This article describes a study protocol for evaluating adherence to oral chemotherapy (OCT) in patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) in Spain.
MethodsThis multicenter, observational, prospective study will be conducted by 6 hospital pharmacists from 6 Spanish hospitals. The study will include men and women aged 18 years or older with a diagnosis of locally advanced or metastatic NSCLC who are being treated or have been prescribed OCT. Once included, the patient will be active and prospectively followed up for 3 months, including 4 study visits to record information on sociodemographic variables, antineoplastic treatment and adherence, pharmaceutical care, clinical variables, and patient-reported outcomes (PRO) (the 3-level version of EQ-5D, the EORTC Core Quality of Life Questionnaire, the Brief Illness Perception Questionnaire, the Treatment Satisfaction with Medicines Questionnaire, and the PRO version of Common Terminology Criteria for Adverse Events). Twelve months after patient inclusion, we will record information on the disease progression status and dispensed prescriptions. The primary outcome is the percentage of treatment adherence that will be calculated based on the pill count as follows: the difference between the number of pills dispensed minus the number of unused pills will be divided by the number of days of treatment multiplied by the number of pills/day prescribed by the oncologist; this quotient will be multiplied by 100 to obtain the percentage of adherence. Based on the that pill count reconciliation, those with a percentage adherence >80% will be primarily categorized as adherent. Secondarily, treatment adherence will be also calculated based on the proportion of days covered and the 4-items Morisky Green Levine Medication Adherence Scale. To analyze the impact of patients' and treatment characteristics on adherence, bivariate analyses will be performed using different adherence cut-off points. To evaluate the impact of adherence on treatment efficacy as evaluated by progression-free survival, we will be using the Kaplan–Meier method and compare it with the log-rank test and univariate Cox regression analysis.
ConclusionsWe expect that our study will provide initial information on key aspects of adherence to OCT (i.e., measurement, facilitators, and barriers) and its relationship with patients' and clinically relevant outcomes in the setting of NSCLC, and that this information will help in designing pharmaceutical interventions to improve adherence.
Este artículo describe un protocolo de estudio para evaluar la adherencia a la quimioterapia oral (CTO) en pacientes con cáncer de pulmón no microcítico (CPNM) localmente avanzado o metastásico en España.
MétodosEste estudio multicéntrico, observacional y prospectivo será realizado por seis farmacéuticos hospitalarios de seis hospitales españoles. El estudio incluirá hombres y mujeres de 18 años o más con un diagnóstico de CPNM localmente avanzado o metastásico que estén en tratamiento o se les haya prescrito CTO. Una vez incluido, el paciente tendrá un seguimiento prospectivo durante 3 meses, incluidas cuatro visitas de estudio para registrar información sobre variables sociodemográficas, tratamiento y adherencia antineoplásica, atención farmacéutica, variables clínicas y resultados comunicados por el paciente (PRO) (la versión de 3 niveles del EQ-5D, el EORTC Core Quality of Life Questionnaire, el Brief Illness Perception Questionnaire, el Treatment Satisfaction with Medicines Questionnaire, y la versión PRO del Common Terminology Criteria for Adverse Events). Doce meses después de la inclusión del paciente, registraremos información sobre el estado de progresión de la enfermedad y las recetas dispensadas. El resultado primario es el porcentaje de adherencia al tratamiento que se calculará en función del recuento de unidades de medicamentos de la siguiente manera: la diferencia entre el número de unidades de medicamentos dispensadas menos el número de unidades de medicamentos no utilizadas se dividirá por el número de días de tratamiento multiplicado por el número de unidades de medicamentos/día prescritas por el oncólogo; este cociente se multiplicará por 100 para obtener el porcentaje de adherencia. Basándose en el recuento de medicación, aquellos con un porcentaje de adherencia >80% serán los que primariamente serán considerados adherentes. De forma secundaria, la adherencia también se estimará con la proporción de días cubiertos y la Escala de Adherencia a la Medicación de 4-ítems de Morisky Green Levine. Para analizar el impacto de las características de los pacientes y del tratamiento sobre la adherencia, se realizarán análisis bivariados utilizando diferentes puntos de corte de adherencia. Para evaluar el impacto de la adherencia en la eficacia del tratamiento en términos de supervivencia libre de progresión, utilizaremos el método de Kaplan–Meier y lo compararemos con la prueba log-rank y el análisis de regresión de Cox univariado.
