The introduction of the concepts of persistence in optimal response (POR) and persistence in suboptimal response (PSR) by Husein-ElAhmed et al.1 represents a significant advance in the measurement of persistence in chronic diseases. Supported by established clinical scales such as PASI 100 and PASI 90 for psoriasis, these metrics go beyond the limited notion of ‘treatment survival’ by linking treatment retention among patients with clinically relevant outcomes, such as sustained remission or inflammation control.
We believe that these concepts should be extended to and validated in other chronic immune-mediated inflammatory diseases (IMIDs), including rheumatoid arthritis, inflammatory bowel disease, hidradenitis suppurativa, and multiple sclerosis. This proposal is based on our previous work on the inclusion of persistence as the sixth ‘P' in the 5P Medicine model,2 as well as on the modulating role of adherence in persistence outcomes.3
Traditionally, persistence has been defined as the time from the start of treatment to its discontinuation, based on dispensing records or electronic medical records.4 However, this metric does not indicate whether therapeutic goals are being achieved or the reasons patients remain on treatment. The POR and PSR metrics address this limitation: POR is associated with sustained remission, whereas PSR reflects sufficient control to justify continuation of treatment.
In the context of IMIDs, disease control is an ongoing process rather than a one-off goal. The objective is not only to achieve remission but also to sustain it over time while minimizing tissue damage, disability, and complications. POR aligns with the treat-to-target strategy as the ultimate therapeutic goal, whereas PSR represents clinically acceptable control in situations where complete remission is not feasible, such as in refractory patients or those with comorbidities (Table 1). Unlike traditional survival rates, which may be influenced by non-clinical factors such as funding decisions or patient preferences, POR and PSR provide a more focused assessment of the actual efficacy and utility of therapy.
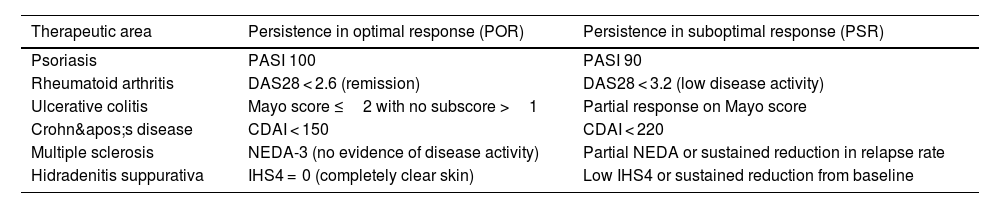
Proposed generic definitions of persistence for chronic immune-mediated inflammatory diseases.
| Therapeutic area | Persistence in optimal response (POR) | Persistence in suboptimal response (PSR) |
|---|---|---|
| Psoriasis | PASI 100 | PASI 90 |
| Rheumatoid arthritis | DAS28 < 2.6 (remission) | DAS28 < 3.2 (low disease activity) |
| Ulcerative colitis | Mayo score ≤2 with no subscore >1 | Partial response on Mayo score |
| Crohn's disease | CDAI < 150 | CDAI < 220 |
| Multiple sclerosis | NEDA-3 (no evidence of disease activity) | Partial NEDA or sustained reduction in relapse rate |
| Hidradenitis suppurativa | IHS4 = 0 (completely clear skin) | Low IHS4 or sustained reduction from baseline |
IMID, immune-mediated inflammatory diseases; POR, persistence in optimal response; PSR, persistence in suboptimal response; PASI, Psoriasis Area and Severity Index; DAS, Disease Activity Score; CDAI, Clinical Disease Activity Index; NEDA, No Evidence of Disease Activity; IHS4, International Hidradenitis Suppurativa Severity Score System.
In our 6P Medicine model, we propose adding persistence as the sixth dimension, alongside personalisation, prediction, prevention, participation, and the population perspective.2 Within this framework, persistence refers to the duration of treatment and is a comprehensive metric that reflects sustained clinical effectiveness (POR or PSR), tolerability, patient adherence, and perceived experience. Incorporating these metrics enables a more accurate assessment of the overall value of a therapy for inflammatory diseases.
Persistence should be interpreted in conjunction with adherence. Low adherence can artificially reduce persistence levels, whereas high adherence combined with low persistence may indicate potential limitations in treatment efficacy or tolerability.3 We therefore recommend that future studies stratify POR and PSR results by adherence levels, baseline severity, and prior treatment lines. They should also incorporate Patient-Reported Outcome Measures and Patient-Reported Experience Measures to reflect clinical outcomes and patient experience. We believe that these metrics have clear practical applications: they help clinicians to make more informed therapeutic decisions; empower patients by providing them with easy-to-understand indicators that align with their expectations; make it easier for funding bodies to establish outcome-based contracts; and provide regulators with a tool that enables them to better model long-term cost-effectiveness.
In conclusion, POR and PSR transform persistence into a genuine marker of objective clinical remission, which can be incorporated into healthcare strategies focused on results, sustainability, and personalized treatment.
CRediT authorship contribution statementJoaquín Borrás-Blasco: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Methodology, Formal analysis, Conceptualization. Vicente Merino-Bohorquez: Writing – review & editing, Writing – original draft, Validation, Supervision, Methodology, Formal analysis. Esther Ramírez-Herráiz: Writing – review & editing, Writing – original draft, Validation, Supervision, Methodology, Formal analysis. Andrés Navarro-Ruiz: Writing – review & editing, Writing – original draft, Validation, Supervision, Methodology, Formal analysis.
FundingNone declared.
None declared.