To present results of the 2022 SEFH-Survey on Spanish Hospital Pharmacy Departments covering care, staffing, resources, technology, education, and research.
MethodsA cross-sectional descriptive study via a voluntary online survey sent to 353 hospitals in Spain. Data were collected from July–December 2022. Long-stay hospitals and correctional facilities were excluded.
ResultsResponse rate was 54.1%. Public hospitals represented 62.6%. Only 10.1% of departments operated 24/7, rising to 39.3% in larger hospitals. Half lacked continuous care service. Outpatients' services operated in mornings and afternoons in 54.8% of them (78.6% in larger centers). Telepharmacy for outpatients reached 57.7% of hospitals. ISO 9000 standards were followed by 70.7% and 14.4% adopted the Joint Commission model.
Each department had on average 7.0 specialist pharmacists (8.8 in public; 3.9 in private), increasing to 13.4 in larger hospitals. Of these, 3.8 pharmacists worked at least half-time in clinical units. Pharmacy technicians were the most common non-pharmacist professionals (mean: 6.9). Including residents, 9.936 professionals worked in Hospital Pharmacy Departments nationwide.
Automated dispensing carousels averaged 0.4 (horizontal) and 1.1 (vertical) per department. Automated dispensing systems covered 19.8% of beds. Robotic outpatient dispensing existed in 20.0% of hospitals. Technology for sterile workflow was used in 45.3%, 10.0% had robotics for cytostatic compounding and 61.7% smart infusion pumps.
Pharmaceutical care was provided in emergency services in 39.8% of hospitals, rising to 67.4 in larger ones. In home hospitalization, it was offered at 32.5% of departments, rising to 60.7% in centers with over 1000 beds.
Sterile formulations were prepared in 82.3% of departments; 15.7% managed advanced therapies. Drug level monitoring was measured in 16.1%, and 43.1% issued pharmacokinetics reports. Pharmacogenetic reports were produced in 8.7%.
On average pharmacy departments attended 3.635 outpatients, totaling 1,28 million nationwide. Cytostatic preparations averaged 31,199 and 46,263 in hospitals with over 500 and 1000 beds, respectively. Clinical trials per department averaged 424.
A total of 321 pharmacists were associate university professors, 401 held board certifications, and there were 2.3 PhD holders per department.
ConclusionHospital Pharmacy Departments are advancing in clinical integration, pharmacokinetics, automation, traceability, and outpatient care, though staffing remains limited and disparities persist. Teaching is strong, yet research remains modest.
Presentar los resultados de la Encuesta SEFH-2022 sobre los Servicios de Farmacia Hospitalaria en España, incluyendo actividad asistencial, personal, recursos, tecnología, docencia e investigación.
MétodosEstudio descriptivo transversal mediante encuesta online voluntaria enviada a 353 hospitales en España. Recogida de datos: julio-diciembre 2022. Se excluyeron centros de larga estancia y penitenciarios.
ResultadosTasa de respuesta: 54,1%. El 62,6% centros públicos. El 10,1% de los servicios ofrecía atención 24/7, alcanzando el 39,3% en hospitales grandes. 50% con atención continuada. 54,8% con atención ambulatoria en turno de mañana y tarde (78,6% en hospitales grandes). El 57,7% disponía de telefarmacia. El 70,7% aplicaba normas ISO 9000 y el 14,4% el modelo Joint Commission.
Cada farmacia contaba con una media de 7,0 farmacéuticos especialistas (8,8 en públicos; 3,9 en privados), alcanzando 13,4 en hospitales grandes. De ellos, 3,8 trabajaban al menos media jornada en unidades clínicas. Los técnicos de farmacia representaban la categoría no facultativa más común (media: 6,9). En total, 9.936 profesionales trabajaban en las farmacias a nivel nacional, incluidos residentes.
Disponían de 0,4 carruseles horizontales y 1,1 verticales por farmacia. Los sistemas automatizados de dispensación cubrían el 19,8% de las camas. El 20,0% tenía robotización ambulatoria. Un 45,3% usaba tecnología para preparación estéril, un 10,0% robótica para citostáticos y un 61,7% bombas inteligentes.
La atención farmacéutica en urgencias se prestaba en el 39,8% de los centros (67,4% en grandes) y en hospitalización a domicilio en el 32,5% (60,7% en hospitales de >1.000 camas). El 82,3% elaboraba preparados estériles, el 15,7% gestionaba terapias avanzadas, el 16,1% monitorizaba niveles plasmáticos, el 43,1% emitía informes farmacocinéticos y el 8,7% farmacogenéticos.
Cada servicio de farmacia atendía a 3.635 pacientes externos (1,28 millones a nivel nacional). Las preparaciones citostáticas eran 31.199 y 46.263 en hospitales de >500 y >1.000 camas, respectivamente. La media de ensayos clínicos fue de 424. Había 321 profesores asociados, 401 farmacéuticos acreditados por el Board, y 2,3 doctores por servicio.
ConclusiónLos Servicios de Farmacia Hospitalaria progresan en integración clínica, automatización y atención ambulatoria, aunque persisten desigualdades y limitaciones de personal. La docencia es sólida; la investigación, aún mejorable.
