The main objective was to compare the persistence between dolutegravir/lamivudine (DTG/3TC) and bictegravir/emtricitabine/tenofovir-alafenamide (BIC/FTC/TAF) and to analyze reasons for discontinuation.
MethodsWe conducted a retrospective, non-interventional, descriptive, and longitudinal study. All human immunodeficiency virus (HIV) patients over 18 years treated with DTG/3TC or BIC/FTC/TAF in our center were included.
Persistence after first year was compared using the χ2 test. Kaplan–Meier survival analysis was performed.
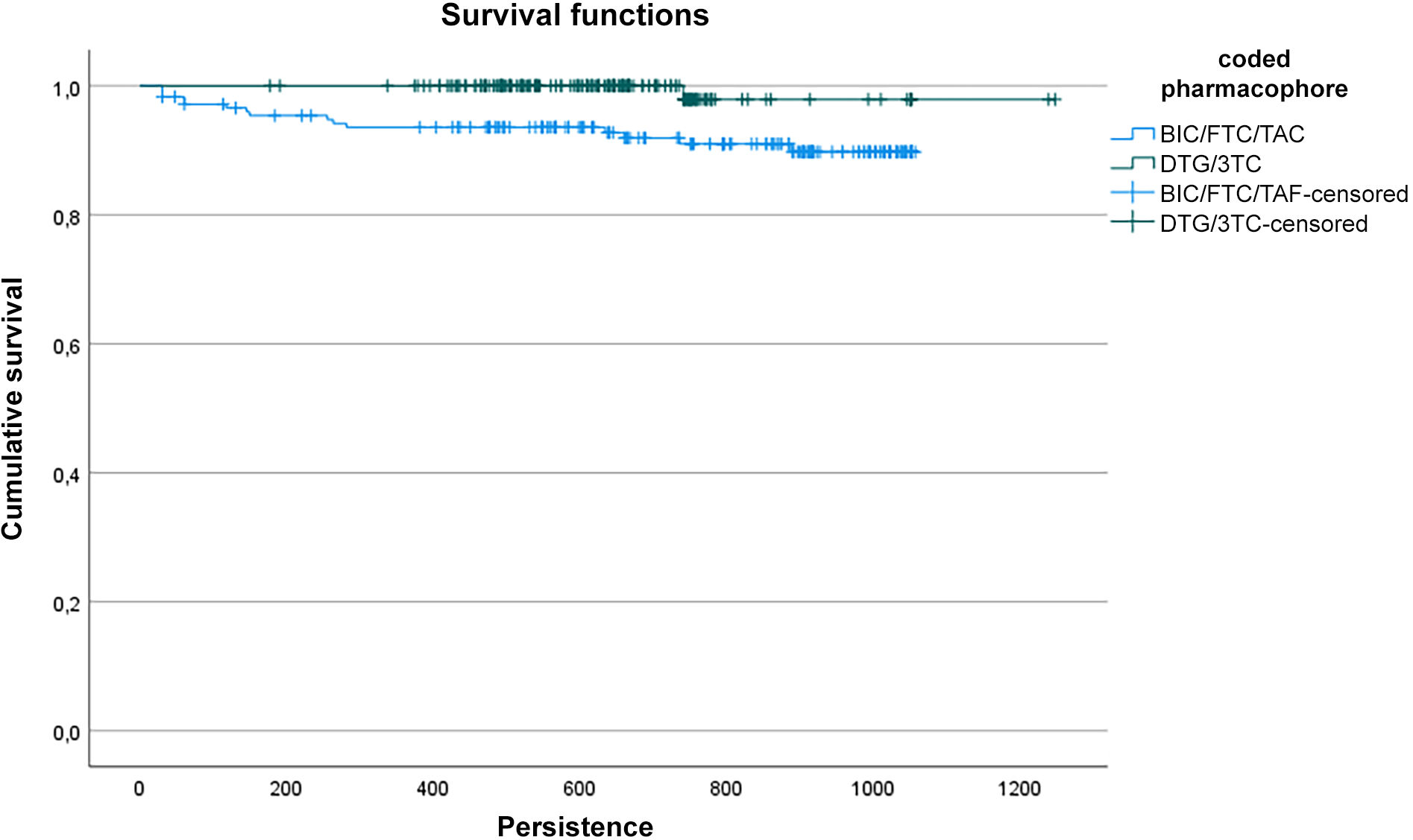
ResultsThree hundred fifty-eight patients were included. 99.5% versus 90.99% of patients were persistent after the first year for DTG/3TC and BIC/FTC/TAF respectively (p=.001).
