Surgical antibiotic prophylaxis is one of the most useful measures to prevent surgical wound infection.
ObjectiveThe aim of this project is to evaluate the appropriateness of the use of antibiotic prophylaxis in surgical procedures performed in Spanish hospitals, both globally and according to the type of surgery performed.
MethodFor this purpose, an observational, retrospective, cross-sectional, and multicentre study has been designed to collect all the variables that allow the evaluation of the appropriateness of surgical antibiotic prophylaxis by comparing the prescribed treatment, the recommendations included in the local guidelines, and the consensus document of the Spanish Society of Infectious Diseases and Clinical Microbiology and the Spanish Association of Surgeons. Indication, choice of antimicrobial, dose, route and duration of administration, timing, re-dosing, and duration of the prophylaxis will be taken into account. The sample will consist of patients who underwent scheduled or emergency surgery, either as inpatients or outpatients, in hospitals in Spain. A sample size of 2335 patients has been established to estimate, with 95% confidence and 80% power, a percentage of appropriateness that is expected to be around 70%. Differences between variables will be analysed using Student's t-test, Mann–Whitney U test, Chi-square test, or Fisher's test, as appropriate. The degree of agreement between the antibiotic prophylaxis recommended by the guidelines of the different hospitals and that recommended in the literature will be analysed by calculating the Cohen's kappa indicator. Binary logistic regression analysis using generalised linear mixed models will be performed to identify possible factors associated with differences in the appropriateness of antibiotic prophylaxis.
DiscussionThe results of this clinical study will allow us to focus on specific surgical areas with higher rates of inappropriateness, identify key points of action and guide future strategies for antimicrobial stewardship programs in the area of antibiotic prophylaxis.
La profilaxis antibiótica quirúrgica es una de las medidas más útiles para la prevención de la infección de la herida quirúrgica.
ObjetivoEl objetivo de este proyecto es evaluar la adecuación del uso de profilaxis antibiótica en procedimientos quirúrgicos realizados en centros hospitalarios españoles, tanto de forma global como en función del tipo de cirugía realizada.
MetodologíaPara ello, se ha diseñado un estudio observacional, retrospectivo, transversal y multicéntrico, donde se recopilarán todas aquellas variables que permitan evaluar la adecuación de la profilaxis antibiótica quirúrgica mediante la comparación del tratamiento prescrito, las recomendaciones recogidas en las guías locales y el documento de consenso de la Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC) y la Asociación Española de Cirujanos (AEC). Se tendrán en cuenta la indicación, elección del antimicrobiano, dosis, vía de administración y tiempo de infusión, momento de la administración de la primera dosis, redosificación y la duración de la profilaxis. La muestra estará constituida por pacientes que hayan sido intervenidos de forma programada o urgente, en régimen de hospitalización o ambulatorio en centros hospitalarios de España. Se ha establecido un tamaño muestral de 2.335 pacientes para estimar con una confianza del 95% y una potencia del 80% un porcentaje de adecuación que se espera esté en torno al 70%. Las diferencias entre variables se analizarán mediante la prueba t-Student, U de Mann–Whitney, el test Chi2 o test de Fisher, según proceda. El grado de concordancia entre la profilaxis antibiótica recomendada por las guías de los distintos hospitales y la recomendada en la literatura se analizará mediante el cálculo del indicador Kappa de Cohen. Con el fin de identificar posibles factores que puedan asociarse con diferencias en la adecuación de uso de profilaxis antibiótica, se llevará a cabo un análisis de regresión logística binario y mediante modelos lineales mixtos generalizados.
DiscusiónLos resultados de este proyecto nos permitirán poner el foco en determinadas áreas quirúrgicas con mayor porcentaje de inadecuación de tratamientos, detectar puntos clave de actuación y dirigir las futuras estrategias de los programas de optimización del uso de antimicrobianos en el ámbito de la profilaxis antibiótica.
Surgical antibiotic prophylaxis comprises the perioperative systemic administration of antibiotics that is initiated before surgery. It is one of the most useful measures for the prevention of surgical wound infection and has an efficacy ranging from 18% to 81%, depending on the type of intervention.1,2 Recommendations for the prescription of antibiotic prophylaxis in Spain have been available since 2002. These recommendations have recently been updated by the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) and the Spanish Association of Surgeons (AEC),3 who have adapted the prescription protocols to each type of surgical intervention and to current epidemiology.