Over the past decades, Hospital Pharmacy Departments (HPDs) in Spain have undergone significant growth and transformation, driven by the increasing demand for specialized pharmaceutical care and the dedication of hospital pharmacists. Notable recent advancements include the integration of pharmacists into clinical teams, the adoption of advanced technologies not only in logistics and dispensing but also in processing and traceability, as well as the pharmacist's leadership in clinical pharmacokinetics and pharmacogenetics.1–3 More recently, pharmacists have also taken on a key role in the management of advanced therapies.4
Surveys by the Spanish Society of Hospital Pharmacists (SEFH) have assessed these specific developments. In 2015, the Report on the Situation of Hospital Pharmacy Services in Spain: Infrastructure, Resources, and Activities, known as the White Paper on Hospital Pharmacy, was published. Its goal was to inform the administration, society, and pharmacy services about the scope and characteristics of hospital pharmacy as a specialty, focusing on healthcare, technology, education, and research. The report highlighted the high level of development of hospital pharmacy in Spain while identifying areas for improvement and strategies for growth.5
The most relevant findings of the second edition, published in 2019 in two issues of the Journal of Farmacia Hospitalaria highlighted the limited number of hospital pharmacists in Spain, despite their growing involvement in clinical units. Progress in logistics automation was noted, but there remains significant potential for improvement in robotic systems and preparation traceability. Non-hospitalized patient care and medication preparation received considerable attention, while clinical pharmacokinetics lagged behind. Despite a strong commitment to teaching, scientific output remained limited, even with an annual increase in PhD-trained professionals.6,7
Given the value of previous editions, a third survey was conducted in 2022 with a similar focus on infrastructure, resources, professional development, and activities. This edition introduced an interactive approach, allowing participants to review their results and compare them with other hospitals.
The purpose of this article is to present the results of SEFH's 2022 national survey and to discuss the evolution of Spanish Hospital Pharmacy Departments, in terms of healthcare, staffing, material resources and technology, information systems, education and research.
MethodsDesign and methodsA cross-sectional descriptive study was conducted by SEFH through a voluntary online survey addressed to the heads of HPD across Spain. Long-stay hospitals, socio-healthcare facilities and correctional institutions were excluded due to their specific characteristics.
Questionnaire designThe survey was based on the version published in 2020 by the SEFH Board of Directors (LB 2019), updated and formatted for online completion. This allowed automatic data collection and easier subsequent analysis. The same working group that revised the questionnaire also validated it by applying it to their own hospitals and making the necessary adjustments to ensure clarity and usability.
The questionnaire included an introductory section outlining the voluntary nature of participation, the response deadline, and completion instructions. Each hospital was to submit one response based on its situation in 2022, except for section six, which refers to quantitative activity in 2021. The survey comprised 53 questions across six sections: (1) hospital and HPD characteristics, including quality and accreditations; (2) human resources; (3) material resources; (4) information systems; (5) training and research; (6) services portfolio and activities.
Open-ended questions were used for general descriptions and comments, while most items were closed-ended with binary (yes/no) or single-choice predefined answers. Some questions allowed multiple selections. Quantitative indicators (resources, activity) were expressed as numbers or percentages. Conditional questions were included, appearing based on prior responses.
Survey distributionThe online questionnaire was sent by SEFH to HPD heads listed in the SEFH member directory and widely disseminated throughout all Spanish regions via SEFH regional delegates.
Data collection took place from July to December 2022. Email reminders were sent in late September to non-respondents.
Data analysisParticipation and representativeness were weighted according to the SEFH member directory and the 2022 National Hospital Catalog (CNH) classification. Responses were adjusted for two variables: hospital ownership (public/private) and bed count, grouped into five strata defined by the CNH. Underrepresented hospitals were up-weighted to match their proportion in the general population, with a 3% margin of error. Weighting also accounted for item-specific response rates to correct for inconsistencies in sample representation.
Data were compiled and analyzed using IBM SPSS® Statistics (version 22.0). A descriptive analysis was performed for all responses. Qualitative variables were reported as frequency distributions, and quantitative ones as means, standard deviations, and other dispersion measures.
ResultsA total of 191 responses were received, resulting in a 54.1% response rate. Publicly owned hospitals accounted for 62.6% of the total, with most having 101–250 beds (26.2%). The regions most represented were Madrid and Catalonia (18.3% each) followed by the Basque country (9.0%).
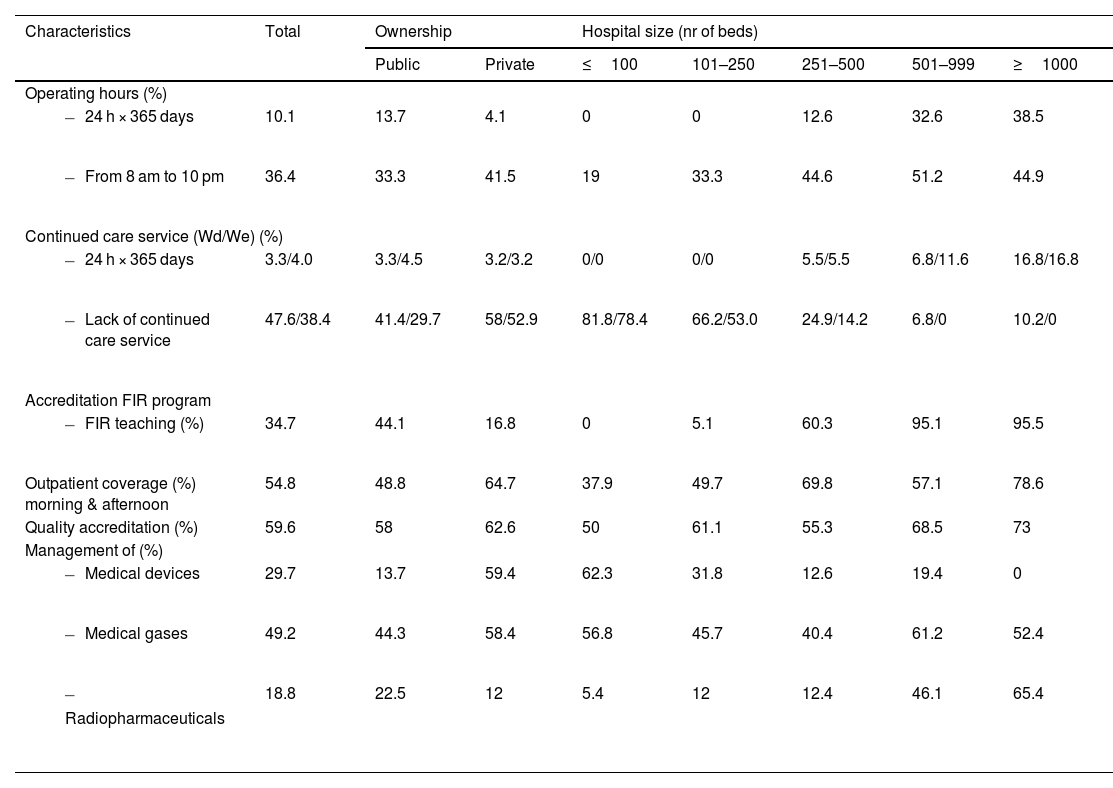
Hospital and HPD characteristics, quality and accreditationTable 1 summarizes the main characteristics of HPD, including operating hours, care models, teaching, accreditation, and management of special services. 10.1% of HPD remained open round the clock. 34.1% were open either in the morning or until 5 pm.