Persistence with DGT/3TC was 1237 days (IC95% 1216–1258) and persistence with BIC/FTC/TAF was 986 days [(IC95% 950–1021); p<.001]. The difference was remained after adjusting for covariates with the cox regression model [HR=8.2 (IC95% 1.03–64.9), p=.047].
The main reasons for discontinuation for BIC/FTC/TAF were toxicity/tolerability.
ConclusionIn our study, patients have a high persistence. Patients on DTG/3TC treatment are more persistent compared to BIC/FTC/TAF, although BIC/FTC/TAF have worse baseline characteristics. The main reason for discontinuation of BIC/FTC/TAF is tolerability/toxicity.
Los objetivos fueron comparar la persistencia entre dolutegravir/lamivudina (DTG/3TC) y bictegravir/emtricitabina/tenofovir-alafenamida (BIC/FTC/TAF) y analizar los factores que influyen en la discontinuación del tratamiento.
MétodosSe realizó un estudio retrospectivo, observacional, descriptivo y longitudinal. Se incluyeron todos los pacientes con el virus de la inmunodeficiencia humana (VIH) mayores de 18 años tratados con DTG/3TC o BIC/FTC/TAF en nuestro centro.
La persistencia tras el primer año se comparó mediante la prueba χ2. Se realizó un análisis de supervivencia de Kaplan–Meier.
ResultadosSe incluyeron 358 pacientes. El 99,5% frente al 90,9% de los pacientes fueron persistentes después del primer año para DTG/3TC y BIC/FTC/TAF respectivamente (p < 0,001).
La persistencia con DGT/3TC fue de 1.237 días (IC95% 1.216–1.258) y la persistencia con BIC/FTC/TAF fue de 986 días [(IC95% 950–1.021); p < 0,001]. La diferencia se mantuvo cuando se ajustó por las covariables con el modelo de regresión de Cox [HR = 8,2 (IC95% 1,03-64,9), p = 0,047].
El principal motivo de interrupción del BIC/FTC/TAF fue la toxicidad/tolerabilidad.
ConclusionesEn nuestro estudio los pacientes tienen una persistencia elevada. Los pacientes en tratamiento con DTG/3TC son más persistentes en comparación con BIC/FTC/TAF, aunque éstos tienen peores características basales. La principal razón para la interrupción de BIC/FTC/TAF es la tolerabilidad/toxicidad.
Infection with the human immunodeficiency virus (HIV) is a chronic condition which now, as a result of the advent of antiretroviral treatment (ART), results in low mortality rates in the developed world.1 Comparative persistence with ARTs across patient populations is a surrogate marker for effectiveness and safety, which can provide the data required for treatment individualization.2 Moreover, single-tablet regimens have been shown to improve adherence and to be associated with longer persistence than multiple-tablet schedules.3–5
The most usual ART regimens in our hospital are dolutegravir/lamivudine (DTG/3TC) and bictegravir/emtricitabine/tenofovir-alafenamide (BIC/FTC/TAF). Other schedules tend to be avoided due to their lower efficiency or, as in the case of the dolutegravir/abacavir/lamivudine (DTG/ABC/3TC) combination, which requires prior determination of HLA-B*5701, due to the need to delay initiation of treatment.1
The main goal of this study was to provide an estimation of the persistence achieved with the 2 most common ARTs in the Spanish setting and analyze the factors leading to changes to, or discontinuations of, treatment.
