Immunotherapy has emerged as a therapeutic alternative to chemotherapy (CT) for perioperative treatment of resectable non-small cell lung cancer (NSCLC). The objective is to perform a network meta-analysis comparing the perioperative efficacy of immunotherapies in resectable NSCLC taking into account tumor expression of programmed death ligand 1 (PD-L1).
MethodA review was performed in Pubmed® and EMBASE® until September 17, 2024. Phase III clinical trials on perioperative immunotherapies (P-) for resectable NSCLC with ≥50 patients were included. The selected endpoint was progression-free survival (PFS) according to different levels of PD-L1 expression. The statistical analysis used Bayesian methods. Fixed- or random-effects models were assessed using deviance information criteria (DIC). A sensitivity analysis was developed to evaluate the influence of heterogeneous studies.
ResultsFour trials were included. Immunotherapeutic schemes with P-toripalimab, P-nivolumab, P-pembrolizumab and P-durvalumab were selected. Only P-toripalimab included a cycle of adjuvant toripalimab + CT. The remaining perioperative combinations contained the neoadjuvant immunotherapeutic agent + CT (4 cycles) regimen followed by adjuvant immunotherapy. The common comparator was neoadjuvant placebo + CT with adjuvant placebo (P-placebo). P-toripalimab was evaluated in a population with heterogeneous characteristics. Fixed effects model was selected for DIC values with irrelevant differences. P-toripalimab obtained greater magnitude of effect in PFS for populations with PD-L1 < 1% and 1–49% (reference treatment). No benefit of any immunotherapeutic combination over P-placebo was observed in resectable NSCLC with PD-L1 expression <1%. P-toripalimab was statistically superior to the other regimens [except P-pembrolizumab, HR = 1.6 (95%CrI: 0.84–3.2)] for PD-L1 expression 1–49%. Immunotherapeutic schemes were superior to p-placebo for PD-L1 expression ≥50%. Sensitivity analysis showed results compatible with the primary analysis.
ConclusionsOur network meta-analysis provides reliable evidence on the efficacy of perioperative immunotherapy in resectable NSCLC according to PD-L1 expression levels, and may favor competition between therapeutic alternatives. A sensitivity analysis supported these results.
La inmunoterapia ha surgido como alternativa terapéutica a la quimioterapia (CT) para el tratamiento perioperatorio de cáncer de pulmón no microcítico (CPNM) resecable. El objetivo es realizar un metaanálisis en red comparando la eficacia perioperatoria de las inmunoterapias en CPNM resecable, considerando la expresión tumoral de ligando 1 de muerte programada (PD-L1).
MétodoSe realizó una revisión en Pubmed® y en EMBASE® hasta el 17 de septiembre de 2024. Se incluyeron ensayos clínicos en fase III sobre las inmunoterapias perioperatorias (P-) para el CPNM resecable con un número mayor o igual a 50 pacientes. La variable seleccionada fue la supervivencia libre de progresión, según los diferentes niveles de expresión de PD-L1. El análisis estadístico usó métodos bayesianos. Se valoraron los modelos de efectos fijos o aleatorizados, usando el Criterio de Información de la Devianza (DIC). Un análisis de sensibilidad fue desarrollado para evaluar la influencia de estudios heterogéneos.
ResultadosSe incluyeron 4 ensayos. Los esquemas inmunoterápicos con P-toripalimab, P-nivolumab, P-pembrolizumab y P-durvalumab fueron seleccionados.Solo P-toripalimab incluyó un ciclo de toripalimab + CT adyuvante. Las combinaciones perioperatorias restantes incluyeron el esquema de agente inmunoterápico + CT (4 ciclos) neoadyuvante, seguido de la inmunoterapia adyuvante. El comparador común fue placebo + CT neoadyuvante con el placebo en adyuvancia (P-placebo). P-toripalimab fue evaluado en una población con características heterogéneas. Se seleccionó el modelo de efectos fijos por diferencias irrelevantes entre los valores de DIC. P-toripalimab obtuvo la mayor magnitud de efecto en la supervivencia libre de progresión para la población con PD-L1 < 1% y 1–49% (tratamiento de referencia). No se observó beneficio de ninguna combinación inmunoterapéutica sobre P-placebo en CPNM resecable con expresión de PD-L1 < 1%. P-toripalimab fue estadísticamente superior a los demás regímenes (excepto P-pembrolizumab, HR = 1,6 [ICr 95%: 0,84–3,2]) para expresión de PD-L1 1–49%. Los esquemas inmunoterapéuticos fueron superiores a P-placebo para expresión de PD-L1 ≥ 50%. En el análisis de sensibilidad se mostraron resultados compatibles con el análisis primario.
ConclusionesEl metaanálisis en red aporta evidencia fiable sobre la eficacia de la inmunoterapia perioperatoria en CPNM resecable según los niveles de expresión de PD-L1, pudiendo favorecer la competencia entre alternativas terapéuticas. Un análisis de sensibilidad respaldó estos resultados.
Lung cancer is responsible for the highest number of deaths worldwide, accounting for 18.8% of all cancer-related deaths.1 Non-small-cell lung cancer (NSCLC) accounts for approximately 80–85% of lung cancer diagnoses.2 Approximately 30% of NSCLC cases are eligible for surgical resection, as they are detected at an early stage.3 To date, surgical resection has remained the main curative intervention in these cases.4 However, disease recurrence occurs in over one-third of patients who undergo resection.5–7
Historically, adjuvant chemotherapy following surgical resection has been used as a therapeutic option to improve the survival rate of patients with resectable NSCLC.8 Regimens involving immune checkpoint inhibitors, particularly those targeting programmed cell death protein 1 (PD-1) or programmed death-ligand 1 (PD-L1), were initially used in the treatment of advanced or metastatic NSCLC.9,10 Subsequently, this type of immunotherapy was also found to be beneficial as a maintenance treatment following adjuvant chemotherapy for resectable NSCLC.11
Other research has suggested that neoadjuvant immunotherapies can delay disease progression.12 Therefore, clinical trials were developed to evaluate the use of immunotherapy during the perioperative period in patients with resectable NSCLC. This therapeutic approach consisted of administering certain drugs (or combinations) before and after surgery to assess the clinical benefit achieved. The NADIM II study found that the perioperative regimen of nivolumab with chemotherapy resulted in a higher rate of complete pathological response than chemotherapy alone.13
Since then, several clinical trials have evaluated the perioperative use of different immunotherapeutic agents for treating resectable NSCLC.14,15 To date, the efficacy results obtained show a significant increase in progression-free survival (PFS). However, no studies have been designed to directly compare one immunotherapy regimen with another. This considerably limits the ability to select the most effective and efficient therapeutic alternatives. Network meta-analyses (NMAs) are statistical techniques that enable the direct and indirect comparison of the effects of various treatments in a clinical context.16 These techniques can therefore facilitate therapeutic positioning in the absence of data on direct comparisons of perioperative immunotherapy treatments in resectable NSCLC.