ConclusionesEsperamos que nuestro estudio proporcione información inicial sobre aspectos clave de la adherencia a la CTO (es decir, medición, facilitadores y barreras) y su relación con los desenlaces relevantes para el clínico y el paciente en el ámbito del CNMP, y que esta información ayude en el diseño de intervenciones farmacéuticas para mejorar la adherencia.
The availability and use of oral chemotherapy (OCT) has increased in recent years.1,2 A recent study showed that the overall use of OCT from 2008 to 2020 increased by almost 60% in dispensed units and by over 800% in patients with non-small cell lung cancer (NSCLC).1 Moreover, this trend is expected to increase; thus, of the 20 novel anticancer active substances launched in the USA in 2021, 9 were oral agents.2 OCT has several advantages for patients, such as greater convenience of administration, reduction in the number of office visits, and a sense of control over their own cancer care.3 Available evidence consistently indicates that patients with cancer prefer oral to intravenous therapy.4 However, the increasing availability of OCT is associated with several threats to patients, including toxicity monitoring and medication adherence.3 In a small survey among OCT users, participants were largely satisfied with OCT but raised concerns regarding their lack of preparedness for side effects.5
According to a systematic review, poor adherence to OCT varies widely, from 46% to 100%, depending on the study, medication type, duration of follow-up, and evaluation tool, among other factors.6 The factors associated with adherence to oral medications may differ across several diseases, including cancer. In that systematic review focused on adherence to OCT, the most frequent factors associated with poor adherence reported in the literature were age (both younger and older age), not married/living alone, depression, number of comorbidities, medication toxicity, number of concomitant prescriptions, and costs.6 Although scarcely investigated, poor adherence has been associated with poor outcomes in patients with cancer. In patients with chronic myeloid leukemia, poor adherence to imatinib was associated with poor molecular responses, and no major molecular responses were observed when adherence was <80%.7 Medication non-adherence is generally associated with higher healthcare costs across different diseases, including cancer.8 Importantly, it has been reported that overall ‘increasing the effectiveness of adherence interventions may have a far greater impact on the health of the population than any improvement in specific medical treatments.”9
Patients reported outcomes (PROs) are any report of the status of a patient's health condition that comes directly from the patient without interpretation of the patient's response by a clinician or anyone else. The integration of symptom monitoring using a PRO tools during the treatment of metastatic cancer was associated with increased survival compared with usual care.10
Medication adherence measures include direct measures such as measuring drug/metabolite levels, and indirect measures such as those involving secondary database analysis, electronic medication packaging devices, pill count, and clinician assessments and self-report using questionnaires (e.g., Morisky Green Levine Medication Adherence Scale). A review on these methods could be found elsewhere.11
Despite its key role in the management of patients with NSCLC, adherence to OCT in this setting has scarcely been investigated. Thus, in the systematic review of adherence to OCT mentioned above, of the 51 studies that evaluated the frequency and correlation with OCT, 32 studies focused on endocrine therapy for cancer, 9 on chronic myeloid leukemia, 5 on a mix of cancers, and 5 on cancers other than NSCLC.6 The primary objective of this study is to evaluate adherence to OCT in patients with locally advanced or metastatic NSCLC in Spain. Secondary objectives include analyzing the impact of patients' and treatment characteristics on adherence; evaluating the potential relationship between adherence and some PROs such as health-related quality of life, disease perception, treatment satisfaction, and toxicity perception; evaluating the relationship between three different methods of measuring adherence; evaluating the potential association between adherence and health resource utilization; and evaluating the impact of adherence on treatment efficacy as evaluated with progression-free survival.