General characteristics of HPD, according to the criteria in the National SEFH 2022 Survey.
| Characteristics | Total | Ownership | Hospital size (nr of beds) | |||||
|---|---|---|---|---|---|---|---|---|
| Public | Private | ≤100 | 101–250 | 251–500 | 501–999 | ≥1000 | ||
| Operating hours (%) | ||||||||
| 10.1 | 13.7 | 4.1 | 0 | 0 | 12.6 | 32.6 | 38.5 |
| 36.4 | 33.3 | 41.5 | 19 | 33.3 | 44.6 | 51.2 | 44.9 |
| Continued care service (Wd/We) (%) | ||||||||
| 3.3/4.0 | 3.3/4.5 | 3.2/3.2 | 0/0 | 0/0 | 5.5/5.5 | 6.8/11.6 | 16.8/16.8 |
| 47.6/38.4 | 41.4/29.7 | 58/52.9 | 81.8/78.4 | 66.2/53.0 | 24.9/14.2 | 6.8/0 | 10.2/0 |
| Accreditation FIR program | ||||||||
| 34.7 | 44.1 | 16.8 | 0 | 5.1 | 60.3 | 95.1 | 95.5 |
| Outpatient coverage (%) morning & afternoon | 54.8 | 48.8 | 64.7 | 37.9 | 49.7 | 69.8 | 57.1 | 78.6 |
| Quality accreditation (%) | 59.6 | 58 | 62.6 | 50 | 61.1 | 55.3 | 68.5 | 73 |
| Management of (%) | ||||||||
| 29.7 | 13.7 | 59.4 | 62.3 | 31.8 | 12.6 | 19.4 | 0 |
| 49.2 | 44.3 | 58.4 | 56.8 | 45.7 | 40.4 | 61.2 | 52.4 |
| 18.8 | 22.5 | 12 | 5.4 | 12 | 12.4 | 46.1 | 65.4 |
Wd/We weekdays/weekends; FIR: farmacéutico Interno residente (pharmacy resident); HPD: hospital pharmacy department.
34.7% of HPD had pharmacy residency program, and 78.7% of hospitals with over 250 beds were accredited. 56.6% of HPD with teaching accreditation trained two residents per year, while 17.6% of larger hospitals trained three residents annually, accounting for 3.0% of all centers. The percentage of such hospitals with a (resident-staffed) continuing care module in place was 22.6%.
Afternoon outpatient coverage was more common in larger and privately owned hospitals.
ISO 9000 standards were applied in 70.7% of HPD, while 14.4% were accredited under the Joint Commission model.
The inclusion of medical devices, radiopharmaceuticals, and medical gases in HPD activities varied significantly by hospital ownership and size. Regarding the management of advanced therapies, 23.6% of hospitals reported involvement, rising to 89.2% in larger hospitals.
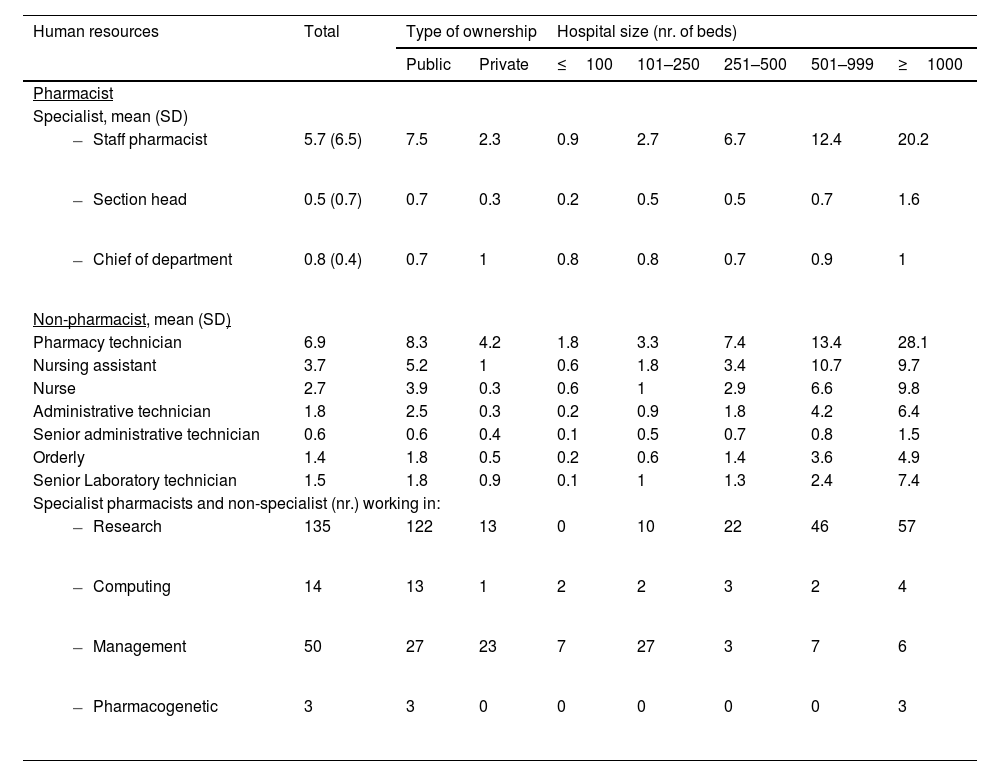
Human resourcesTable 2 summarizes the composition of human resources in HPD. The mean number of specialist pharmacists per department was 7.0 (8.8 in public hospitals and 3.9 in private hospitals), reaching an average of 22.8 in hospitals with over 1000 beds. A total of 15.8% of pharmacists held managerial roles. Regarding employment status, 27.5% were temporary civil servants, and 16.2% held fixed-term contracts. The ratio of statutory to contractual employment was 7:3. In terms of age distribution, 65.7% of pharmacists were between 30 and 50 years old, followed by 20.8% aged 51 to 60, and 5.3% over 61. In the private sector, 51.2% of pharmacists were under the age of 40.