On the other hand, developing reliable NMAs requires systematic reviews with appropriate data selection criteria. Subgroup analysis enables the data obtained from different populations undergoing a treatment to be evaluated according to a specific characteristic.17 Tumour PD-L1 tumour expression levels have previously been used as a predictive biomarker of response to immunotherapy in NSCLC.18 Therefore, evaluating outcomes according to subgroups based on this factor in the perioperative context could be of interest. Thus, populations with high PD-L1 tumour expression, which have a potentially higher response to immunotherapy, would not be mixed with populations with lower levels of the biomarker, which have a lower response. Using results from heterogeneous studies and populations in an NMA could lead to erroneous conclusions and negatively influence clinical decision-making.19 The objective was to develop an NMA of the efficacy of perioperative immunotherapy regimens in patients with resectable NSCLC according to PD-L1 tumour expression levels.
MethodsSystematic review of the literatureThe PICOS strategy was applied to develop the research question20: population, intervention, comparator, outcome variable, and study design. The target population consisted of patients diagnosed with resectable NSCLC with measured PD-L1 tumour expression levels. The selected intervention comprised perioperative regimens (both neoadjuvant and adjuvant) that included immunotherapy agents. Any comparator was accepted. The outcome variable selected was PFS. Phase III comparative studies with a sample size of more than 50 patients from the target population were included, as these studies have sufficient maturity and statistical power to avoid premature conclusions.
The literature search followed the recommendations of the Preferred Reporting Items for a Systematic Review and Meta-analysis (PRISMA) guide.21 This review was conducted across 2 databases (PubMed and EMBASE) on September 17, 2024. In PubMed, the filter “randomized controlled trial” was applied with the following search strategy: “perioperative non-small-cell lung cancer.” In EMBASE, the filters “study types: randomized controlled trial” and “publication types: article” were applied, with the search descriptors: perioperative resectable non-small-cell lung cancer OR (perioperative AND resectable AND non-small AND [cell/exp. OR cell] AND [‘lung’/exp. OR lung] AND [cancer/exp. OR cancer]).
Screening and study selection criteria.Two researchers independently conducted the systematic search. During the screening process, the titles and abstracts of the search results were reviewed to exclude publications that did not meet the established inclusion criteria. The full text of the publications was examined during the eligibility process. A citation search was subsequently performed. Any inconsistencies regarding the inclusion or exclusion of search results were resolved through discussion between the 2 reviewers and a third party. The search results included were those that met the PICOS criteria described above. The search results excluded were those that did not meet the above criteria, as well as those published in a language other than Spanish or English.
Data extractionThe following data were collected from the selected clinical trials: study identification and publication date, intervention arm design, comparator treatment, duration and periods of treatment administration, median patient follow-up, relevant population characteristics (age, functional status, disease stage, histology, PD-L1 tumour expression, lymph node involvement), sample sizes, hazard ratio (HR) values for PFS, and 95% credible interval (CrI).
