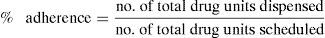To describe the efficacy, safety, compliance and cost savings of lopinavir/ritonavir monotherapy.
MethodObservational, descriptive and retrospective study evaluating monotherapy. Adherence was calculated using an objective method. We estimated the direct costs of dispensing non-triple therapy.
ResultsWe identified 17 patients. Interval adherence was >95% in 9 patients, 90%–95% in 2 patients, 90%–85% in 2 patients, and less than 85% in 4 patients. Viral load was undetectable during weeks 12, 24, 36, and 48, except in 2 patients. The CD4 count in most analytical tests remained at >350cells/ml, only 1 patient had a lower figure. The average savings was 4819 Euros/patient/year (range 1116–8700).
ConclusionsIn selected patients, monotherapy can be a cost-effective treatment option.
Describir la efectividad, seguridad, adherencia y ahorro económico de la monoterapia basada en lopinavir/ritonavir.
MétodoEstudio observacional, descriptivo y retrospectivo que evaluó la monoterapia. La adherencia se calculó utilizando un método objetivo. Se estimaron los costes directos derivados de la no dispensación de la triple terapia.
ResultadosIdentificamos 17 pacientes. La adherencia por intervalos fue: >95%, 9 pacientes; 90-95%, 2 pacientes; 90-85%, 2 pacientes; inferior al 85%, 4 pacientes. La carga viral fue indetectable durante las semanas 12, 24, 36 y 48 excepto en 2 pacientes. Las cifras de CD4 se mantuvieron en la mayor parte de las analíticas >350 cél./μl, y solo un paciente tuvo una cifra inferior. El ahorro medio fue 4.819 euros/paciente/año (rango 1.116-8.700).
ConclusionesEn pacientes seleccionados la monoterapia puede ser una opción terapéutica coste-efectiva.
After more than 20 years of experience with the human immunodeficiency virus (HIV), it seems that there are two keys to antiretroviral treatment (ART) being effective in the long term1,2: being able to inhibit viral replication and having a high genetic barrier in order to prevent resistant mutations in the viral genome.
It is now obvious that the first ART regimens based on monotherapy with nucleoside analogue (NA) reverse transcriptase inhibitors were lacking in both areas, and as new drugs were developed, combination therapy was the next logical step. Beginning in 1997, it was clearly understood that a combination of at least 3 drugs—2 NARTIs and a protease inhibitor (PI) or 2 NARTIs and a non-analogue nucleoside (NN) RTI—was necessary in order to achieve effective, long-lasting viral replication inhibition, and this became the treatment of choice for HIV.1,2
The concept of triple therapy would have remained unquestioned longer if it were not for the fact that these drug combinations, which are very effective as antivirals, give rise to significant toxicities in the medium and long term,3 in addition to being expensive.4 For these reasons, clinical research in recent years has examined the possibility of using simpler, less toxic and less costly treatments, and this has even shed doubts on the utility of triple therapy.On this subject, studies of PI pharmacokinetic properties, efficacy and genetic barrier in association with low doses of ritonavir appear to be promising. Co-formulations of lopinavir/ritonavir (LPV/r), atazanavir/ritonavir (ATZ/r) and darunavir/ritonavir (DRV/r) have been examined in non-inferiority studies.2 As LPV/r is currently the most widely used combination, due to having appeared first, it is an excellent combination for evaluating the possibility of treating HIV with a single drug on a practical level, without the limitations intrinsic in clinical trials.5–7
The fact that HIV may be considered as a chronic disease when treatment adherence is at a maximum, given the decrease in morbidity and mortality provided by ART,8 means that monotherapy can prevent resistance to other drugs in the future, side effects associated with treatment, and even decrease drug treatment costs as a result of simplifying treatment.
The aim of our study was to describe the effectiveness, safety, adherence (ADH) and cost reduction compared with triple therapy in patients in our hospital district who are currently undergoing treatment with LPV/r-based monotherapy.
MethodObservational, descriptive and retrospective study carried out in a general tertiary care hospital (611 beds) that evaluated the effectiveness, safety, and economic savings of monotherapy with LPV/r 200/50mg dosed at 2 tablets every 12h in all patients receiving that treatment between treatment onset and the date when data was collected (April 2010).