The antibiotic prophylaxis used must not only be active against the pathogens that most frequently cause infection, but must also involve correct dosage, administration route, and timing of administration and duration. By contrast, the inappropriate use of antibiotic prophylaxis can increase the risk of infection and toxicity as well as lead to bacterial resistance.3
Currently, 72% of Spanish hospitals are developing initiatives to optimise the use of antibiotics for both treatment and prophylaxis.4 In 40% of cases, these initiatives are included in Antimicrobial Stewardship Programmes (ASP). A fundamental strategy of ASPs is the monitoring of antimicrobial consumption and prescription quality indicators that enables the detection of differences in usage patterns over time between intra- and inter-hospital services.5
Previous studies have analysed the appropriateness of antibiotic prophylaxis in different settings.6–11 A study conducted in 14 hospitals in Madrid (Spain), showed that the percentage of appropriate surgical prophylaxis was 72.5% and that duration was the most frequent cause of inappropriateness.12 Another study conducted at the Complejo Hospitalario Universitario A Coruña showed a high overall appropriateness rate of 83%–98%. The lowest rates of appropriateness were obtained at the time of administration of the first dose and duration, with rates ranging from 72% to 85%, respectively.13
Monitoring the use of antimicrobials in surgical prophylaxis is essential to ensure the administration of appropriate prophylaxis regimens and to avoid surgical wound infections. However, in Spain, there are no data on the evaluation of the appropriateness of antibiotic prophylaxis at the national level.
We present the protocol of the ProA-Q observational study, which will assess the appropriateness of antibiotic prophylaxis use in surgical procedures performed in Spanish hospitals, both from an overall perspective as well as according to the type of surgery performed.
MethodologyDesignObservational, retrospective, cross-sectional, multicentre study.
PopulationAdult and paediatric inpatients or outpatients who underwent elective or emergency surgery in hospitals in Spain.
Inclusion criteriaInpatients or outpatients who have undergone elective or emergency surgery in Spanish hospitals and in the following surgical areas: angiology and vascular surgery, cardiac surgery, general and digestive surgery, maxillofacial surgery, paediatric surgery, thoracic surgery, gynaecology and obstetrics, neurosurgery, otorhinolaryngology, traumatology, and urology.
Exclusion criteriaPatients who required ophthalmological, dermatological, or plastic surgery, and surgery for the implantation of central vascular catheters in which the administration of surgical antibiotic prophylaxis by the systemic route is very infrequent.
HospitalsSpanish hospitals will participate in the study. A preliminary list of participating hospital is included in Table S1 of the supplementary data.
RecruitmentThe research team will assign each participating centre the number of patients to be included by each surgical area based on the size of the centre. The data collection time-point will be set on the day chosen by each centre during the week assigned by the research team based on the following considerations: - Hospitals that do not reach the assigned number of patients on the day of the data collection time-point may continue to include patients for a maximum of 4 consecutive days from the first day of the time-point onward. - Hospitals in which the number of patients who underwent surgery on the day of the time-point exceeds the number assigned to the centre will include patients in the order in which they entered the operating theatre.
- -
Sociodemographic: date of birth, and sex.
- -
Anthropometric and clinical: weight, height, surgical area/service, type of procedure (scheduled or urgent), type of surgery, regime (inpatient or outpatient), date and time of procedure, history of allergy to antimicrobials, history of infection or colonisation by multidrug-resistant bacteria (methicillin-resistant Staphylococcus aureus and multidrug-resistant Gram-negative bacteria) in the last year, and renal function (creatinine clearance value).
- -
Related to antibiotic prophylaxis: prophylactic antimicrobial, dose, route of administration and duration of infusion (if intravenous), timing of administration of the first dose in relation to the start of surgery, surgical complications that may justify the need for a second dose of prophylactic antibiotic, administration of a second intraoperative dose, and duration of antibiotic prophylaxis.