Specialist pharmacists and non-pharmacist personnel working in HPD.
| Human resources | Total | Type of ownership | Hospital size (nr. of beds) | |||||
|---|---|---|---|---|---|---|---|---|
| Public | Private | ≤100 | 101–250 | 251–500 | 501–999 | ≥1000 | ||
| Pharmacist | ||||||||
| Specialist, mean (SD) | ||||||||
| 5.7 (6.5) | 7.5 | 2.3 | 0.9 | 2.7 | 6.7 | 12.4 | 20.2 |
| 0.5 (0.7) | 0.7 | 0.3 | 0.2 | 0.5 | 0.5 | 0.7 | 1.6 |
| 0.8 (0.4) | 0.7 | 1 | 0.8 | 0.8 | 0.7 | 0.9 | 1 |
| Non-pharmacist, mean (SD) | ||||||||
| Pharmacy technician | 6.9 | 8.3 | 4.2 | 1.8 | 3.3 | 7.4 | 13.4 | 28.1 |
| Nursing assistant | 3.7 | 5.2 | 1 | 0.6 | 1.8 | 3.4 | 10.7 | 9.7 |
| Nurse | 2.7 | 3.9 | 0.3 | 0.6 | 1 | 2.9 | 6.6 | 9.8 |
| Administrative technician | 1.8 | 2.5 | 0.3 | 0.2 | 0.9 | 1.8 | 4.2 | 6.4 |
| Senior administrative technician | 0.6 | 0.6 | 0.4 | 0.1 | 0.5 | 0.7 | 0.8 | 1.5 |
| Orderly | 1.4 | 1.8 | 0.5 | 0.2 | 0.6 | 1.4 | 3.6 | 4.9 |
| Senior Laboratory technician | 1.5 | 1.8 | 0.9 | 0.1 | 1 | 1.3 | 2.4 | 7.4 |
| Specialist pharmacists and non-specialist (nr.) working in: | ||||||||
| 135 | 122 | 13 | 0 | 10 | 22 | 46 | 57 |
| 14 | 13 | 1 | 2 | 2 | 3 | 2 | 4 |
| 50 | 27 | 23 | 7 | 27 | 3 | 7 | 6 |
| 3 | 3 | 0 | 0 | 0 | 0 | 0 | 3 |
HPD: hospital pharmacy department; SD: standard deviation.
Non-pharmacist staff included mainly pharmacy technicians, nursing assistants, and nurses. The total number of professionals nationwide in 2022 was 9936, with an average of 26.7 per HPD.
Concerning the number of pharmacists dedicating at least half of their working day to clinical activities, the average per HPD was 3.8. In larger hospitals, with over 500 and 1000 beds, the averages reached 6.5 and 12.4 pharmacists, respectively. The most common clinical areas were oncology (254 pharmacists), hematology (176), infectious diseases (116), and emergency and geriatric care, with 86 and 82 pharmacists, respectively.
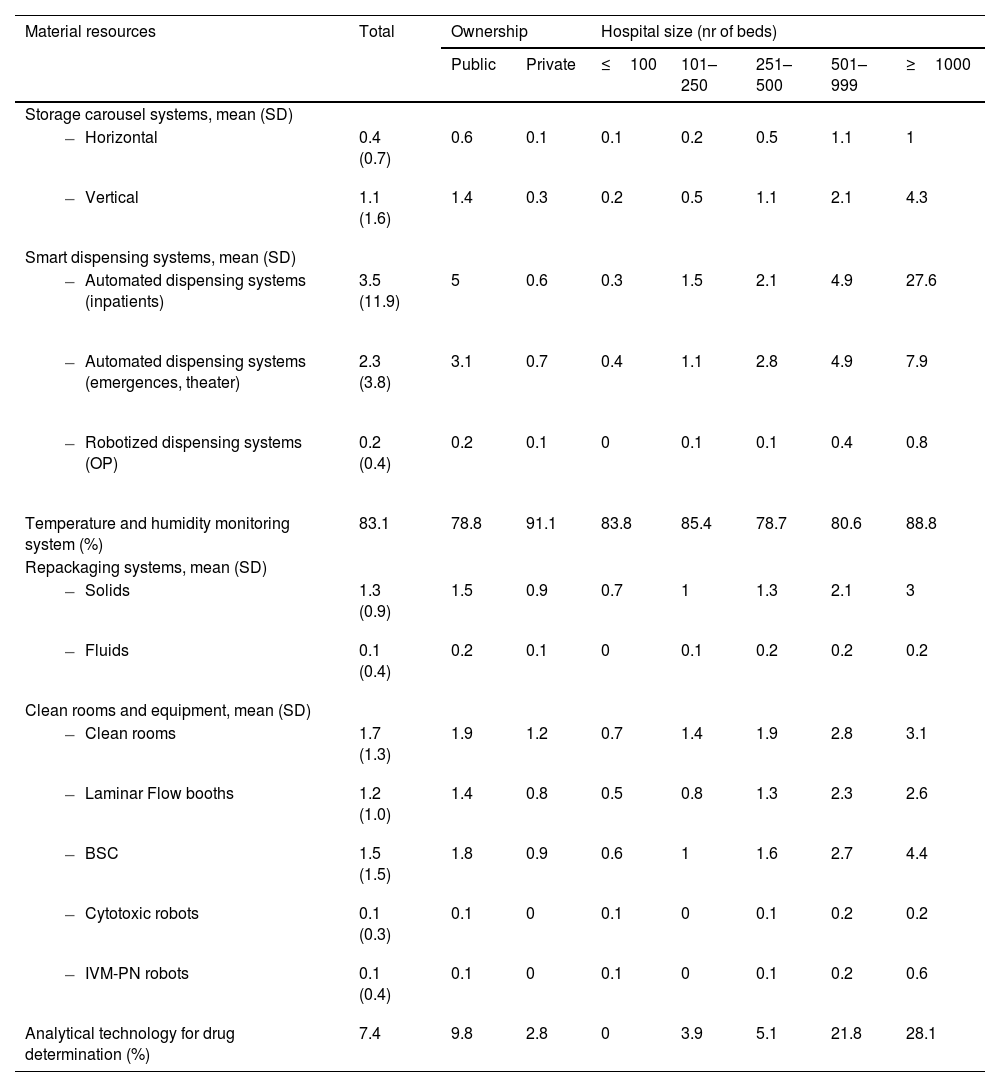
Material resourcesHPD reported the use of both horizontal and vertical automated carousel systems. Automated dispensing systems (ADS) were available for both inpatient and outpatient use, including in non-hospitalization areas such as emergency rooms and operating theaters. HPD reported the availability of clean rooms and laminar flow equipment, including biosafety cabinets. Robots for compounding cytotoxic and/or intravenous mixtures were available in 10.0% of HPD.
