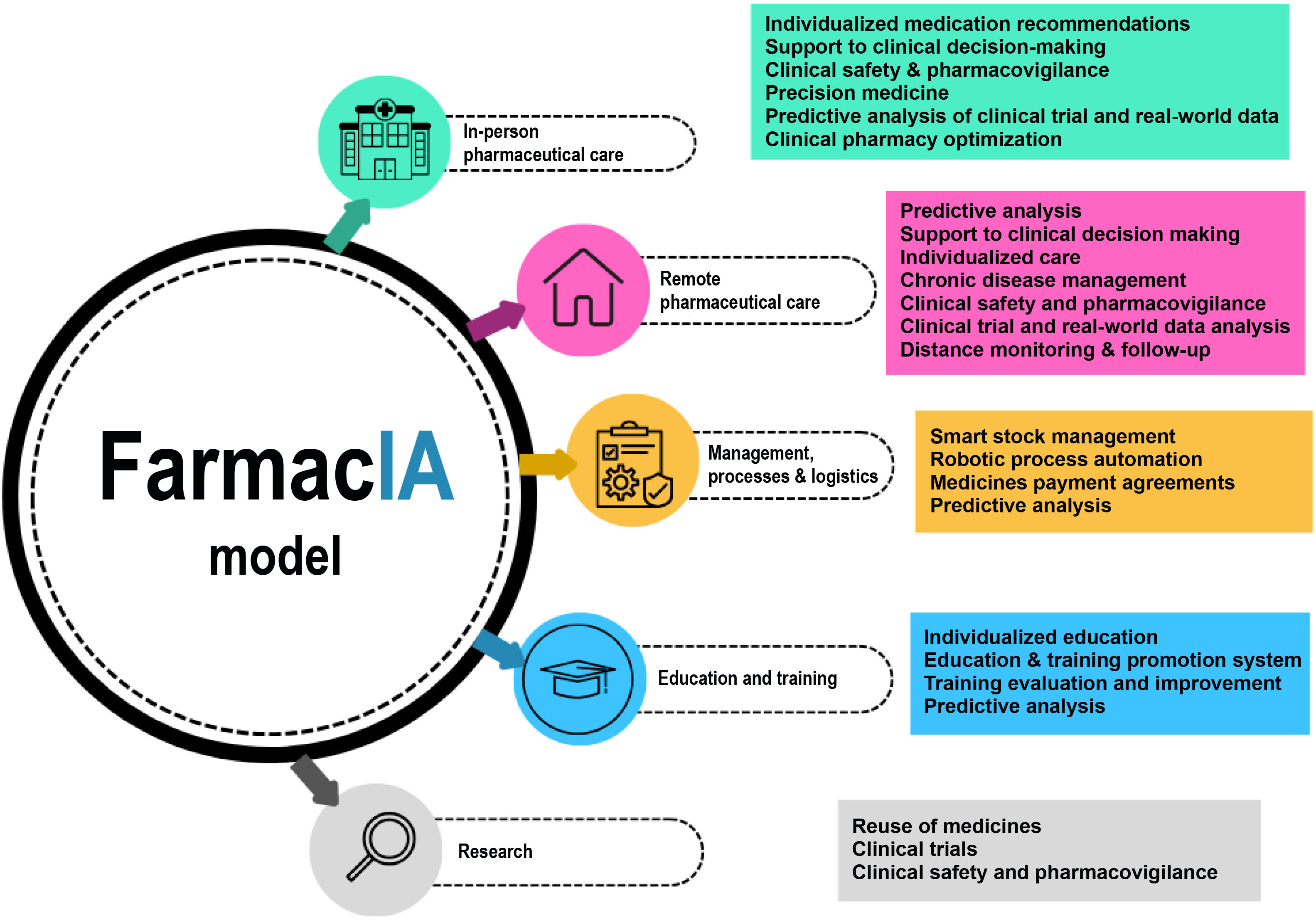
The advent of technologies such as DL and neural networks or tools such as LLMs are poised to enable great advances in the realm of HP. Fig. 3 shows a classification of the most significant benefits of AI for HP into 5 domains.
In-person pharmaceutical carePharmacotherapeutic managementAn analysis of patients' data and clinical characteristics makes it possible to make recommendations based on scientific evidence. Consequently, AI algorithms may help optimize drug therapy, identify the most appropriate pharmacological alternatives, develop more precise dosing schemes, and supervise the effectiveness of treatments.38 The data generated should allow more informed decision-making, improve health outcomes and reduce the incidence of adverse events.