Risk of biasPossible sources of bias were evaluated, including those related to the heterogeneity of the designs of the included clinical trials, the characteristics of the recruited populations, and the common comparators used in the NMA. The impact of prolonged follow-up on the identification of clinical events was taken into account, as was the influence of insufficient sample sizes on the accuracy of the results. Differences in the profiles of the recruited patients were analysed due to their potential effect on data quality and on the heterogeneity of the results. The variables considered included age, functional status, disease stage, histology, and lymph node involvement. Heterogeneity in the regimens forming the NMA nodes was also assessed due to its relationship with the uncertainty of the results.
Network meta-analysisAn NMA was developed based on the results of the systematic search using the HRs for PFS along with the upper and lower bounds of the 95% CrI. The network was formed through connections via the common comparator. The reference regimen used to evaluate the results was the regimen that had the greatest effect size. A primary analysis was performed, including all the selected studies. A sensitivity analysis was subsequently performed using a network that excluded the studies which, despite meeting the established inclusion criteria, could introduce some heterogeneity and bias in relation to the rest of the network. The Graphical Models for Meta-Comparison (GEMTC) package for R Statistics22 and the JAGS23 programme were used for the NMA, which was based on Bayesian methods combining direct and indirect evidence. According to the data and models used by Bayesian methods, the CrI obtained showed that there was a 95% probability that the true parameter lies between the upper and lower bounds. The Deviance Information Criterion (DIC) was used to compare fixed-effects and randomised-effects models. We selected the model that best fitted the network. This was defined as the model with the lowest DIC score, establishing a value of 5 as the minimum relevant difference between competing models.24 If the minimum difference was not reached, the option providing the most accurate data was selected. In addition, the consistency and heterogeneity of the NMA were analysed using the Q statistic.25 The I2 statistic was used to determine the proportion of variability in the results attributable to heterogeneity.26 Heterogeneity was categorised as low when the I2 value was less than 25%, medium when it was between 25% and 50%, and high when it was more than 50%.27 The random-effects model was used in cases of high heterogeneity.28
Finally, an analysis was performed using the aggregated data from the regimens involving immunotherapy agents in those PD-L1 tumour expression subgroups in which no differences were observed compared to standard therapy. The evaluation aimed to assess the effect of reduced statistical power when using results by subgroups with smaller sample sizes, rather than the overall results of the clinical trials. The calculations were performed based on the HRs and the variances of their natural logarithms.29
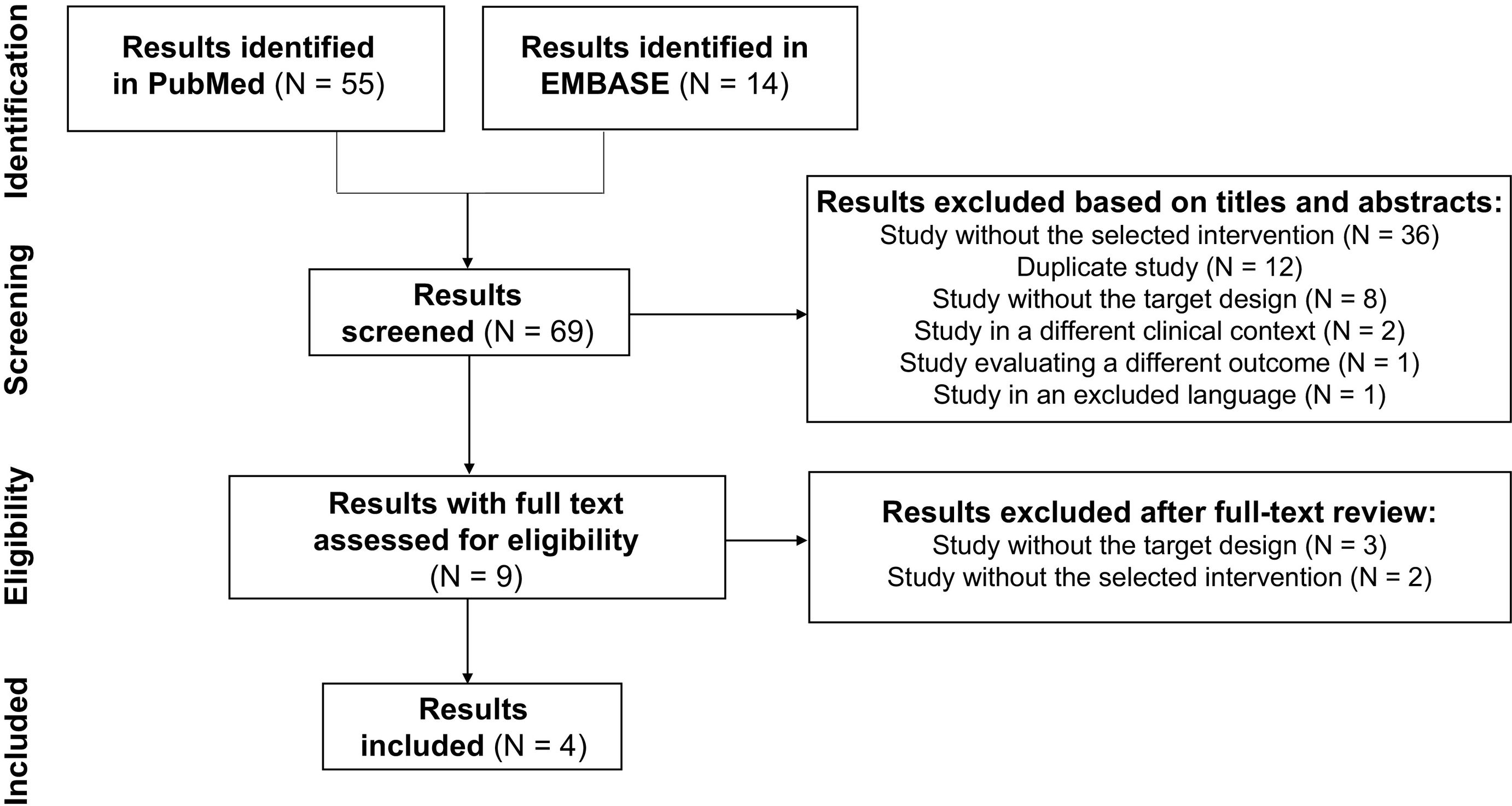
ResultsSystematic review of the literature and selection of studiesA total of 69 search results were found across the PubMed and EMBASE databases. Of these, 65 results were excluded for the following reasons: 38 studies did not include the selected intervention; 12 were duplicate publications; 11 studies did not have a target design; 2 studies were on different clinical contexts; 1 evaluated different variables; and 1 used an excluded language. No studies that met the established selection criteria were found in the citation search. Therefore, four clinical trials were included.14,15,30,31Fig. 1 depicts the systematic review, which was developed in accordance with the PRISMA recommendations.
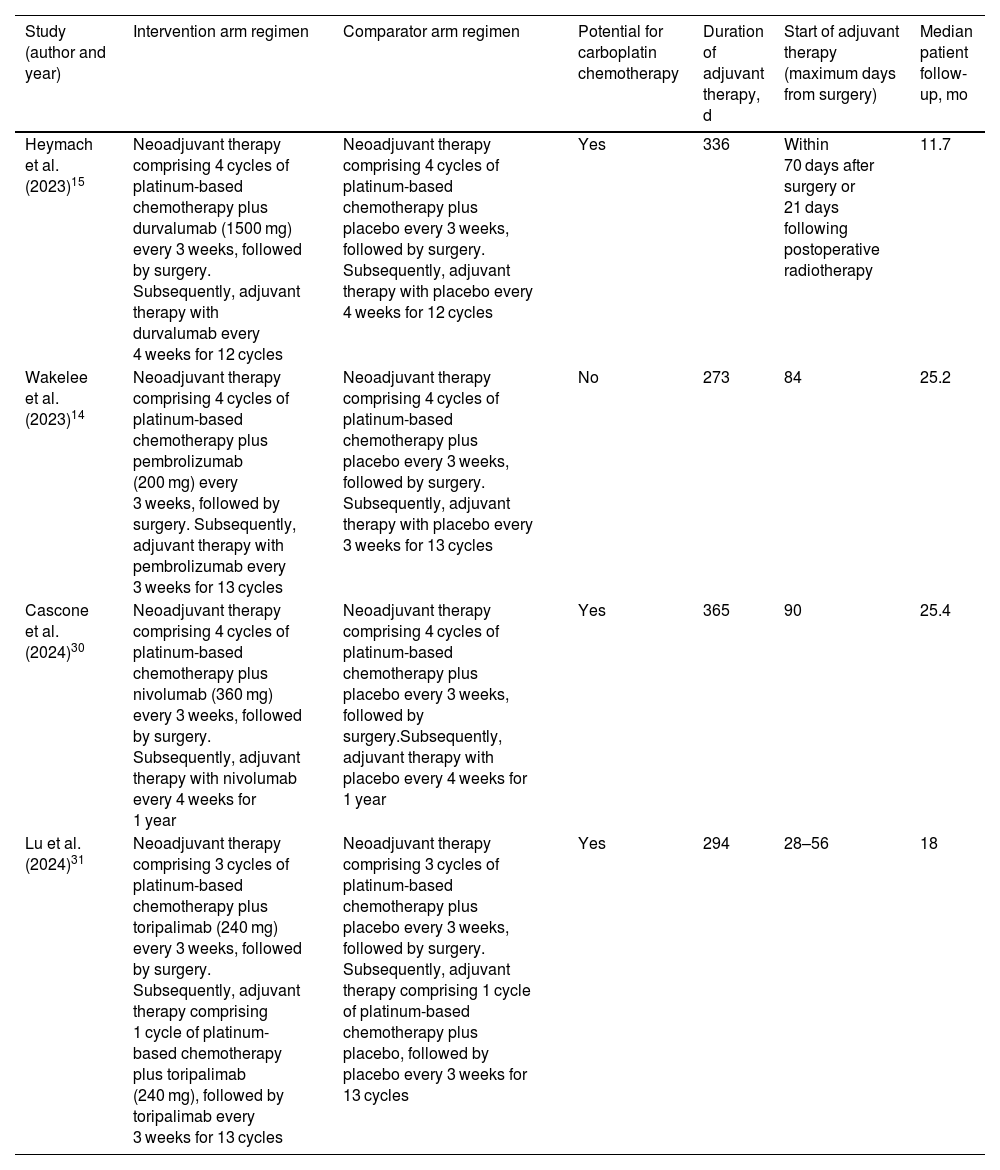
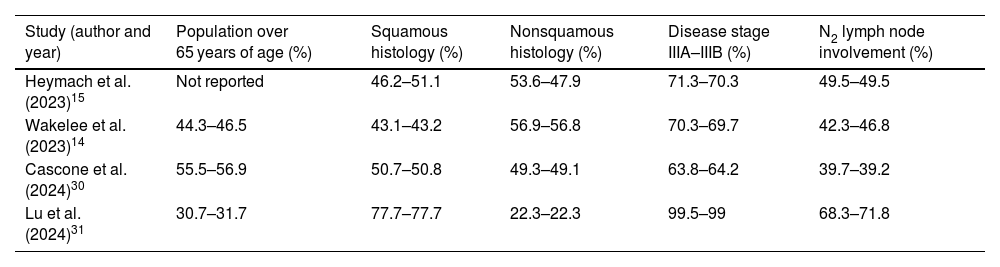