Various systems related to medication, traceability and safety were implemented across HPD. Linear barcode systems were more commonly used than 2D barcodes in all areas, particularly for outpatient dispensing (36.7%) and clinical trial sample management (20.9%). Traceability systems were most frequently applied to cytotoxic compounding (45.3%), followed by other sterile preparations (24.5%) and non-sterile compounding (30.5%). Smart infusion pumps were available in 61.7% of hospitals, with higher adoption in larger facilities. Further details on automation, traceability and safety-related technologies are presented in Table 3.
Material resources associated to the compounding and distribution of medications in Spanish HPD.
| Material resources | Total | Ownership | Hospital size (nr of beds) | |||||
|---|---|---|---|---|---|---|---|---|
| Public | Private | ≤100 | 101–250 | 251–500 | 501–999 | ≥1000 | ||
| Storage carousel systems, mean (SD) | ||||||||
| 0.4 (0.7) | 0.6 | 0.1 | 0.1 | 0.2 | 0.5 | 1.1 | 1 |
| 1.1 (1.6) | 1.4 | 0.3 | 0.2 | 0.5 | 1.1 | 2.1 | 4.3 |
| Smart dispensing systems, mean (SD) | ||||||||
| 3.5 (11.9) | 5 | 0.6 | 0.3 | 1.5 | 2.1 | 4.9 | 27.6 |
| 2.3 (3.8) | 3.1 | 0.7 | 0.4 | 1.1 | 2.8 | 4.9 | 7.9 |
| 0.2 (0.4) | 0.2 | 0.1 | 0 | 0.1 | 0.1 | 0.4 | 0.8 |
| Temperature and humidity monitoring system (%) | 83.1 | 78.8 | 91.1 | 83.8 | 85.4 | 78.7 | 80.6 | 88.8 |
| Repackaging systems, mean (SD) | ||||||||
| 1.3 (0.9) | 1.5 | 0.9 | 0.7 | 1 | 1.3 | 2.1 | 3 |
| 0.1 (0.4) | 0.2 | 0.1 | 0 | 0.1 | 0.2 | 0.2 | 0.2 |
| Clean rooms and equipment, mean (SD) | ||||||||
| 1.7 (1.3) | 1.9 | 1.2 | 0.7 | 1.4 | 1.9 | 2.8 | 3.1 |
| 1.2 (1.0) | 1.4 | 0.8 | 0.5 | 0.8 | 1.3 | 2.3 | 2.6 |
| 1.5 (1.5) | 1.8 | 0.9 | 0.6 | 1 | 1.6 | 2.7 | 4.4 |
| 0.1 (0.3) | 0.1 | 0 | 0.1 | 0 | 0.1 | 0.2 | 0.2 |
| 0.1 (0.4) | 0.1 | 0 | 0.1 | 0 | 0.1 | 0.2 | 0.6 |
| Analytical technology for drug determination (%) | 7.4 | 9.8 | 2.8 | 0 | 3.9 | 5.1 | 21.8 | 28.1 |
SD: standard deviation; OP: outpatients; BSC: biosafety cabinet; IVM-PN: intravenous mixtures and parenteral nutrition; HPD: hospital pharmacy department.
Electronic prescriptions were implemented in 95.0% of hospitals for hospitalized patients, reaching 57.7% for outpatients and 61.9% for day-hospital patients. The administration of medication was electronically recorded in 60.8% of hospitalized patients and 49.5% of day-hospital patients.
Telepharmacy for outpatients reached 57.7% of hospitals. In hospitals with more than 500 beds, this figure exceeded 91.0%. On average, 9.3% of outpatients were treated through this method, with ranges varying between 3.0% and 23.5%, depending on the hospital's size.
Teaching activities and researchTeaching activities in HPD were carried out at both undergraduate and specialized healthcare training levels. In 2021, the average number of agreements between universities and HPD was 1.3. The average number of pharmacy students completing supervised internships in HPD was 5.5. Regarding teaching staff, HPD had an average of 0.9 associate university professors, totaling 321 across the system. Additionally, 17 centers had staff university professors (0.2 on average in hospitals with more than 1000 beds), although there were currently no full professors in the field.