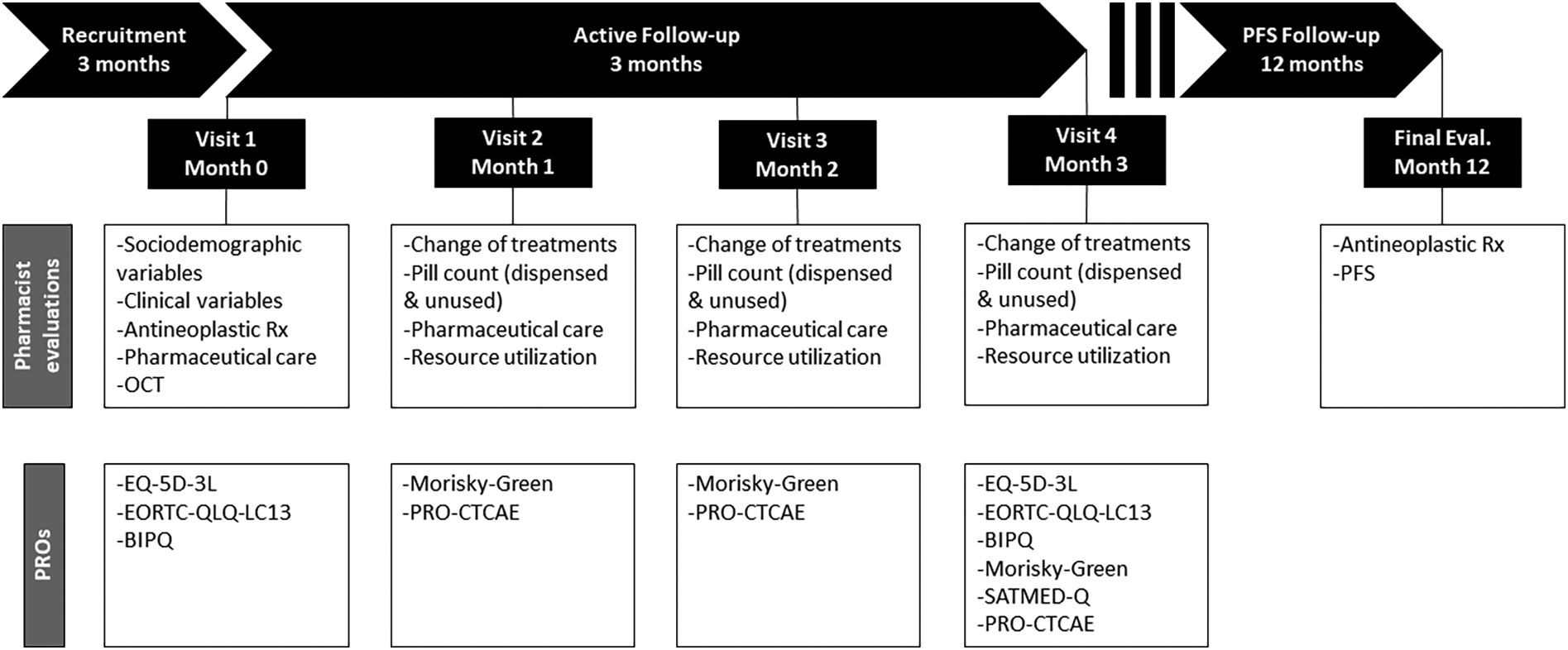
MethodsDesignThis is a multicenter, observational, prospective study. The decision to prescribe treatment is fully separate from the decision to include a patient. Treatment will be prescribed as usual by the responsible oncologists, while the decision to include the patient in the study will be the responsibility of the participating pharmacist. Once included, the patient will be actively and prospectively followed up for 3 months, including 4 study visits to record information on sociodemographic variables, antineoplastic treatment and adherence, pharmaceutical care, clinical variables, and PROs (see below). Twelve months after inclusion of the last patient, we will record information on the progression status and dispensed prescriptions. The overall design is illustrated in figure. The study has been approved by the Ethics Committee for Research with Medicines from Galicia (Santiago de Compostela, Spain).
Study settingThe study will be conducted by 6 Spanish hospitals with experience in managing patients with NSCLC.
Eligibility criteriaThe study will include men and women aged 18 years or older with a diagnosis of locally advanced or metastatic NSCLC, who are being treated or have been prescribed OCT for the management of NSCLC according to the standards and criteria of the participating centers, and who provide written informed consent. Patients will be excluded if, according to the investigator's judgment, they are unable to understand the questionnaires administered during the study or if the patient is currently included in a clinical trial.