Information was obtained through the pharmacy department's outpatient dispensing programme Dominion Transtool®4.3.rev.7.0.8, review of patients’ medical histories, and the CLINET programme providing digital medical histories.
The study population included all HIV-infected patients treated with LPV/r monotherapy over a minimum duration of 3 months.
We analysed compliance with criteria recommending use of monotherapy,2 which are as follows:
- •
Patients with no prior history of PI failure.
- •
Patients whose plasma viral load (PVL) was undetectable at least 6 months before the change to monotherapy.
- •
Patients showing signs or symptoms of NA toxicity.
Data was collected using a form that included the following information:
- •
Demographic variables referring to the LPV/r monotherapy period (age, sex, stage of the disease and onset of ART).
- •
Virological response variable: This is the primary effectiveness variable, defined as PVL values. This is a discrete quantitative variable.
The following were defined as secondary effectiveness variables:
- •
Immunological response variable: defined as CD4 T-cell values in cells/μl. This is a discrete quantitative variable.
- •
Clinical response variable: defined as the appearance of infections and/or related opportunistic neoplasia. This is a dichotomous qualitative variable.
Other variables were:
- •
Safety variable: defined by the appearance of adverse drug reactions (ADRs). This is a nominal qualitative variable. Adverse reactions were classified by intensity according to the World Health Organisation's Common Toxicity Criteria for Adverse Events, version 3.0. The cause of adverse reactions was established by using a modified Karch–Lasagna algorithm.
- •
Adherence variable: may be expressed as a continuous or dichotomous variable since it contains 2 categories:
- (a)
Good compliance if adherence >95%.
- (b)
Poor compliance if adherence <95%.
- (a)
The calculation was made based on the dispensing records and using the following formula:
Scheduled units were defined as those necessary to comply with treatment in the days between the first and the last drug dispensed.
- •
Cost savings variable: defined as the savings arising from not using triple therapy. This is a continuous quantitative variable. Savings were calculated using the cost of the period (in days) with LPV/r monotherapy compared to the cost of triple therapy for the same period of time. We used the laboratory sale price plus VAT for each of the drugs to make these calculations.
A descriptive statistical analysis was applied to the data. Variable values were expressed as means, medians, percentages, and frequencies.
Patients were informed about all matters involved in the new treatment strategy, and their consent forms were included in their medical histories. Throughout the entire study, participants were guaranteed anonymity and that their data would be kept confidential.
ResultsA total of 17 patients in our centre received treatment with LPV/r in monotherapy: 12 men and 5 women. Patients’ mean age was 43 years (range: 39–56 years).
Their clinical stages were category 3 (82.35%) and category 2 (17.64%). The mean time elapsed from the onset of ART until beginning monotherapy was 10.87 years (range: 9–11). In our patients, the mean duration of LPV/r monotherapy was 13 months (range: 3–45 months) prior to the date on which the study data were collected.
Adherence by intervals was as follows: ADH>95%, 9 patients; 90%–95%, 2 patients; 90%–85%, 2 patients; less than 85%, 4 patients.
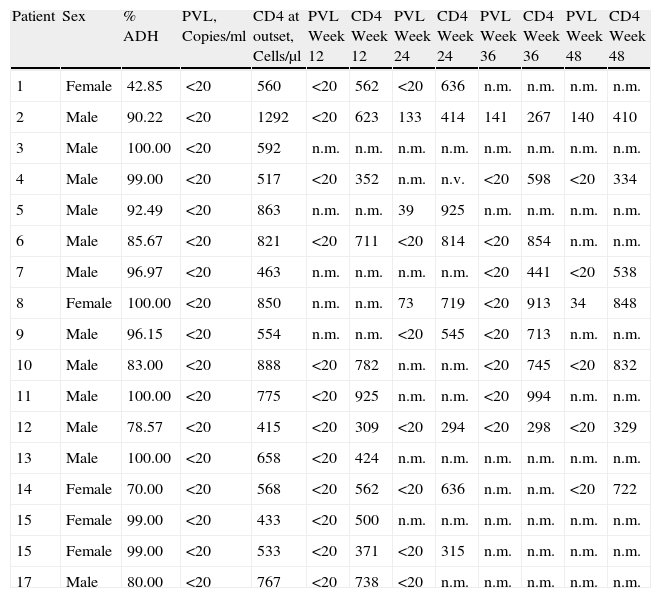
All of the patients had an undetectable PVL and a mean CD4 cell count of 683cells/μl (range: 415–1292cells/μl). PVL remained undetectable (below 20 copies/ml) during weeks 12, 24, 36, and 48 in all of our patients except for 2 patients in weeks 24, 36 and 48 (always <150 copies/ml).