Regarding the Board of Pharmacy Specialties (BPS) certifications, 402 hospital pharmacists held at least one certification. Among them, 51.5% specialized in oncology, 21.6% in pharmacotherapy, and 14.7% in nutrition. The remaining 49 hospital pharmacists were certified in seven other specialties, including mental health, infectious diseases, pediatrics, and critical care, among others.
The number of research projects involving a hospital pharmacist as an investigator included 746 national projects and 33 international ones. Regarding the impact factor of publications, the overall average reached 29. On average, each HPD had 2.3 PhD holders. Public hospitals had an average of 2.7 PhD holders, while private hospitals had 1.3.
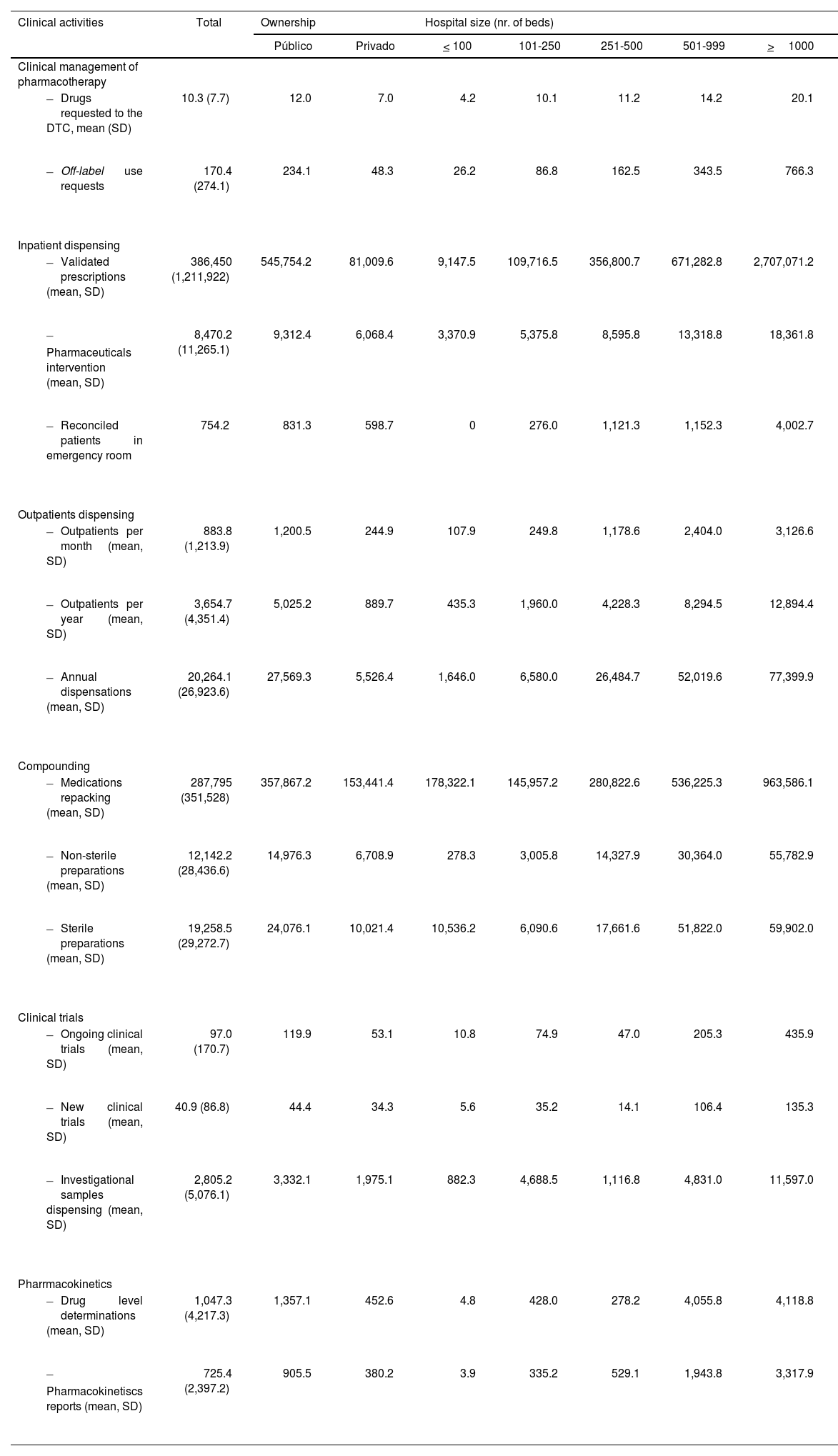
Portfolio/healthcare activityThe service portfolio activities for 2021, based on survey questions with a weighted response rate above 50%, are summarized in Table 4. Overall, 51.0% of HPD dispensed medications to home hospitalization units, a practice also widespread in the private hospitals (42.4%). The service was included in 100% of hospitals with more than 1000 beds and exceeded 65% in hospitals with more than 250 beds.
Clinical activity related to the portfolio of services of Hospital Pharmacy Services during the year 2021, according to the criteria of the SEFH-2021 National Survey.