Precision medicineAI is capable of analyzing specific patient data such as genetic profiles, clinical and lab test results, and identifying response biomarkers and subpopulations at risk of drug inefficacy, thus anticipating the patients' response to a given drug, and detecting molecular targets and interactions.39
As regards pharmacokinetic monitoring, AI can be a useful adjunct to traditional systems,40 assisting in the decision-making process (exposure-concentration prediction and dose optimization), in the development of models (covariate selection, model validation, and patient classification), and in the administration of precise doses (selection of the most appropriate model and estimation of parameters). The goal is to prepare more accurate therapeutic plans, predict each individual patient's response to treatment and, as a result, optimize drug selection and dosing, based on the knowledge extracted from large and complex sets of molecular, pharmacokinetic, epidemiological, clinical, and demographic data.
Clinical safetyMethods such as DL allow us to predict adverse drug reactions with a good AUC value (around 0.85).41 They also help discover new reactions by analyzing the chemical structure and mechanism of action of the drug, as well as the effects associated with polypharmacy.42
AI is capable of rapidly analyzing the patients' profiles, including their medical history and the drugs they are taking, in order to identify potential drug–drug interactions.43 This is an effective way of avoiding harmful combinations, thus promoting patient safety.
AI-based predictions allow identification of high-risk patients and early optimization of treatments, thereby improving patient safety and reducing hospital readmissions.
Remote pharmaceutical careFollowing up chronic patientsThe use of conversational agents44 able to interact with patients in a personalized and contextualized way has all but revolutionized patient follow-up as, when combined with other technologies, they can provide health data such as vital signs, patient-reported outcomes, adverse events, etc., which are extremely useful for clinical and pharmacotherapeutical decision-making.
The collection and analysis of patients' health data makes for more proactive interventions, e.g., when a treatment plan needs to be adjusted, and allows clinicians to offer lifestyle recommendations for effectively managing chronic conditions.
AI has also been shown to be an effective tool for evaluating and optimizing adherence.45 AI systems may act as a coach, generating automatic medication reminders, sending text and voice messages and collecting information on patients' lifestyles, beliefs, and opinions.
The use of AI in these scenarios simplifies patient follow-up, provides real-time information and improves medication adherence and persistence, which typically results in better health outcomes.
Selection of pharmaceutical interventionsGenerative AI is extremely useful in remote pharmaceutical care. AI-based selection systems46 draw on datasets containing patient symptoms, drug information, and medical records among others to generate personalized questions. These systems allow a more effective characterization of health problems, making recommendations including referring patients to a health center, changing a pharmaceutical appointment, sending a personalized message, or contacting a hospital pharmacist. In these circumstances, it is possible to adopt a proactive stance in order to prevent or at least minimize errors and adverse events.
Management, processes, and logisticsStorage and distribution of medicationsAn analysis of large datasets allows algorithms to predict the demand for medications, identifying expiry dates and optimizing stock levels to allow a stable supply, the reduction of waste and the prevention of shortages, all the while improving operating efficiency and the quality of care.38 Use of AI in HP departments should reduce inefficiencies, freeing up pharmacists' time so that they can focus on patient care and clinical decision-making.
Funding systems and medication reimbursement agreementsFunding and reimbursement systems have in the last decade moved away from traditional practices based on fixed unit costs to more innovative result- and value-based solutions with an emphasis on the attainment of specific therapeutic goals.
Applying NLP to medical records may provide precise information on the activity or the effectiveness/safety of a given drug vis-à-vis its competitors in the real world. Moreover, AI can also be used to determine the risk inherent in a certain agreement or contract.47
The new Center for Artificial Intelligence in Pharmaceutics (CIAM), a pioneering initiative in Europe coordinated by the Health and Social Care Consortium of Catalonia and developed by Catalonia's Department of Health in collaboration with the Agency for Health Quality and Evaluation, the TIC-Salud Social Foundation and the Catalan Health Service, has recently been established. CIAM's mission is to use AI to optimize all processes related to effectiveness, patient safety, and the sustainability of the health system.
Education and trainingPatient educationThe design of virtual agents44 may provide patients with the information they need as well as meet their informative needs with respect to their medications. This should help them make better informed decisions on how to manage their health.
AI solutions may be designed to monitor adherence, reduce the waiting times associated with the dispensing of drugs, collect data on patients' habits and opinions, and remotely manage their drug regimens in order to improve convenience and their overall experience. AI also empowers patients to effectively manage their health, thereby improving their health outcomes.