Data synthesisTable 1 shows the characteristics of the treatments evaluated in the studies and the duration of patient follow-up. The selected regimens included the perioperative use of durvalumab (P-durvalumab) from the AEGEAN study,15 pembrolizumab (P-pembrolizumab) from the KEYNOTE-671 trial,14 nivolumab (P-nivolumab) from the CheckMate 77T study,30 and toripalimab (P-toripalimab) from the Neotorch trial.31 In the intervention arms of the studies, perioperative treatments comprised 4 neoadjuvant cycles of immunotherapy plus platinum-based chemotherapy, followed by surgery, and subsequent adjuvant monotherapy with the immunotherapy agent. The only exception was P-toripalimab. In this case, 3 neoadjuvant cycles of toripalimab plus platinum-based chemotherapy were administered, followed by surgery, and then an adjuvant cycle of toripalimab in combination with chemotherapy. Toripalimab was subsequently administered as monotherapy. Compared to the other trials, this difference was also reflected in the control arm of the Neotorch study,31 which involved 3 neoadjuvant cycles of placebo plus platinum-based chemotherapy, and 1 cycle of this combination as adjuvant therapy after surgery. All other control arms used platinum-based chemotherapy combined with a placebo for 4 cycles of neoadjuvant therapy. The selected studies included patients with an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1. Subgroups of patients were defined based on PD-L1 tumour expression, with cut-offs of less than 1%, between 1% and 49%, and at least 50%. Table 2 shows the most relevant differences in the characteristics of the populations recruited for the trials.
Treatment characteristics and patient follow-up.
| Study (author and year) | Intervention arm regimen | Comparator arm regimen | Potential for carboplatin chemotherapy | Duration of adjuvant therapy, d | Start of adjuvant therapy (maximum days from surgery) | Median patient follow-up, mo |
|---|---|---|---|---|---|---|
| Heymach et al. (2023)15 | Neoadjuvant therapy comprising 4 cycles of platinum-based chemotherapy plus durvalumab (1500 mg) every 3 weeks, followed by surgery. Subsequently, adjuvant therapy with durvalumab every 4 weeks for 12 cycles | Neoadjuvant therapy comprising 4 cycles of platinum-based chemotherapy plus placebo every 3 weeks, followed by surgery. Subsequently, adjuvant therapy with placebo every 4 weeks for 12 cycles | Yes | 336 | Within 70 days after surgery or 21 days following postoperative radiotherapy | 11.7 |
| Wakelee et al. (2023)14 | Neoadjuvant therapy comprising 4 cycles of platinum-based chemotherapy plus pembrolizumab (200 mg) every 3 weeks, followed by surgery. Subsequently, adjuvant therapy with pembrolizumab every 3 weeks for 13 cycles | Neoadjuvant therapy comprising 4 cycles of platinum-based chemotherapy plus placebo every 3 weeks, followed by surgery. Subsequently, adjuvant therapy with placebo every 3 weeks for 13 cycles | No | 273 | 84 | 25.2 |
| Cascone et al. (2024)30 | Neoadjuvant therapy comprising 4 cycles of platinum-based chemotherapy plus nivolumab (360 mg) every 3 weeks, followed by surgery. Subsequently, adjuvant therapy with nivolumab every 4 weeks for 1 year | Neoadjuvant therapy comprising 4 cycles of platinum-based chemotherapy plus placebo every 3 weeks, followed by surgery.Subsequently, adjuvant therapy with placebo every 4 weeks for 1 year | Yes | 365 | 90 | 25.4 |
| Lu et al. (2024)31 | Neoadjuvant therapy comprising 3 cycles of platinum-based chemotherapy plus toripalimab (240 mg) every 3 weeks, followed by surgery. Subsequently, adjuvant therapy comprising 1 cycle of platinum-based chemotherapy plus toripalimab (240 mg), followed by toripalimab every 3 weeks for 13 cycles | Neoadjuvant therapy comprising 3 cycles of platinum-based chemotherapy plus placebo every 3 weeks, followed by surgery. Subsequently, adjuvant therapy comprising 1 cycle of platinum-based chemotherapy plus placebo, followed by placebo every 3 weeks for 13 cycles | Yes | 294 | 28–56 | 18 |
The most relevant differences in the characteristics of the populations recruited for the trials.