| Clinical activities | Total | Ownership | Hospital size (nr. of beds) | |||||
|---|---|---|---|---|---|---|---|---|
| Público | Privado | < 100 | 101-250 | 251-500 | 501-999 | >1000 | ||
| Clinical management of pharmacotherapy | ||||||||
| 10.3 (7.7) | 12.0 | 7.0 | 4.2 | 10.1 | 11.2 | 14.2 | 20.1 |
| 170.4 (274.1) | 234.1 | 48.3 | 26.2 | 86.8 | 162.5 | 343.5 | 766.3 |
| Inpatient dispensing | ||||||||
| 386,450 (1,211,922) | 545,754.2 | 81,009.6 | 9,147.5 | 109,716.5 | 356,800.7 | 671,282.8 | 2,707,071.2 |
| 8,470.2 (11,265.1) | 9,312.4 | 6,068.4 | 3,370.9 | 5,375.8 | 8,595.8 | 13,318.8 | 18,361.8 |
| 754.2 | 831.3 | 598.7 | 0 | 276.0 | 1,121.3 | 1,152.3 | 4,002.7 |
| Outpatients dispensing | ||||||||
| 883.8 (1,213.9) | 1,200.5 | 244.9 | 107.9 | 249.8 | 1,178.6 | 2,404.0 | 3,126.6 |
| 3,654.7 (4,351.4) | 5,025.2 | 889.7 | 435.3 | 1,960.0 | 4,228.3 | 8,294.5 | 12,894.4 |
| 20,264.1 (26,923.6) | 27,569.3 | 5,526.4 | 1,646.0 | 6,580.0 | 26,484.7 | 52,019.6 | 77,399.9 |
| Compounding | ||||||||
| 287,795 (351,528) | 357,867.2 | 153,441.4 | 178,322.1 | 145,957.2 | 280,822.6 | 536,225.3 | 963,586.1 |
| 12,142.2 (28,436.6) | 14,976.3 | 6,708.9 | 278.3 | 3,005.8 | 14,327.9 | 30,364.0 | 55,782.9 |
| 19,258.5 (29,272.7) | 24,076.1 | 10,021.4 | 10,536.2 | 6,090.6 | 17,661.6 | 51,822.0 | 59,902.0 |
| Clinical trials | ||||||||
| 97.0 (170.7) | 119.9 | 53.1 | 10.8 | 74.9 | 47.0 | 205.3 | 435.9 |
| 40.9 (86.8) | 44.4 | 34.3 | 5.6 | 35.2 | 14.1 | 106.4 | 135.3 |
| 2,805.2 (5,076.1) | 3,332.1 | 1,975.1 | 882.3 | 4,688.5 | 1,116.8 | 4,831.0 | 11,597.0 |
| Pharrmacokinetics | ||||||||
| 1,047.3 (4,217.3) | 1,357.1 | 452.6 | 4.8 | 428.0 | 278.2 | 4,055.8 | 4,118.8 |
| 725.4 (2,397.2) | 905.5 | 380.2 | 3.9 | 335.2 | 529.1 | 1,943.8 | 3,317.9 |
DTC: drug therapy committee; SD: standard deviation.
The preparation of sterile and non-sterile compounds was carried out in 82.3% and 85.4% of HPD, respectively. Ophthalmic preparations were conducted in all hospitals with more than 250 beds. Advanced therapy medications were prepared or packaged in 15.7% of HPD, increasing to 50.5% in hospitals with more than 1000 beds.
PC for inpatients exceeded 75% of patients in 60.6% of hospitals. For outpatients, 66.3% of hospitals with more than 1000 beds reported providing PC to over 75% of patients. Regarding day hospital patients, a compliance rate of 53.6% was observed in hospitals with 251–500 beds.
In emergency settings, 39.8% of hospitals provided PC to patients who visit and remain in the emergency department. Among larger hospitals, this figure rose to 55.8% in those with 501–999 beds and to 67.4% in hospitals with more than 1000 beds. Overall, 14.1% of HPD provided PC to more than 50% of the patients.
Pharmacogenetic reports were produced in 8.7% of HPD, with the highest involvement in hospitals with more than 500 and 1000 beds (19.4–39.3%, respectively).
Medication safety activities, including the detection and reporting of adverse drug reactions and medication errors, were regularly performed in 33.3% and 58.9% of HPD, respectively.
In 2021, a total of 59,132 off-label drug use requests were processed. Additionally, 134,777,159 treatment lines were validated for hospitalized patients, and pharmaceutical interventions reached 2,666,804. The average number of interventions in larger hospitals ranged between 12,950 and 20,414 annually.
Medication reconciliation at hospital admission or discharge was performed by HPD in 19.8% of total admissions, with a higher rate in private hospitals (26.0%) compared to public ones (16.1%). Provision of discharge medication information remains very limited, covering only 1.2% of all hospital discharges. Hospitals with 501–1000 beds and those with more than 1000 beds reported average rates of 2.3% and 5.4%, respectively.
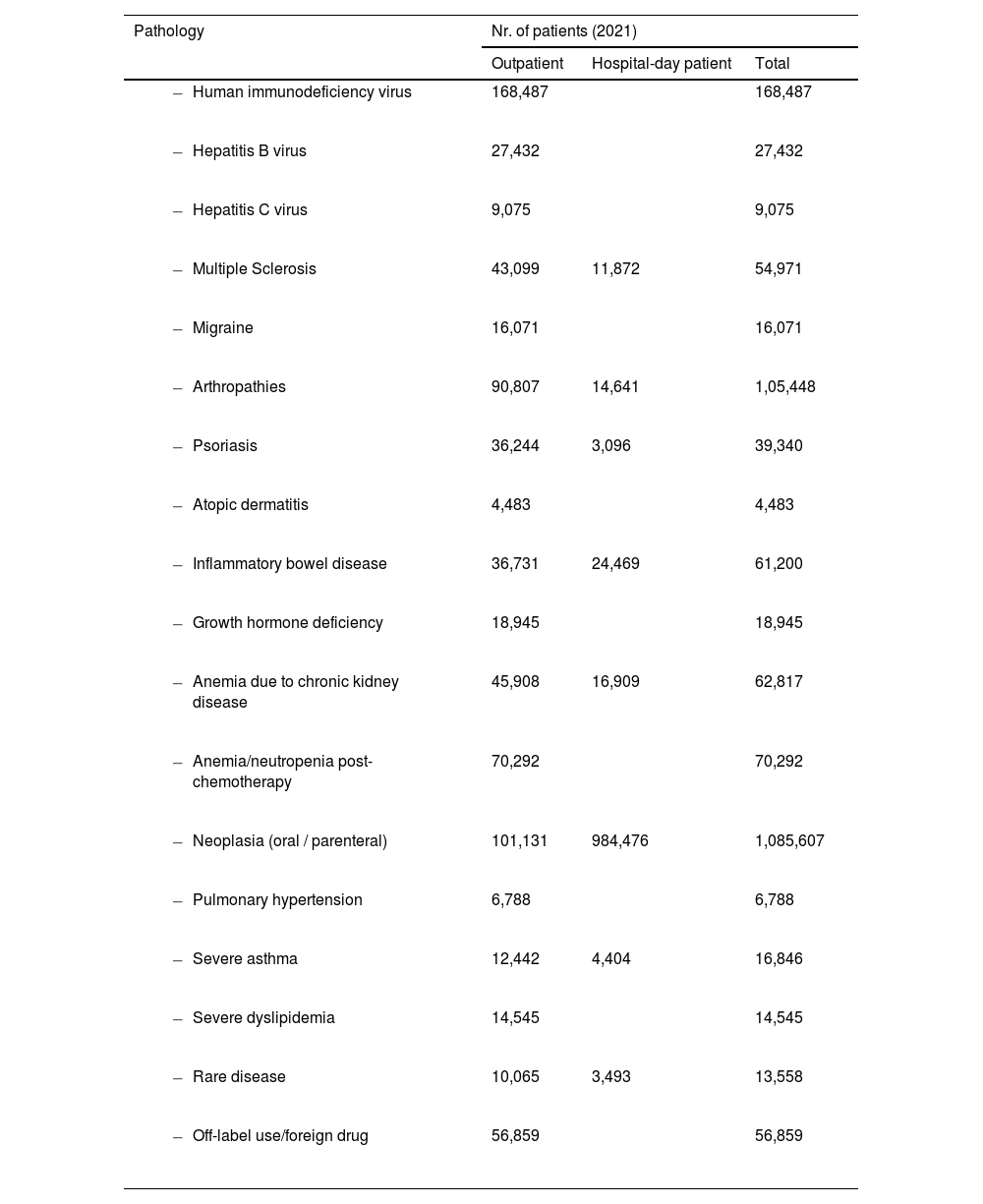