ExposurePatients will be considered exposed to the intervention of interest if they are currently receiving or have been prescribed OCT. OCT should be prescribed in routine clinical practice and in accordance with the summary of product characteristics by the oncologist responsible for the management of patients who will not participate in the study as part of the research team.
Study assessments and outcomes measuresThere will be 4 visits during the active follow-up period of 3 months, at baseline, and every month until month 3 (Fig. 1); these visits could be substituted by telephone contact. At visit 1, at the time of the inclusion of the patient in the study, the pharmacist will record the following information: demographics including age, sex, race, studies, employment status, and tobacco use; disease-related variables such as date of diagnosis, disease stage at the time of diagnosis, histology, ECOG performance status, number and location of metastases, and biomarkers (specifically ALK, EGFR, EGFR-T790M, ROS-1, BRAF); and treatment-related variables including previous antineoplastic, concomitant medication, and OCTs, for the later recording of information posology and date of dispensation. At visit 1, the patient will complete the 3-level version of the EQ-5D (EQ-5D-3L),12 13-item lung cancer-specific questionnaire module of the EORTC Core Quality of Life Questionnaire (EORTC QLQ-LC13),13 and Brief Illness Perception Questionnaire (BIPQ).14 At visits 2, 3, and 4, the pharmacist will record information on the OCT pill count (i.e., dispensed and unused pills), treatment changes of concomitant medications and OCT, pharmaceutical care interventions, and use of resources (emergency department visits and admissions). During these visits, the patient will fulfill the 4-items Morisky Green Levine Medication Adherence Scale (MGLS-4)15 and the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE®), and the quality of life questionnaires, BIPQ, and Treatment Satisfaction with Medicines Questionnaire (SATMED-Q)16 will be self-administered again at visit 4. The final evaluation will take place 12 months after inclusion of the patient, and the pharmacist will record information on disease progression and dispensed OCT prescriptions. We will use the Spanish validated version of PROs, whose characteristics are briefly described below and that will be administered in a paper-based format.
Study periods and assessments.
BIPQ, Brief Illness Perception Questionnaire; EORTC QLQ-LC13, 13-item lung cancer-specific questionnaire module of the EORTC Core Quality of Life Questionnaire; EQ-5D-3L, 3-level version of the EQ-5D; MGLS-4, 4-items Morisky Green Levine Medication Adherence Scale; OCT, oral chemotherapy; PRO-CTCAE®, Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events; PFS, progression-free survival; SATMED-Q, Treatment Satisfaction with Medicines Questionnaire.
The EQ-5D is a 5-item generic measure of quality of life that comprises 5 dimensions: movement, self-care, daily life activities, pain/discomfort, and anxiety/depression. We will administer the Spanish version of the EQ-5D 3-level version, where the degree of impairment in each dimension is evaluated as having no problems, some problems, or extreme problems.12 The instrument includes a Visual Analog Scale (EQ-5D VAS) to evaluate self-rated health from 0 (worst imaginable health status) to 100 (best imaginable health status).
The EORTC QLQ-LC13 comprises both a multi-item scale for evaluating dyspnea and single-item measures of lung cancer-associated symptoms such as pain, coughing, sore mouth, dysphagia, peripheral neuropathy, alopecia, and hemoptysis.13 The extent to which the patient has experienced these symptoms or problems during the past week is rated as ‘not at all,’ a ‘little,’ ‘quite a bite,’ or ‘very much.’
The Brief Illness Perception Questionnaire (Brief IPQ) is a 9-item questionnaire that assesses cognitive and emotional representations of illness.14 It comprises 5 items on the cognitive representation of illness perception (consequences, timeline, personal control, treatment control, and identity), 2 items on emotional representation (concern and emotions), 1 item on illness comprehensibility, and 1 item on the perceived cause of illness, in which respondents list the 3 most important causal factors in their illness. The first 8 items are rated on a scale from 0 (minimum) to 10 (maximum), and summing up produced the total score ranges from 0 to 80.