| Study (author and year) | Population over 65 years of age (%) | Squamous histology (%) | Nonsquamous histology (%) | Disease stage IIIA–IIIB (%) | N2 lymph node involvement (%) |
|---|---|---|---|---|---|
| Heymach et al. (2023)15 | Not reported | 46.2–51.1 | 53.6–47.9 | 71.3–70.3 | 49.5–49.5 |
| Wakelee et al. (2023)14 | 44.3–46.5 | 43.1–43.2 | 56.9–56.8 | 70.3–69.7 | 42.3–46.8 |
| Cascone et al. (2024)30 | 55.5–56.9 | 50.7–50.8 | 49.3–49.1 | 63.8–64.2 | 39.7–39.2 |
| Lu et al. (2024)31 | 30.7–31.7 | 77.7–77.7 | 22.3–22.3 | 99.5–99 | 68.3–71.8 |
Each cell contains 2 numbers separated by a dash, representing the intervention and control arms of each study, with the following distribution: percentage of the study population with the specified characteristic in the intervention arm, and percentage of the study population with the specified characteristic in the control arm.
The designs of the included clinical trials were similar, with randomisation stratified according to disease stage and PD-L1 tumour expression. The study on the use of P-durvalumab had the shortest patient follow-up period, at 11.7 months.15 The immunotherapy regimen with the smallest sample size was P-toripalimab in subgroups with PD-L1 tumour expression of less than 1% (N = 51) and between 1% and 49% (N = 69).31 However, the smallest sample size was recruited for the P-nivolumab subgroup with PD-L1 tumour expression of at least 50% (N = 45).30
Patients recruited for the Neotorch trial differed somewhat from those in the other studies.14,15,30,31 P-toripalimab was used in approximately 31% of patients of more than 65 years of age, whereas this subpopulation accounted for between 45% and 55% of cases in the other studies. A total of 99% of patients recruited to evaluate the effect of P-toripalimab had stage IIIA–IIIB disease, compared to approximately 64–70% with this stage in the other trials. Similarly, a squamous histology predominated in the Neotorch study, accounting for 78% of cases, whereas this percentage ranged from 43% to 50% in the other studies. On the other hand, 22% of patients treated with P-toripalimab had a nonsquamous histology, compared with 49–56% of patients treated with the other regimens. N2 lymph node involvement was observed in 77% of patients receiving P-toripalimab, compared with 39–49% in other studies.
The different perioperative distribution of the immunotherapy-plus-chemotherapy combination in the Neotorch study compared to the other treatments was also considered a possible source of bias. These differences resulted in some heterogeneity in the common comparator node of the NMA: the perioperative use of placebo plus platinum-based chemotherapy (P-placebo). On the other hand, all perioperative regimens involved the use of carboplatin or cisplatin chemotherapy, except for the P-pembrolizumab regimen, which only included cisplatin therapy. Given the differences in the recruited population and perioperative drug distribution, the Neotorch study was excluded from the NMA in the sensitivity analysis.