- -
Related to local guidelines or protocols and the SEIMC/AEC consensus document on surgical antibiotic prophylaxis3: recommendation or otherwise of antibiotic prophylaxis, recommended antimicrobial, dose, route of administration and duration of infusion, timing of administration of the first dose, timing of administration of the second intraoperative dose, and recommended duration of antibiotic prophylaxis.
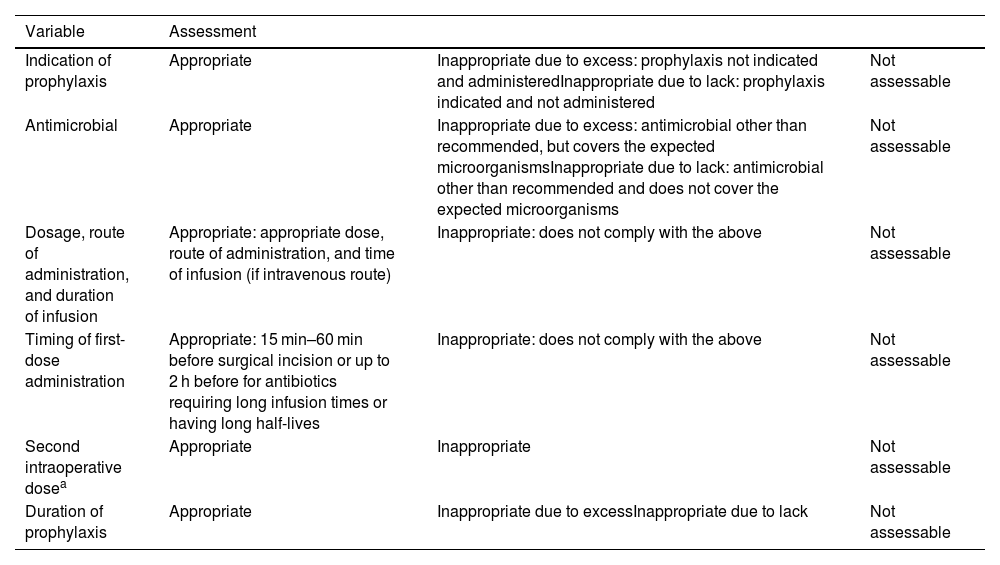
Assessment of appropriateness based on indication, chosen antimicrobial, dose, route of administration and duration of infusion, timing of first dose, second intraoperative dose, and duration of antibiotic prophylaxis (Table 1).
Assessment of appropriateness.
| Variable | Assessment | ||
|---|---|---|---|
| Indication of prophylaxis | Appropriate | Inappropriate due to excess: prophylaxis not indicated and administeredInappropriate due to lack: prophylaxis indicated and not administered | Not assessable |
| Antimicrobial | Appropriate | Inappropriate due to excess: antimicrobial other than recommended, but covers the expected microorganismsInappropriate due to lack: antimicrobial other than recommended and does not cover the expected microorganisms | Not assessable |
| Dosage, route of administration, and duration of infusion | Appropriate: appropriate dose, route of administration, and time of infusion (if intravenous route) | Inappropriate: does not comply with the above | Not assessable |
| Timing of first-dose administration | Appropriate: 15 min–60 min before surgical incision or up to 2 h before for antibiotics requiring long infusion times or having long half-lives | Inappropriate: does not comply with the above | Not assessable |
| Second intraoperative dosea | Appropriate | Inappropriate | Not assessable |
| Duration of prophylaxis | Appropriate | Inappropriate due to excessInappropriate due to lack | Not assessable |
For each item, a rating of “appropriate” will be assigned if the medical prescription and the recommendation match, “inappropriate” if the medical prescription and the recommendation do not match, and “not assessable” if insufficient information is available to assess appropriateness.
For the purposes of the overall assessment, the following variables will be considered appropriate: antibiotic prophylaxis that adheres with all the recommendations in terms of indication, antimicrobial, dose, route of administration and duration of infusion, timing of administration of the first dose, second intraoperative dose, and duration of antibiotic prophylaxis.