MethodsThis was a retrospective observational descriptive longitudinal study of HIV patients started on BIC/FTC/TAF and DTG/3TC before April 1, 2021. To be included, patients had to be over 18 years of age, have a diagnosis of HIV infection, and have been treated with DTG/3TC or BIC/FTC/TAF.
The main variable analyzed was persistence, defined as a continuous variable when considering the number of days elapsed from the date the medication was collected, without exceeding the predefined washout period. On the other hand, persistence was defined as a categorical variable when, after a given time, the prescription was found to be active and patients had collected their medication at intervals not exceeding the washout period. One-year treatment persistence was measured in this manner. The washout period is the time that extends from the end of one drug regimen and the first dispensation of the next. A maximum washout period of 90 days was established.6 Changes aimed at simplification or exclusively at increasing efficiency were excluded from the study.
The secondary variables considered were as follows: age at the start of treatment; sex at birth; plasma viral load (pVL) at the start of treatment; baseline CD4 T-cell count; being naive, number of previous ART lines, occurrence of a change or discontinuation during treatment, reason for such changes or discontinuations, Charlson Comorbidity Index, duration of treatment, and adherence, measured using the medication possession ratio (MPR).
In the statistical analysis, qualitative variables were expressed by means of absolute and relative frequencies while quantitative variables were expressed in the form of means+standard deviations or medians and interquartile ranges (IQRs). The discontinuation rate for the treatments was calculated based on their incidence density for every 1000 patients per year. As regards the characteristics of the populations analyzed, qualitative variables were compared using the chi-squared test while quantitative variables were compared using the Mann–Whitney U test and Student's distribution t test, as deemed appropriate according to the normality analysis performed. A Kaplan–Meier survival analysis was carried out and the factors influencing survival were determined using Cox regression analysis. The statistical analysis was conducted using SPSS v27.0 software.
The study was approved by our hospital's Research Ethics Committee.
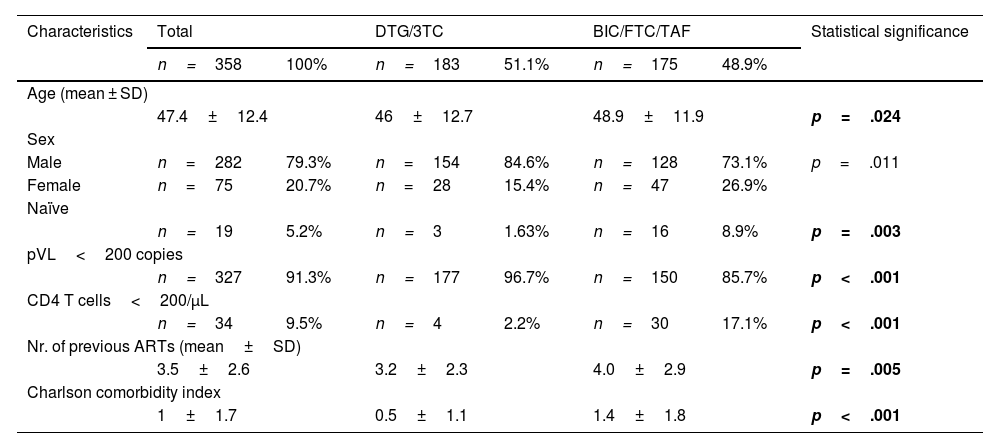
ResultsThe study included 358 patients, of whom 79.3% were male; 5.2% of them naïve. Mean age was 47.4±12 years. A total of 48.9% of subjects were treated with BIC/FTC/TAF. Baseline pVL stood at less than 200 copies/mL in 91.3% of patients; and the CD4 T-cell count was under 200 cells/μL in 9.5% of patients. The mean number of previous ARTs was 3.5±2.6 and the Charlson Comorbidity Index was 1±1.7. The patients' demographic and clinical characteristics are shown in Table 1.