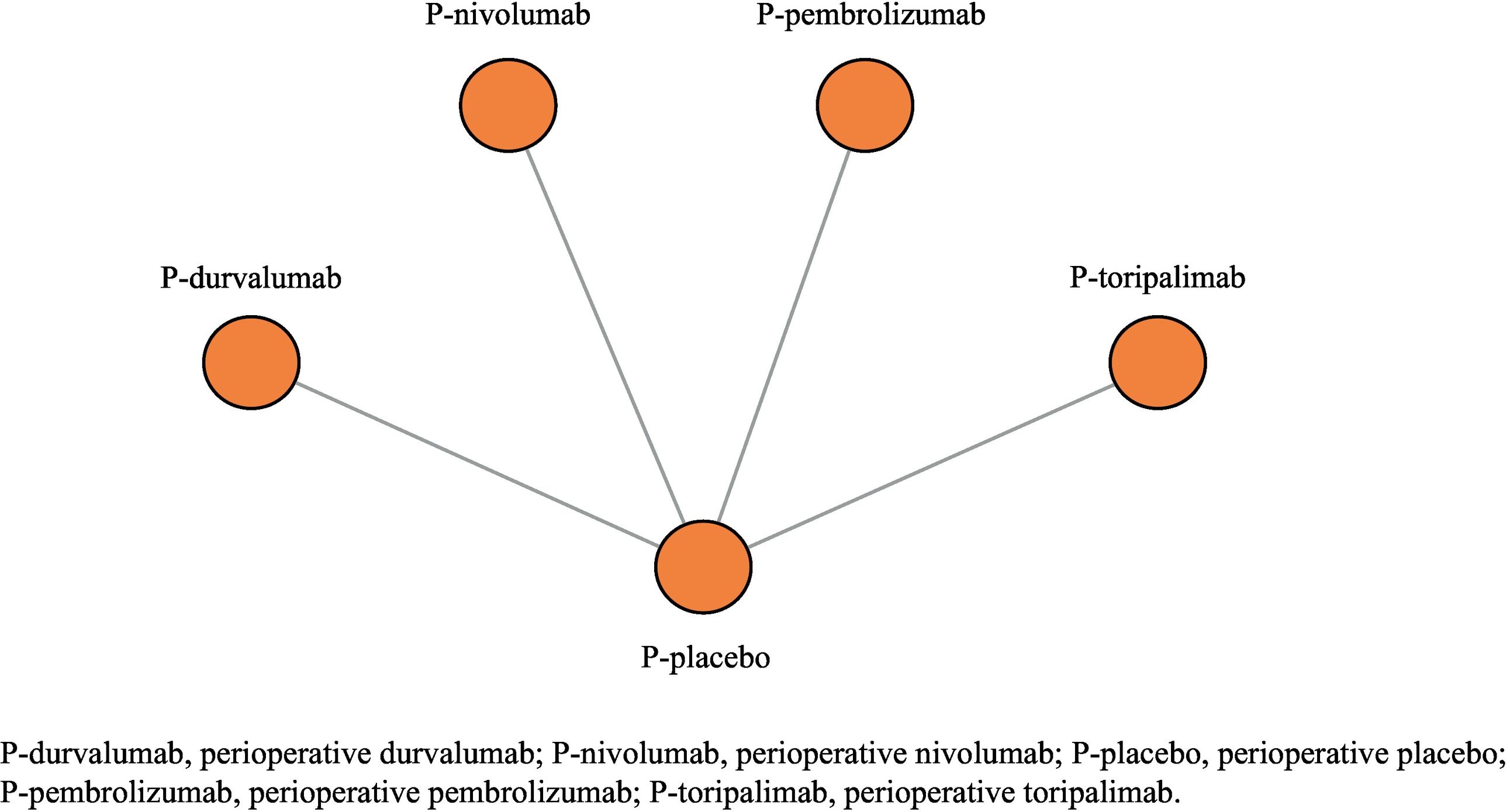
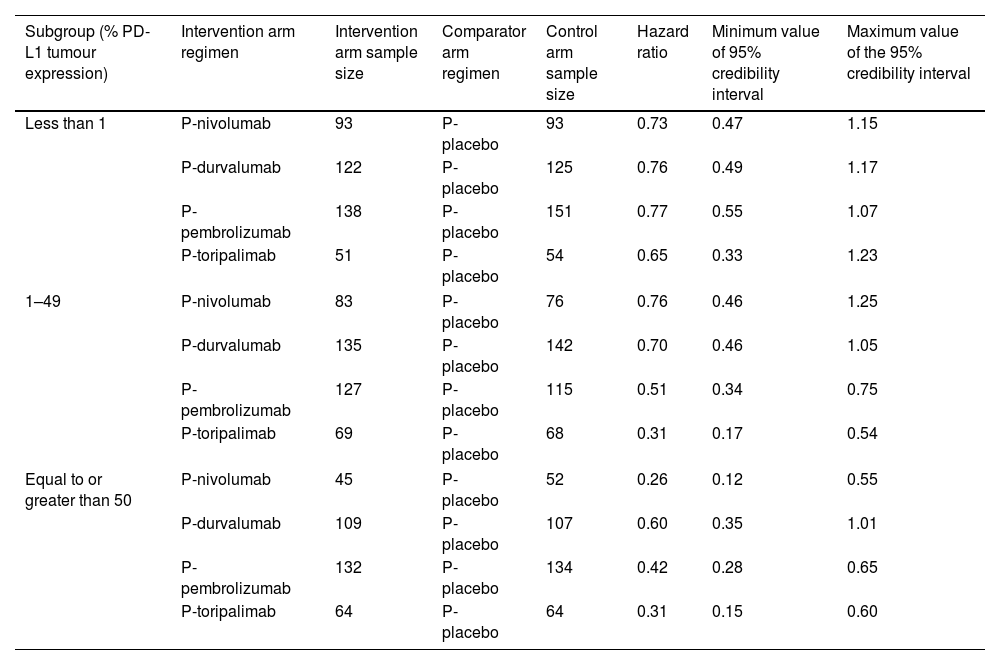
Data analysisFig. 2 shows the NMA network. Table 3 shows the sample sizes and efficacy values of the treatments used to develop the NMA, according to PD-L1 tumour expression. The primary analysis included all perioperative immunotherapy regimens, whereas the sensitivity analysis did not include P-toripalimab.31
Sample sizes and treatment efficacy values according to programmed death-ligand 1 expression.
| Subgroup (% PD-L1 tumour expression) | Intervention arm regimen | Intervention arm sample size | Comparator arm regimen | Control arm sample size | Hazard ratio | Minimum value of 95% credibility interval | Maximum value of the 95% credibility interval |
|---|---|---|---|---|---|---|---|
| Less than 1 | P-nivolumab | 93 | P-placebo | 93 | 0.73 | 0.47 | 1.15 |
| P-durvalumab | 122 | P-placebo | 125 | 0.76 | 0.49 | 1.17 | |
| P-pembrolizumab | 138 | P-placebo | 151 | 0.77 | 0.55 | 1.07 | |
| P-toripalimab | 51 | P-placebo | 54 | 0.65 | 0.33 | 1.23 | |
| 1–49 | P-nivolumab | 83 | P-placebo | 76 | 0.76 | 0.46 | 1.25 |
| P-durvalumab | 135 | P-placebo | 142 | 0.70 | 0.46 | 1.05 | |
| P-pembrolizumab | 127 | P-placebo | 115 | 0.51 | 0.34 | 0.75 | |
| P-toripalimab | 69 | P-placebo | 68 | 0.31 | 0.17 | 0.54 | |
| Equal to or greater than 50 | P-nivolumab | 45 | P-placebo | 52 | 0.26 | 0.12 | 0.55 |
| P-durvalumab | 109 | P-placebo | 107 | 0.60 | 0.35 | 1.01 | |
| P-pembrolizumab | 132 | P-placebo | 134 | 0.42 | 0.28 | 0.65 | |
| P-toripalimab | 64 | P-placebo | 64 | 0.31 | 0.15 | 0.60 | |
According to the level of PD-L1 tumour expression, no relevant differences were found between the fixed-effects model and the random-effects model in each of the scenarios. The DIC values for the fixed-effects model and the random-effects model were 7.98 and 8.03, respectively, in the analysis with PD-L1 tumour expression of less than 1%; 7.98 and 8 with PD-L1 tumour expression between 1% and 49%; and 7.97 and 7.98 with PD-L1 tumour expression of at least 50%.
As the differences were below the 5-point threshold, which was defined as the minimum relevant difference, the fixed-effects model was selected to provide more accurate information. Consistency data could not be obtained due to the absence of indirect evidence between the regimens (star-shaped network). The value of the I2 statistic was 25% in all analyses according to PD-L1 tumour expression (<1%, 1–49%, and ≥50%) for the primary analysis. Given the low level of heterogeneity, the use of the randomised effects model was completely ruled out.