All the above should result in more effective pharmacist-patient communication and in the availability of valuable information on patients' preferences, concerns, and levels of satisfaction, which is bound to result in more personalized and patient-centered care.
Training of health practitionersGenerative AI solutions are set to become a valuable research and scientific publishing tool that plays a key role in the process of reading, drafting, analyzing, and proofreading scientific papers.
Their participation is also crucial in the realm of specialized health training as a vehicle to evaluate the students' progression in real time. This should enable identification of areas for improvement, the offering of guidance, the development of personalized training programs, the prediction of future performance, and the detection of potential problems.48 Thanks to AI, training will become a fully personalized experience providing healthcare professionals with better skills and more in-depth knowledge, which will unquestionably result in improved patient outcomes.
ResearchClinical trialsAI is able to analyze large volumes of clinical and research data, to identify patterns, facilitate patient recruitment and accelerate the various processes involved in clinical trials.49 It will therefore facilitate the identification of potential subjects and the analysis of results, and it will contribute to the development of evidence-based practices, boosting the advancement of medical research and promoting research.
Drug therapy and clinical safetyMethods such as DL are being applied to the reuse of already-approved medications for new uses and indications50 thanks to the analysis of genetic profiles, the search of new drug–protein or drug–receptor interactions, and the discovery of new molecular mechanisms, among others. At the same time, clinical researchers will use DL to discover new adverse events and drug–drug interactions, and to identify new phenotypes and response biomarkers.
However, in spite of AI's huge potential benefits, use cases in HP departments are still scarce. Most applications consist in support systems for the review of prescription orders and in the detection and/or prediction of adverse drug reactions.51–59Table 2 contains 6 real-use cases applicable to HP departments.
Use cases of artificial intelligence in hospital pharmacy departments.
| Use case | AI branch | Characteristic | Results | Benefits |
|---|---|---|---|---|
| Bu et al.54 | Rule-based expert system |
| ||
| ||||
| ||||
| ||||
| ||||
| ||||
| If the prescription was labeled as adequate, the pharmacists reviewed it before dispensing the drug(s). If the prescription was inadequate, both the physician and the pharmacist were informed. |
| |||
| ||||
| According to AI, pharmacists did not reject any of the adequate prescriptions | Increased patient safety.Optimization of the pharmacist's workflow.Optimization of the physician's prescription process.Promotion of pharmacovigilance. | |||
| Knight et al.55 | ML: supervised learning | Goal: to detect irregular or excessive use of narcotics.Model design: a literature review was carried out and, based on the professionals' experience, predictors related with diversions of narcotics were included as input variables for the model: delayed administration | ||
| Errors in the recording of administered, unused or returned doses. | ||||
| Errors in storage and custody procedures | ||||
| Inadequate prescription | ||||
| Inappropriate medication | ||||
| The model as trained iteratively for 12 months with data of past cases from 2 hospitals.Procedure: a comparison was made between the supervised learning model and the existing models to determine which took the longest to identify diversions of narcotics. | Accuracy: 93%Specificity: 95.9%Sensitivity: 96.6%The model detected 22 incidents in 3 hospitals.It is able to detect incidents 160 days faster on average (median: 74 days) | Improving patient safety.Faster detection of drug diversions and minimizing the efforts made by the staff to detect these problems. | ||
| Van Laere et al.56 | ML: random forest | Goal: to predict the risk of QTc prolongation and issue a warning in cases of elevated risk. Model design: algorithms were trained with a set of 512 data and 102 drug–drug interactions that may result in QTc prolongation.Several algorithms were analyzed to determine which was associated to a better performance.Procedure used: A comparison was made between traditional statistical models and ML. | The best performing algorithm was the one based on a random forest model:Accuracy: 82%Specificity: 88%Exhaustivity: 76%Conversely, methods such as logistic regression yielded a performance of 62% overall. | Optimizing the pharmacist's workflow.Improving patient safety.Promoting pharmacovigilance |
| Yaçın et al.57 | ML: random forest | Goal: to predict medication errors in the neonatal ICU.Model design: a univariate analysis was made to include the relevant variables in the algorithm:-variables related with drug therapy- variables related with the staff's workload- demographic and clinical variablesProcedure used: Several methods were used to design the algorithm. The system was trained with historical data (training datasets) to evaluate and validate its performance. | The ML algorithm determined that the 2 most important variables related to the occurrence of medication errors were the total number of drugs taken and the presence of anti-infectious drugs in the patient's regimen.The best performing ML algorithm was the one based on a random forest model as it was capable of predicting adverse events with great accuracy.The performance of the model was as follows:Accuracy: 92%Exhaustivity: 92%Specificity: 92%F1 score: 93%AUROC: 92% | Improving patient safety.Reducing the fatigue resulting from prescription alerts.Promoting pharmacovigilance |