The CD4 count remained above 350cells/μl for most of the tests. The mean CD4 count for the different weeks in which we tested was 537cells/μl in week 12; 588cells/μl in week 24; 647cells/μl in week 36, and 580cells/μl in week 48. Only one patient had a CD4 count below 350cells/μl in week 36 (267cells/μl) and in week 48 (384cells/μl). Values for each of the patients are shown in Table 1.
Data for Patients Treated With Monotherapy Based on LPV/r.
| Patient | Sex | % ADH | PVL, Copies/ml | CD4 at outset, Cells/μl | PVL Week 12 | CD4 Week 12 | PVL Week 24 | CD4 Week 24 | PVL Week 36 | CD4 Week 36 | PVL Week 48 | CD4 Week 48 |
| 1 | Female | 42.85 | <20 | 560 | <20 | 562 | <20 | 636 | n.m. | n.m. | n.m. | n.m. |
| 2 | Male | 90.22 | <20 | 1292 | <20 | 623 | 133 | 414 | 141 | 267 | 140 | 410 |
| 3 | Male | 100.00 | <20 | 592 | n.m. | n.m. | n.m. | n.m. | n.m. | n.m. | n.m. | n.m. |
| 4 | Male | 99.00 | <20 | 517 | <20 | 352 | n.m. | n.v. | <20 | 598 | <20 | 334 |
| 5 | Male | 92.49 | <20 | 863 | n.m. | n.m. | 39 | 925 | n.m. | n.m. | n.m. | n.m. |
| 6 | Male | 85.67 | <20 | 821 | <20 | 711 | <20 | 814 | <20 | 854 | n.m. | n.m. |
| 7 | Male | 96.97 | <20 | 463 | n.m. | n.m. | n.m. | n.m. | <20 | 441 | <20 | 538 |
| 8 | Female | 100.00 | <20 | 850 | n.m. | n.m. | 73 | 719 | <20 | 913 | 34 | 848 |
| 9 | Male | 96.15 | <20 | 554 | n.m. | n.m. | <20 | 545 | <20 | 713 | n.m. | n.m. |
| 10 | Male | 83.00 | <20 | 888 | <20 | 782 | n.m. | n.m. | <20 | 745 | <20 | 832 |
| 11 | Male | 100.00 | <20 | 775 | <20 | 925 | n.m. | n.m. | <20 | 994 | n.m. | n.m. |
| 12 | Male | 78.57 | <20 | 415 | <20 | 309 | <20 | 294 | <20 | 298 | <20 | 329 |
| 13 | Male | 100.00 | <20 | 658 | <20 | 424 | n.m. | n.m. | n.m. | n.m. | n.m. | n.m. |
| 14 | Female | 70.00 | <20 | 568 | <20 | 562 | <20 | 636 | n.m. | n.m. | <20 | 722 |
| 15 | Female | 99.00 | <20 | 433 | <20 | 500 | n.m. | n.m. | n.m. | n.m. | n.m. | n.m. |
| 15 | Female | 99.00 | <20 | 533 | <20 | 371 | <20 | 315 | n.m. | n.m. | n.m. | n.m. |
| 17 | Male | 80.00 | <20 | 767 | <20 | 738 | <20 | n.m. | n.m. | n.m. | n.m. | n.m. |
CD4: CD4 lymphocytes; PVL: plasma viral load; n.m.: not measured.