A pharmacist in consultation with a doctor specialised in anaesthesia, surgery, and/or infectious diseases who belong to the hospital's ASP will assess the appropriateness of the use of antibiotic prophylaxis by comparing the prescribed treatment and the recommendations contained in the local surgical antibiotic prophylaxis guidelines of each hospital. Likewise, this analysis will be conducted using the SEIMC/AEC consensus document on antibiotic prophylaxis in surgery3 as a reference. Participating hospitals without local antibiotic prophylaxis guidelines will perform the assessment using the SEIMC/AEC consensus document3 alone.
Adverse drug reaction reportingAdverse drug reactions will be reported to the Regional Centre for Pharmacovigilance using the yellow card or via the following website: www.notificaRAM.es.
Sources of informationPharmacists will retrospectively collect data from the patients' electronic medical records, in-hospital e-prescribing modules, and operating room records.
Sample sizeThe size of the sample was calculated using data provided by the Ministry of Health in 2019, which showed that 2 428 316 surgical interventions were performed in hospitals within the Spanish National Health System, excluding ophthalmological, dermatological, and plastic surgery. Thus, there were approximately 202 360 surgeries per month and 46 698 surgeries per week. Data from previous studies were also taken into account, which showed that approximately 70% of antimicrobial prescriptions for surgical prophylaxis were appropriate.12
Based on these data, the multicentric character of the study, and the differences in the frequency of interventions within each surgical area, a minimum sample size of 2335 patients was estimated to represent the target population estimate with a minimum of 50 hospitals, a confidence level of 95%, and a statistical power of 80%.
The distribution of the sample size by surgical area is shown in Table S2 of the supplementary data.
Data collection and managementData collection and management will be conducted using an electronic database using Research Electronic Data Capture (REDCap) software. The data will be anonymised and entered by assigning a specific code; this approach will guarantee the confidentiality of the data of all the patients included in the study and ensure adherence with the regulations of the Spanish Organic Law 3/2018 on Personal Data Protection and Guarantee of Digital Rights.
Study periodData collection and assessment of appropriateness will be conducted within 10 calendar days of obtaining the authorisations from the participating hospitals. The results are expected to be published in the second half of 2023.
Statistical analysisA descriptive analysis will be performed: continuous variables will be expressed as measures of central tendency (mean, median), dispersion, and position; qualitative variables will be expressed as frequency distributions and percentages.
Differences between continuous quantitative variables in independent groups will be analysed using the Student t-test or the corresponding Mann–Whitney U-test in the non-parametric case. The Chi2 test or Fisher's exact test will be used to analyse the association between qualitative variables. Cohen's Kappa indicator will be used to analyse the degree of concordance between the antibiotic prophylaxis recommended by the guidelines of the different hospitals and that recommended in the literature for each type of surgical procedure.
A binary logistic regression analysis (univariate and multivariate) will be conducted to determine the characteristics of the patients, hospitals, and areas or types of surgical procedure (independent variables) that may be related to the appropriateness of antibiotic prophylaxis (dependent variable). Generalised linear mixed models will be used to evaluate the cluster effect in relation to the hospital variable.
Two-tailed analyses will be performed and a P-value of less than .05 will be used as a cut-off for statistical significance. All analyses will be conducted using the IBM SPSS 25.0 statistical package and the R statistical package.
DiscussionThe ProA-Q study is a clinical research project whose objective is to fill a need for information in the field of antimicrobial use and ASP. Two previous studies conducted in Spanish hospitals showed overall appropriateness rates of surgical antibiotic prophylaxis of more than 70%. One of the studies was conducted in a single centre, whereas the other was conducted in several hospitals in the same Autonomous Community, which limits the generalisability of their results to other hospitals and populations.12,13 The recent PAUSATE study analysed the prevalence and appropriateness of antimicrobial use in 103 Spanish hospitals. Its results show that 45% of antimicrobial prescriptions could be improved and 19% are inappropriate, although it is not possible to determine what percentage of prophylactic antimicrobial use is appropriate.14
Researchers have to invest a great deal of effort to achieve appropriate sample sizes that permit statistical inference and allow definitive conclusions to be drawn. In this sense, national and international research networks and scientific societies can provide the support and infrastructure required for multicentre studies.15 In our case, the dissemination of the project by means of the Spanish Society of Hospital Pharmacy (SEFH) has facilitated the inclusion of more than 100 public and private hospitals in 16 Autonomous Communities. Moreover, one of the research priorities of ASP is to conduct studies with the aim of improving the use of antimicrobials and antibiotic prophylaxis in populations commonly excluded from clinical trials.16 Our proposal to assess the appropriateness of prophylaxis in paediatric surgery will attempt to fulfil that objective.