The MGLS-4 includes 4 questions with elements of forgetfulness and symptom severity, which are answered in a yes/no format. The score ranges from 0 to 4, with 0 indicating perfect adherence and a score equal to or greater than 1 indicating some degree of non-adherence.15
The SATMED-Q is a self-administered tool with 17 items that evaluates treatment satisfaction in six dimensions: treatment effectiveness, convenience of use, impact on daily activities, medical care, global satisfaction, and undesirable side effects.16 A global score for satisfaction with drug treatment can be obtained by summing the scores of all domains, with scores ranging from 0 to 68, with higher scores indicating higher satisfaction.
PRO-CTCAE is a PRO measurement system developed by the National Cancer Institute of the USA to evaluate symptomatic toxicity in patients in cancer clinical trials. It comprises 124 items representing 78 symptomatic toxicities drawn from the CTCAE. The investigator evaluated the attributes of frequency, severity, interference, amount, and presence/absence (each symptomatic adverse event was assessed by 1–3 attributes depending on the symptom), and 21 toxicities were selected for this study.
Statistical analysisSample size has been calculated assuming a finite population (N=19 141 cases of NSCLC estimated for Spain17), maximum data variability among the participants (p=q=0.5), and a precision error of 10% with a 95% level of confidence. The maximum variability criterion is generally assumed when the final objective is to estimate the proportions.
We applied the following formula18:
Where N is the size of the study population (N=19 141); Zα is 95% of the normal distribution (Zα=1.96); p=q=0.5; e is the precision error (10%).
Under these assumptions, and considering a 10% of losses, the sample size is 106 subjects (18–22 patients/hospital).
The primary outcome is the percentage of treatment adherence that will be calculated based on the pill count as follows: the difference between the number of pills dispensed minus the number of unused pills will be divided by the number of days of treatment multiplied by the number of pills/day prescribed by the oncologist; this quotient will be multiplied by 100 to obtain the percentage of adherence. Those with a percentage adherence >80% will be primarily categorized as adherent. The percentage of treatment adherence will be presented using the mean and standard deviation and the median and interquartile range.
Secondarily, adherence based on a cut-off value of 90% for the pill count will be also calculated. Treatment adherence will be also calculated based on the proportion of days covered (PDC) and the MGLS-4. PDC will be calculated as the number of days the patient was covered by the drug based on the prescription fill date and days of supply divided by the number of days of the treatment period (counted as the number of the index prescription date until the end of the study, study discontinuation, or death); the resultant quotient be multiplied by 100. Patients will be considered adherent if the PDC is ≥80%. Patients will be considered adherent according to the MGLS-4 if the score is 0. The proportion of adherent patients according to dichotomous criteria for each cut-off point of the pill count, PDC, and MGLS-4 will be presented using absolute and relative frequencies with the corresponding 95% confidence interval.
The adherence results will be reported for the overall sample and the following subgroups: first- and second-generation EGFR-TKIs (Erlotinib, Afatinib, and Gefitinib), third-generation EGFR-TKIs (osimertinib), and ALK inhibitors (Alectinib, Crizotinib, and Lorlatinib).
To analyze the impact of patients' and treatment characteristics on adherence, bivariate analyses will be performed using the different cut-off points for adherence to pill count, PDC, and MGLS-4, quantitative variables will be compared using the Student’s t-test or a non-parametric test, and qualitative variables will be compared using the chi-square test. To evaluate the potential relationship between adherence and some PROs, such as health-related quality of life, disease perception, treatment satisfaction, toxicity perception, and health resource utilization, bivariate analyses will be performed in a similar manner. Agreement between the 3 measures of adherence will be evaluated using Fleiss' kappa index. Finally, to evaluate the impact of adherence (i.e., being treatment adherent as defined above) on treatment efficacy as evaluated by progression-free survival, we will be using the Kaplan–Meir method and compare it with the log-rank test and univariate Cox regression analysis.
Statistical analyses will be performed using IBM SPSS Statistics 26.0 or a subsequent version. All comparisons will be considered significant if p<.05.