Demographic and clinical characteristics.
| Characteristics | Total | DTG/3TC | BIC/FTC/TAF | Statistical significance | |||
|---|---|---|---|---|---|---|---|
| n=358 | 100% | n=183 | 51.1% | n=175 | 48.9% | ||
| Age (mean ± SD) | |||||||
| 47.4±12.4 | 46±12.7 | 48.9±11.9 | p=.024 | ||||
| Sex | |||||||
| Male | n=282 | 79.3% | n=154 | 84.6% | n=128 | 73.1% | p=.011 |
| Female | n=75 | 20.7% | n=28 | 15.4% | n=47 | 26.9% | |
| Naïve | |||||||
| n=19 | 5.2% | n=3 | 1.63% | n=16 | 8.9% | p=.003 | |
| pVL<200 copies | |||||||
| n=327 | 91.3% | n=177 | 96.7% | n=150 | 85.7% | p<.001 | |
| CD4 T cells<200/μL | |||||||
| n=34 | 9.5% | n=4 | 2.2% | n=30 | 17.1% | p<.001 | |
| Nr. of previous ARTs (mean±SD) | |||||||
| 3.5±2.6 | 3.2±2.3 | 4.0±2.9 | p=.005 | ||||
| Charlson comorbidity index | |||||||
| 1±1.7 | 0.5±1.1 | 1.4±1.8 | p<.001 | ||||
Overall MPR was 95.4±11.1 (96.5±6.4 with DTG/3TC and 94.0±14.7 with BIC/FTC/TAF), without any statistically significant differences between the groups. A total of 99.5% of patients on DTG/3TC remained persistent at 1 year from the onset of treatment. In contrast, persistence at 1 year from the start of treatment among patients treated with BIC/FTC/TAF was 90.9% (p<.001). The overall discontinuation rate for every 1000 patients per year was 0.09 (0.02 for DTG/3TC and 0.22 for BIC/FTC/TAF).
Mean overall persistence was 1197 days (1.172–1.221, 95% CI). Persistence with DTG/3TC was 1237 days (1216–1258, 95% CI) and persistence with BIC/FTC/TAF was 986 days ([950–1.021, 95% CI]; p<.001) (Fig. 1). The difference persisted after adjusting for covariates such as adherence, age, sex, viral load, CD4-T-cell count, naivety and the Charlson comorbidity index, using the Cox regression model (HR=8.2 [1.03–64.9 95 CI], p=.047).
No statistically significant differences were found with the other variables analyzed.
The reasons for discontinuing BIC/FTC/TAF were as follows: toxicity/intolerability (n=8), emergence of comorbidities (n=3), lack of adherence (n=2), patient's request (n=1), and absence of efficacy (n=1). In the group of patients treated with DTG/3TC, only one subject discontinued the treatment due to toxicity/intolerability.
DiscussionThe present study compared DGT/3TC with BIC/FTC/TAF and found high levels of persistence with both integrase strand transfer inhibitor (INSTI)-based therapies.
An analysis by Korten et al. found that 5.6% of patients had discontinued their INSTI treatment at 12 months. Moreover, persistence was found to be higher than with protease inhibitors (PIs) and non-nucleoside analog reverse transcriptase inhibitors (NNRTIs).7
Several authors have compared persistence with single-tablet regimens with persistence with multiple-tablet regimens.3–5 Nonetheless, few have compared the persistence achieved with the various single-tablet regimens available. Wang et al. reported that 72.5% of patients on DTG were persistent at 12 months but these authors failed to analyze the reasons for discontinuation of the treatment. Other studies analyzing other, both single- and multiple-tablet combinations containing DTG. Wang et al.8 obtained lower persistence with DTG/3TG at 1 year from the start of treatment than the present report (99.5% of patients).
Moreover. the rate of discontinuation of DTG/3TC among our patients was also low (0.02 for every 1000 patients per year). Suárez-García et al. observed low discontinuation rates with DGT/3TC, with 4 of their 255 patients discontinuing the treatment because of adverse events during the first 48 weeks.9
As regards bictegravir-containing regimens, Molina et al. reported that less than 1% of patients discontinued the treatment before the first 48 weeks.10 In a real-world practice analysis, Nasreddine et al. observed that 6.5% of subjects discontinued BIC/FTC/TAF within the first 48 weeks (7.4 discontinuations per 1000 patients/year).11In our study, the discontinuation rate for BIC/FTC/TAF was far lower (0.22 for every 1000 patients per year).