The primary endpoint of this study is the rate of appropriateness of antibiotic prophylaxis, which is one of the most recognised process indicators within ASP. This indicator is especially relevant when analysing the degree of implementation of strategies promoted by ASP teams and their long-term maintenance. The assessment of prescribing quality requires the individualised analysis of patients; for this reason, it is less frequent and is limited by the high workload involved and the lack of standardisation.5 Other strengths of this study include the use of a standardised methodology and the assessment of appropriateness based on the SEIMC/AEC consensus document,3 which will also facilitate the assessment of concordance between local protocols and updated evidence. In addition, we aim to ensure recruitment success and reduce the subjectivity inherent to the evaluation of prescription quality by the formation of local multidisciplinary teams comprising professionals from various specialties with expertise in ASP.
However, this study is not without limitations. According to current recommendations on the design of ASP studies, process indicators should be accompanied by clinical and microbiological indicators that provide information on the impact of the evaluated strategy on patients.17 According to previous studies, the efficacy of antibiotic prophylaxis in the prevention of surgical site infections varies according to the type of surgery.18,19 The main preventive measures for surgical infection are 2% chlorhexidine/alcohol scrub, correct hair removal, and maintenance of normothermia and normoglycaemia.18 Therefore, although correct antibiotic prophylaxis is one of the recommended measures to reduce the risk of surgery-related infection, it is not the only one, and so unequivocal and unbiased relationships cannot be established that would allow us to draw robust conclusions from the analysis of clinical variables in this study. Future studies that included all these variables could analyse their effect on surgical wound infection rates. In addition, the voluntary participation of the hospitals may have led to a potential selection bias, given that it is likely that the hospitals with the highest motivation for appropriate antimicrobial use are the ones that will have volunteered to participate in the study. However, this limitation will be largely controlled for given the large number of participating hospitals.
In conclusion, the results of this project will allow us to determine the rate of appropriateness of antibiotic prophylaxis in Spanish hospitals, both globally and by surgical area, and will provide relevant information to detect key points of action and promote strategies at local and national levels within the ASP. Deficiencies identified will be addressed with the development of new working procedures, new technologies, educational software, data use modules, and other tools with the potential for industrial property rights registration and exploitation.
FundingThis study was funded by the Fundación Española de Farmacia Hospitalaria (FEFH) through the call of Grants 2022–2023 for the SEFH Working Groups. Ana Belén Guisado-Gil receives funding from the Subprograma Juan Rodés, Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Ciencia, Innovación y Universidades, Spain (JR21/00017).
Declaration of authorshipAll authors participated in the conception and design of the study. Ana Belén Guisado-Gil and Didiana Jaramillo were responsible for writing the manuscript. The critical revision of the text was conducted by José María Gutiérrez-Urbón, Almudena Ribed-Sánchez, Sonia Luque-Pardos, Abraham Sánchez-Cadena, Beatriz Mejuto, Germán Peñalva, and José Miguel Cisneros. All authors approved the final version for publication.
Ethical responsibilitiesThis study will be conducted in accordance with Royal Decree 957/2020 of 3 November, 2020, which regulates observational studies on medicinal products for human use. The study will fully comply with the principles of good clinical practice and the Declaration of Helsinki. The study was approved by the CEIm Provincial de Sevilla (Spain) on 12 January, 2023 (sponsor code: ProA-Q, CEIm code: 1654-N-22). Due to the characteristics of the study, the CEIm approved the request to waive informed consent.
The authors would like to thank the SEFH, the Pharmaceutical Care in Infectious Diseases working group (AFinf group), the Specialist Pharmacists in the Surgical Patient working group (Faquir group) of the SEFH, and all the participating hospitals for their support.