DiscussionThe increasing availability and consumption of OCT will significantly impact all aspects of oncology care and affect the roles of healthcare professionals involved in patient care.3 For the oncologist, there will be a reduced burden in terms of office visits, but an increased need for coordination with other professionals, such as the hospital pharmacist, who will be increasingly involved in safety monitoring and monitoring treatment adherence.3 This role of the hospital pharmacist is already consistent with the perception of some patients with cancer receiving OCT, who consider the hospital pharmacy service as a facilitator in accessing treatment and obtaining in-depth information on treatment and its side effects.19 In addition, some traditional roles and responsibilities of oncologists, nurses, and pharmacists will be shifted to patients and caregivers.3 It is also likely that part of the ongoing assessment of symptoms, toxicity, and adherence will need to be performed or enhanced remotely.1,6
Our study has several limitations. It is being conducted in a country where chemotherapy is reimbursed, and the cost of medications/copayment has been identified as a factor affecting adherence to OCT.20 Therefore, when interpreting the factors associated with adherence in our study, they will not be fully representative of other settings. To evaluate the impact of adherence on treatment efficacy as evaluated by progression-free survival, we will be using the 3-month initial period for defining adherence and not the whole study period.
Both patients and healthcare professionals involved in the management of OCT consider that adequate provision of information to patients is a key facilitator, and that toxicity is a key barrier to adherence.19 Despite the paucity of robust data, consistent with other authors, we think that the hospital pharmacist plays a key role in monitoring and improving adherence to OCT and overall improving OCT management.19 We expect that our study will provide initial information on key aspects of adherence to OCT (i.e., measurement, facilitators, and barriers) and its relationship with patients' and clinically relevant outcomes in the setting of NSCLC, and that this information will help in designing pharmaceutical interventions to improve adherence.
Declaration of AuthorshipAll authors were responsible for the concept and design of the study, reviewed and approved the final version of the manuscript and are considered guarantors of it. All authors meet the authorship criteria detailed by the International Committee of Journal Medical Editors.
FundingThis study is funded by AstraZeneca Farmacéutica Spain. Editorial and writing support was provided by Dr. Fernando Rico-Villademoros of APICES, who was contracted and funded by AstraZeneca Farmacéutica Spain. AstraZeneca Farmacéutica Spain had the opportunity to review the manuscript from the point of view of medical and scientific accuracy, as well as possible intellectual property considerations.
AcknowledgementsThe authors thank Juan Luis Sanz and Susana Vara (APICES, Madrid, Spain) for their support with the study design, and Fernando Rico-Villademoros (APICES, Madrid, Spain) for writing a draft of this manuscript.
Ethical responsibilitiesThe protocol of this study has been approved by Ethics Committee for Research with Medicines from Galicia.
Conflicts of interestIrene Mangues-Bafalluy has been advisory board member for Seagen; has received Consulting honorarium from Janssen, Gilead; and has received Speaker honorarium from MSD, GSK.
Beatriz Bernárdez has received payments for lectures, attendance to congresses, travel to courses and congresses, advisory boards, participation in working groups, etc., in the last few years from: Astra Zeneca, BMS, Merck, MSD, Novartis, Astellas, Sanofi Pfizer Takeda, GSK, Pharmamar, Daiichi Sankyo, Amgen, Seagen, Ipsen.
José Manuel Martínez-Sesmero has received consulting honorarium from AZ, Pfizer, Janssen, UCB; and has received speaker honorarium from Roche and GSK.
Andres Navarro-Ruiz has received consulting honorarium from Astra, UCB; and has received speaker honorarium from Roche.
Ana Rosa Rubio-Salvador has been advisory board member for GSK; has received consulting honorarium from Janssen, Lilly, AMGEN; and has received speaker honorarium from MSD, GSK.
Maria Teresa Martín-Conde has no conflict of interest.
CRediT authorship contribution statementIrene Mangues-Bafalluy: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. Beatriz Bernárdez: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. José Manuel Martínez-Sesmero: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. Andres Navarro-Ruiz: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. Ana Rosa Rubio-Salvador: Writing – review & editing, Writing – original draft, Investigation, Conceptualization. Maria Teresa Martín-Conde: Writing – review & editing, Writing – original draft.