Although the discontinuation rate for BIC/FTC/TAF in this study was higher than for DGT/3TC, it must be considered that the populations were completely different. The group treated with BIC/FTC/TAF was older, with more patients with pVLs >200 copies and CD4 T-cell counts < 200 cells. They also presented with higher values on the Charlson comorbidity index than patients on DGT/3TC. Eaton et al. observed that factors such as the type of ART administered, sex, a low CD4 T-cell count, and having begun treatment more recently were significantly associated with a regimen change.12
Adherence to ART typically leads to an undetectable HIV pVL, with increased quality of life and survivorship.13 Moreover, some authors have found a relationship between adherence and persistence with ART.4 In our study, patients on DTG/3TC were more persistent than those treated with BIC/FTC/TAF. However, no correlation was found between adherence and persistence, either because adherence to both regimens was high, or due to the bias inherent in MPR-based estimations, which may in some cases yield exaggerated adherence levels.14
The main reason for discontinuation of BIC/FTC/TAF among our patients was toxicity/intolerability. A cohort study also found that the main reason for discontinuation was toxicity/intolerability.15
The chief limitation of retrospective studies lies in their potential selection bias. In this regard, a key limitation in this study had to do with the differences between the 2 populations analyzed. Considering that patients treated with BIC/FTC/TAF had a poorer baseline status, the fact that they presented with more comorbidities may be related to the appearance of side effects and a lower adherence due to polypharmacy. However, persistence in both populations was extremely high, with the statistically significant difference between them remaining even after adjusting for the various covariates mentioned.
ConclusionsOverall persistence with the therapies under analysis was extremely high. However. patients treated with DTG/3TC were found to be more persistent than those on BIC/FTC/TAF, despite the latter's poorer baseline clinical status (this finding must naturally be validated by further studies of a prospective nature). The main reason for discontinuation of BIC/FTC/TAF was toxicity/intolerability.
Contribution to the scientific literatureComparative persistence among different patient populations is a surrogate marker for the effectiveness and safety of any treatment. Although several studies have compared persistence with single-tablet regimens with persistence with multiple-tablet schedules, few authors have compared different single-tablet regimens in terms of their persistence. This study was aimed at describing persistence with dolutegravir/lamivudine and bictegravir/emtricitabine/tenofovir-alafenamide, currently the two most commonly administered single-tablet regimens.
Ethical responsibilitiesThe authors have followed the bioethical principles set out in the Helsinki Declaration, in the Belmont report and in the Oviedo Convention on Human Rights and Biomedicine.
Informed consentThe authors asked the Ethics Committee for a waiver of informed consent with respect to this study. Given the observational and retrospective nature of the analysis, it would have been extremely difficult to contact each and every patient, due to the large size of the sample and the fact that some of them were no longer being followed-up at the time.
FundingNo funding.
ContributionsAll authors contributed equally to the preparation of the present article.
Presentation at conferenceThe article was presented at the 27th EAHP Congress, held in Lisbon on March 22, 23, and 24, 2023.
Liability and transfer of rightsAll co-authors accept the responsibilities defined by the International Committee of Medical Journal Editors (available at http://www.icmje.org/).
In the event of publication, the authors exclusively transfer to the Revista and, by extension to SEFH, their rights to reproducing, distributing, translating, and publicly communicating their work by any sound, audiovisual or electronic medium or format. A specific right transfer letter will be sent once the paper is submitted through SEFH's online manuscript processing system.
CRediT authorship contribution statementLorena Martín-Zaragoza: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Javier Sánchez-Rubio-Ferrández: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Alberto Onteniente-González: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Marcos Gómez-Bermejo: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Sergio Julio Rodríguez-Álvarez: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Alfonso Monereo-Alonso: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Teresa Molina-García: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.