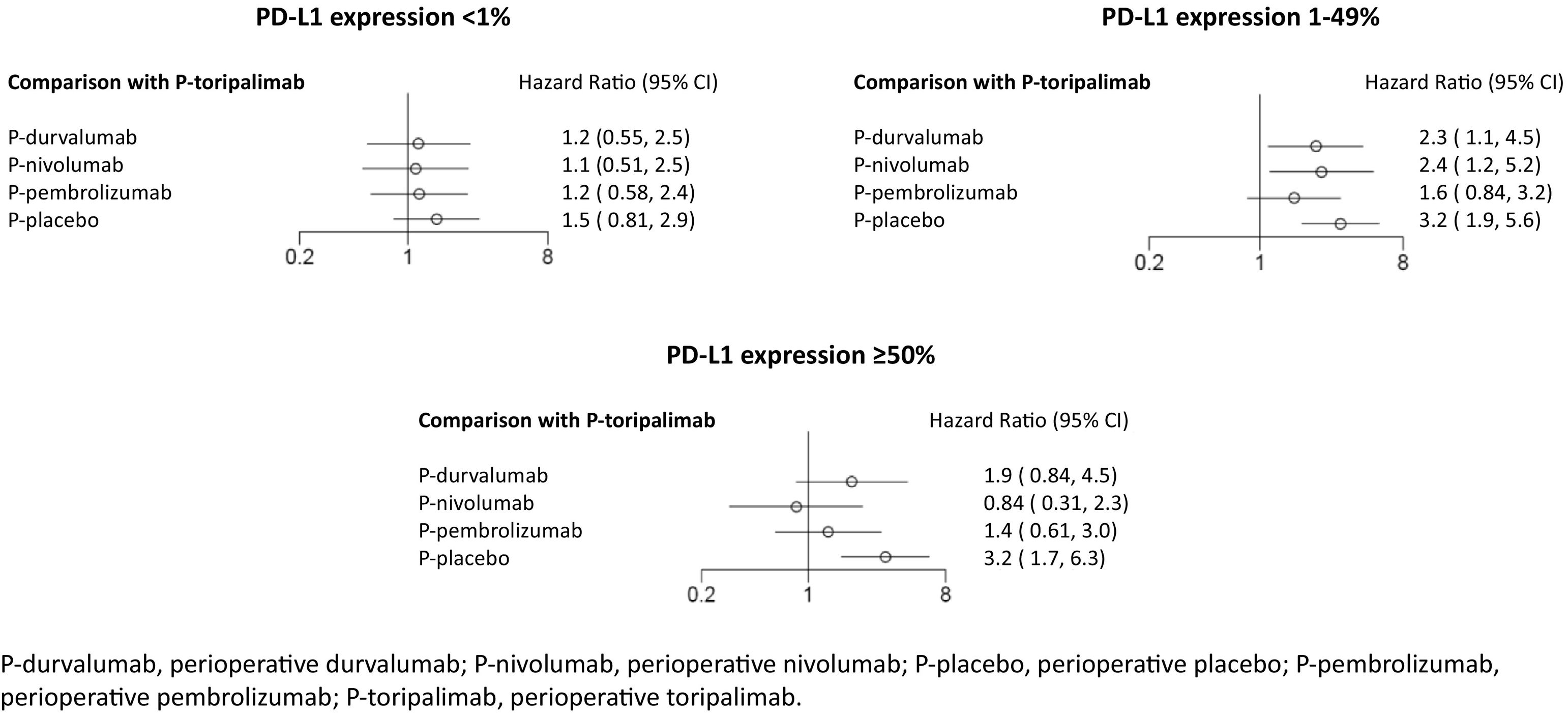
In the primary analysis, P-toripalimab was selected as the reference treatment because it produced the greatest effect size in the PD-L1 tumour expression scenarios of less than 1% and between 1% and 49%. Fig. 3 shows the HR values of the different therapeutic alternatives in relation to this regimen.
In the analysis of PD-L1 levels of less than 1%, no statistically significant differences were observed between any of the perioperative regimens involving immunotherapy agents vs P-placebo. In cases of PD-L1 tumour expression levels between 1% and 49%, the P-toripalimab regimen was not found to be superior to P-pembrolizumab. However, the rest of the regimens were observed to be statistically significantly inferior. In cases of PD-L1 tumour expression levels of at least 50%, a benefit was demonstrated when immunotherapy agents were used in combination with perioperative treatment compared to P-placebo.
For the primary analysis, an HR of 0.74 (95% CrI: 0.60–0.92) was calculated in the evaluation using the aggregated data from the regimens involving immunotherapy agents vs P-placebo in the subgroup with PD-L1 tumour expression of less than 1%. This was the only scenario in which no statistically significant differences were observed for any of the immunotherapy regimens vs P-placebo.
In the sensitivity analysis, the reference treatment was P-nivolumab (with better HR in subgroups with PD-L1 tumour expression <1% and ≥50%). Compared with P-nivolumab, the HR values for the other therapeutic alternatives in the PD-L1 tumour expression scenario of less than 1% were as follows: 1.0 (95% CrI: 0.56–2) for P-durvalumab; 1.1 (95% CrI: 0.6–1.9) for P-pembrolizumab; and 1.4 (95% CrI: 0.87–2.2) for P-placebo. In the 1% to 49% scenario, the values were as follows: 0.67 (95% CrI: 0.36–1.3) for P-pembrolizumab; 0.92 (95% CrI: 0.48–1.7) for P-durvalumab; and 1.3 (95% CrI: 0.8–2.2) for P-placebo. In the at least 50% scenario, the values were as follows: 1.6 (95% CrI: 0.68–3.8) for P-pembrolizumab; 2.3 (95% CrI: 0.93–5.7) for P-durvalumab; and 3.8 (95% CrI: 1.8–8.1) for P-placebo.
For the sensitivity analysis, an HR of 0.76 (95% CrI: 0.60–0.95) was estimated in the evaluation using the aggregated data from the immunotherapy regimens (excluding P-toripalimab) vs P-placebo in the subgroup with PD-L1 tumour expression of less than 1%.
DiscussionNetwork meta-analyses enable treatment regimens to be compared directly and indirectly in the absence of clinical studies that use direct comparisons. This is particularly important in areas such as perioperative immunotherapies in resectable NSCLC. The results of this NMA highlight the relevance of using PD-L1 tumour expression levels as a key biomarker to guide treatment decisions.
In patients with PD-L1 tumour expression between 1% and 49%, perioperative regimens including P-toripalimab and P-pembrolizumab demonstrated greater benefit in terms of PFS, suggesting that these combinations could be positioned as the preferred options for this subgroup. In patients with PD-L1 tumour expression of at least 50%, all evaluated immunotherapy regimens showed benefits compared to the exclusive use of chemotherapy, offering greater flexibility in the selection of therapeutic alternatives. However, no favourable PFS results were observed for any of the perioperative immunotherapy regimens in patients with PD-L1 tumour expression of less than 1% when evaluated individually. This result highlights the need to reevaluate the effectiveness of these treatments for this population using more mature data. Currently, this aspect could justify the continued use of standard chemotherapy. These findings underscore the importance of personalised biomarker-based treatment. These data are highly biologically plausible, given that immunotherapeutic agents all act by interfering with PD-L1 binding to its receptor.32–35 Patients with higher PD-L1 tumour expression may respond better to drugs that act on this therapeutic target. In contrast, patients with lower expression of this biomarker would gain no additional benefit from these treatments.