| Zhao et al.58 | ML: neural network | Goal: to monitor patients at home so as to find out whether they were administering their insulin and using their inhalers properly.Model design: the neural network model was used to teach the algorithm to detect when a drug was administered correctly or incorrectly.The algorithm was trained with 47,788 (positive and negative) examples. Procedure used: a wireless sensor was installed in the patients' home, whose signals were analyzed by AI. The sensor provided information on the location, actions and movements of the patient while taking the medication. In the event of inappropriate administration, the patient received an alert. The reliability of the model for detecting administration errors was analyzed. | The model is able to reliably detect medication administration errors.AUC for insulin: 0.967AUC for inhalers: 0,992 | Finding out and improving adherence. |
| Huang et al.59 | ML: LASSO algorithm | Goal: to predict the clearance rate of tacrolimus and combine it with a population-based model, considering clinical characteristics and genetic polymorphisms to individualize the dose administered to pediatric patients with nephrotic syndrome.Model design: several methods were analyzed to identify the one with the best performance.The selected variables were the same as those in the population-based model for tacrolimus.Procedure used: the model was trained with a test dataset and its performance was later evaluated. | The highest values were achieved by the LASSO model:R2: 0.42 | More accurate predictions of a drug's clearance rate.Optimizing the initial dose so that patients achieved their individual therapeutic target. |
AUC: area under the curve; AI: artificial intelligence; ML: machine learning; ICU: intensive care unit.
Hospital pharmacists with an understanding of AI will be able to contribute significant added value and face new challenges from an innovative perspective. Specifically, they will know how to:
- •
Identify clinical, logistic, and operational challenges and problems that may be addressed by AI.
- •
Lead the development and clinical validation of AI models.
- •
Be active members of research teams working on the application of AI to healthcare.
- •
Liaise between other clinicians and data scientists working on AI projects.
- •
Critically evaluate drug-related models, algorithms and AI systems.
As conclusions, AI has become an invaluable tool for analyzing large volumes of data and obtaining results that may support decision-making, optimize human efforts, and make the best use of time and money, contributing to saving, and improving the quality of patients' lives.
The next generation of AI technologies applied to HP will be based on the creation of expert systems able to identify and prevent medication-related problems using the patient data stored in pharmaceutical databases and in external data repositories. Aided by advancements such as robotization and workflow improvements, AI technologies will make it possible for pharmacists to identify serious medication-related problems in a way that is closer to patients and to the clinical team.
AI is poised to play a decisive role in the transformation of some of the tasks performed by HP departments, such as dispensing and medication management, contributing to the delivery of a wider range of services that should result in more effective patient care.
Although the use cases of AI in the field of HP are still few and far between, it is to be expected that AI systems will play an increasingly important role in optimizing pharmaceutical care and improving safety and efficiency in the hospital setting. Further, and higher quality research is, however, required to examine the usefulness of AI in the HP setting to determine whether it can indeed become a viable tool at the service of HP departments.
Ethical responsibilitiesAll co-authors accept the responsibilities defined by the International Committee of Medical Journal Editors (available at http://www.icmje.org/).
FundingNo funding.
Authorship statementAll three authors participated equally in the conception and design of this article as well as in its writing and critical review, making significant intellectual contributions to the analysis presented. All of them had a say in the approval of the final version submitted for publication.
Transfer of rightsIn the event of publication, the authors exclusively transfer to the Revista and, by extension to SEFH, their rights to reproducing, distributing, translating, and publicly communicating their work by any sound, audiovisual or electronic medium or format. A specific right transfer letter will be sent once the paper is submitted through SEFH's online manuscript processing system.
CRediT authorship contribution statementYared González Pérez: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Conceptualization. Alfredo Montero Delgado: Writing – original draft, Validation, Conceptualization. Jose Manuel Martinez sesmero: Writing – original draft, Validation, Conceptualization.
The authors would like to thank Luis Margusino for entrusting them with preparing the present manuscript.