ADRs were observed in 3 patients: there was 1 case of diarrhoea and 2 cases of hypercholesterolaemia. In addition, we observed an improvement in other ADRs that had been caused by triple therapy in 3 cases of lipodystrophy and 1 episode of nausea and hot flashes. Improvement in cases of lipodystrophy was evaluated by the doctor, who examined the most commonly affected areas (facial fat, temporal fascia, suborbital fat, neck, gluteals and lower limbs). This was reflected in their medical histories, but could not be quantified.
None of the treatments prescribed to patients was discontinued.
The mean economic savings arising from monotherapy use was 4819 Euros/patient/year (range: 1116–8700 Euros).
DiscussionIn patients with no prior history of PI failure, with a PVL that had been undetectable for at least 6 months and who had signs and symptoms of NA toxicity, simplifying treatment to monotherapy with darunavir or lopinavir in combination with ritonavir is possible with an evidence grade of A.2
Monotherapy-based antiretroviral treatment has therefore been shown to be effective for maintaining patients’ clinical condition by keeping an undetectable PVL and a high CD4 count. In addition, it decreases patient exposure to drug combinations with severe and frequent ADRs, reduces the likelihood of generating viral resistance and cuts down on drug consumption per patient. In a few exceptional cases, however, a patient may experience a sustained virological response with a poor immune response.
In one clinical trial, Pulido et al.9 observed that LPV/r was effective in maintaining undetectable PVLs in a high number of selected patients (67%) after 4 years of monotherapy-based treatment. One especially interesting aspect is the fact that in cases in which reintroducing combination therapy was necessary, there are no reports of PI resistance appearing,9 meaning that future treatments were not jeopardised.10
In our study, we saw promising clinical results in patients outside of the context of a clinical trial. The treatment is unquestionably effective given good adherence, and the possibility of avoiding resistances is an excellent strategy to be considered. Patients with poor adherence who experience treatment failure would have the possibility of rescue treatment by reintroducing the NAs that were discontinued upon changing to monotherapy.
Monotherapy with a PI may become an increasingly common treatment in the near future. In fact, reference guidelines clearly show that 3 different PIs (ATZ/r, DRV/r and LPV/r) are no worse than the equivalent combination treatment; however, we lack studies comparing the 3 PIs. Yet again science has moved beyond the recommendations committed to laboratory leaflets and approved by authorised regulatory agencies, since none of the 3 PIs is officially recognised for monotherapy use by the Spanish Ministry of Health and Social Policy. This is also the case for the EMEA and the FDA.
In our case, the number of patients on monotherapy tripled over the last year. It is possible that, compared with the classic treatment of 2 NAs plus a third PI or NN, the option of an enhanced PI in monotherapy will expand to cover a significant percentage of our total number of patients.
In a panorama of continuously increasing medication use, the savings of monotherapy are particularly relevant. In our case, antiretroviral treatment accounts for one third of our outpatient consumption, with a mean of €6880 per patient/year in 2009. The cost of monotherapy could reduce the total consumption of these treatments. Based on the data in our study, the decrease in consumption would be equivalent to cutting each patient's drug use to half. Today, finding drug treatment options that are equally effective, potentially safer, and also less costly might seem illusory.
Nevertheless, we must recognise the fact that, despite the advantage which many of our patients might enjoy by receiving an antiretroviral treatment in monotherapy, and the growing signs that monotherapy will be used more in the near future, we do not currently know which is the best enhanced protease inhibitor for use in monotherapy since there are no comparative studies for lopinavir and darunavir. It would be extremely interesting to understand priorities, based on the effectiveness/safety profile, in order to choose between different PIs for use in monotherapy.
The limits of our study include a short follow-up and the fact that we had no access to studies on resistances in cases of treatment failure. It would therefore be desirable to complete scientific studies designed to provide more evidence.
According to our observations, we were able to conclude that monotherapy could be a cost-effective treatment option in selected patients.
Conflict of interestThe authors affirm that they have no conflicts of interest.
Please cite this article as: González Rivas L, et al. Simplificación del tratamiento antirretroviral: una buena alternativa para nuestros pacientes y para la sostenibilidad de nuestro sistema sanitario. Farm Hosp. 2011;35:317–321.