On the other hand, the evaluation of aggregated data from immunotherapy combinations takes into account the limited statistical power of the subgroup data. This aggregate analysis suggests that adding perioperative immunotherapy to chemotherapy is beneficial for patients with PD-L1 tumour expression of less than 1%. This apparent inconsistency with the individual results obtained from the separate regimens could be due to the small sample sizes and low number of events when subgroup data are used rather than the overall results from clinical trials. Therefore, the use of perioperative immunotherapy for resectable NSCLC with PD-L1 tumour expression of less than 1% should not be completely ruled out.
The sensitivity analysis showed results that were consistent with those of the primary analysis. The combination of perioperative immunotherapy and chemotherapy in patients with resectable NSCLC and high PD-L1 tumour expression was also found to have a positive effect on PFS in the sensitivity analysis. The immunotherapy agents evaluated individually did not show any benefit for patients with low PD-L1 tumour expression. However, a benefit was found in the sensitivity analysis of the aggregated data from the immunotherapy regimens in the subgroup with lower PD-L1 tumour expression. This sensitivity analysis was developed following the exclusion of P-toripalimab from the NMA. This was due to differences in the recruited population compared to those in the other studies. It was also due to a different distribution of the last cycle of immunotherapy combined with chemotherapy in the comparator arm of the Neotorch trial.31 This trial forms a node in the treatment network. These heterogeneous characteristics made assessing the influence of P-toripalimab in the NMA both interesting and necessary.
The review highlighted both the main differences between the Neotorch study31 and the other studies, as well as any other potential minor differences. P-pembrolizumab only included cisplatin-based chemotherapy regimens; P-durvalumab had the shortest follow-up period.
Several reasons were behind the selection of PFS as the variable to be evaluated in the trials. Firstly, no statistically significant differences in overall survival (OS) have been observed between perioperative regimens with or without immunotherapy. This is because studies have focused on the early stages of the disease in which death events are rare.14 Therefore, more reliable assessments would require longer studies with larger sample sizes and more mature OS data. Secondly, the influence of subsequent treatments on the OS must be carefully considered in the context of the evaluation.
Our study selection criteria ensured that phase III clinical trials with adequate sample sizes were included. These selection criteria took into account the subgroup analysis recommendations of Gil-Sierra et al.,17 as the present NMA used population data based on PD-L1 tumour expression levels. Given the limitations of subgroup analyses, comparisons were based only on higher-quality scientific studies with adequate sample sizes.
Some NMAs have been previously published on the perioperative use of immunotherapy in resectable NSCLC. Mei et al. conducted an NMA involving a systematic search up to August 2023.36 This work is now outdated, as it does not include subsequent trials, such as those conducted by Cascone et al.30 The authors suggest that, for patients with negative PD-L1 tumour expression or high expression, respectively, P-toripalimab or P-nivolumab should be the preferred therapeutic alternatives. In fact, their study established rankings for the evaluated regimens. These prioritisations of regimens were not based on solid statistical criteria for relevant variables.
Unlike the study by Mei et al.,36 our study did not identify any basis for prioritising between the different combinations of immunotherapy agents plus chemotherapy for patients with PD-L1 tumour expression levels of less than 1% and at least 50%. Our data interpretation is supported by reliable statistical criteria that rule out the possibility of observed differences being due to chance. From a pharmacoeconomic point of view, this increases the competitiveness of the therapeutic alternatives, which may result in drugs being purchased at lower prices.
Prior to our work, Chen et al. conducted a study. They found an association between P-toripalimab or P-nivolumab and benefit,36 although their NMA had limitations similar to those of Mei et al.37 In this case, the authors did not conduct separate comparisons based on PD-L1 tumour expression levels. We suggest that this is a significant limitation when applying the results of the NMA to the clinical decision-making process for patients with resectable NSCLC who are eligible for perioperative immunotherapy. PD-L1 tumour expression levels are a proven biomarker that must be carefully evaluated in the therapeutic positioning of drugs for NSCLC. Our study evaluated different treatment regimens across various scenarios according to PD-L1 tumour expression levels, an approach that could aid therapeutic personalization. In addition, our NMA found statistically significant differences in PFS between P-toripalimab and P-pembrolizumab compared with other regimens, but only in the scenario involving PD-L1 tumour expression ranging from 1% to 49%. In the other scenarios, no immunotherapy agent regimen was found to be superior to the others. These findings were supported by the results of the NMA sensitivity analysis.
Other studies have evaluated the effects of neoadjuvant and adjuvant treatments, in addition to those that have compared different perioperative immunotherapy regimens for resectable NSCLC. Recently published studies have found no benefit in adding adjuvant regimens to neoadjuvant regimens, with similar results for the perioperative and exclusively neoadjuvant use of immunotherapy.38,39 Furthermore, adding adjuvant immunotherapy may result in more adverse events and increased costs. These types of study complement our work, helping to guide the therapeutic positioning of the different regimens.
In conclusion, this study provides a relevant and reliable NMA of the efficacy of perioperative immunotherapy regimens for resectable NSCLC. P-toripalimab and P-pembrolizumab were found to increase PFS in patients with PD-L1 tumour expression levels ranging from 1% to 49%. No differences were observed between the immunotherapy-chemotherapy combinations in the other subgroups, suggesting that competition between the therapeutic alternatives could be encouraged.
Ethical responsibilitiesThe authors declare that they have fulfilled the instructions for authors and ethical responsibilities. All authors who have signed the manuscript have met the requirements for authorship and declared their respective conflicts of interest.
All necessary ethical responsibilities regarding authorship and the avoidance of redundant publication were fulfilled. The protection of human and animal subjects, as well as informed consent, was not required due to the study design.
All authors accept their responsibilities as defined by the International Committee of Medical Journal Editors (available at http://www.icmje.org/).
CRediT authorship contribution statementGil-Sierra Manuel David: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Briceño-Casado Maria del Pilar: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Moreno-Ramos Cristina: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
FundingNone declared.
None declared.