HPD attended and dispensed medications to 1,279,408 outpatients, with an annual total of 7,031,619 dispensed medications. Outpatient care in the private sector accounted for 12.59% of total patients. The number of patients treated in HPD, both outpatients and those in day hospitals, is summarized in Table 5.
Outpatients and Hospital-day patients treated in HPD by pathology during the year 2021.
| Pathology | Nr. of patients (2021) | ||
|---|---|---|---|
| Outpatient | Hospital-day patient | Total | |
| 168,487 | 168,487 | |
| 27,432 | 27,432 | |
| 9,075 | 9,075 | |
| 43,099 | 11,872 | 54,971 |
| 16,071 | 16,071 | |
| 90,807 | 14,641 | 1,05,448 |
| 36,244 | 3,096 | 39,340 |
| 4,483 | 4,483 | |
| 36,731 | 24,469 | 61,200 |
| 18,945 | 18,945 | |
| 45,908 | 16,909 | 62,817 |
| 70,292 | 70,292 | |
| 101,131 | 984,476 | 1,085,607 |
| 6,788 | 6,788 | |
| 12,442 | 4,404 | 16,846 |
| 14,545 | 14,545 | |
| 10,065 | 3,493 | 13,558 |
| 56,859 | 56,859 | |
The average number of cytotoxic mixtures per HPD was 14,607 (SD: 16,671). In larger hospitals, those with more than 500 and 1000 beds, the average of cytostatic preparations was 31,199 and 46,263, respectively. Other hazardous non-cytostatic mixtures totaled 957,500.
The preparation of ophthalmic formulations stood out, with 1,116,597 units prepared in 2021. Hospitals with 501 to 999 beds averaged 8,171 such preparations. Adult parenteral nutrition mixtures totaled 688,819. In larger hospitals – those with 501 to 999 beds and those with over 1000 beds – the average number of preparations were 5030 and 8908, respectively. Pediatric parenteral nutrition preparations amounted to 119,340, with 28.8% produced in the private hospitals.
Pharmaceutical forms that were repackaged and unit-dose conditioned amounted to 94,615,751. The number of ongoing clinical trials, new trials, and investigational drug sample dispensations in 2021 was 33,126, 14,137, and 1,009,867, respectively.
HPD issued 271,198 clinical pharmacokinetics reports. Larger hospitals reported an average of 3,399 reports.
DiscussionThe last SEFH National Survey in 2019, which had a high response rate, demonstrated the strong engagement and sense of belonging among Spanish HPDs since the first survey in 2014.5–7 This commitment persisted in 2022, with a similar response rate exceeding 50%. Other pharmacist surveys since 2021, whether by SEFH or other scientific societies, reported lower response rates between 14% and 36%.8–12 Our current survey received the same response rate from public and private hospitals.
Effective HPD performance requires adequate staffing, infrastructure, and technology. Only 10% operated 24/7, unchanged from 2019, and nearly half lacked a continuing care module—also consistent with previous data.6 Nearly all hospitals in the sample provided outpatient care and dispensation, which contrasts with the model in the United States (U.S), where outpatients medication supply is completely different.12,13 In the ASHP 2022 National Survey, pharmacists worked in ambulatory or care clinics in 51.6% of hospitals with outpatient services, ranging from 27.0% to 90.9% depending on hospital size.12 In our sample, all HPDs operated at least five days a week, and over 55% offered care both morning and afternoon, over 30% more than in 2019.6
A strong interest in continuous improvement was evident, with a 13% increase in HPD accredited to a quality standard compared to 2019.6 ISO 9000 certifications were the most common, achieved by over two-thirds of HPD. Notably, nearly 15% obtained Joint Commission International accreditation in 2021, underscoring pharmacists' strong commitment to medication and patient safety in Spanish hospitals.14
Management of medical devices by HPD has remained stable since 2019,6 not exceeding 15% in public hospitals. In contrast, medical gas management increased by nearly 18%, reflecting greater public hospital involvement, as also seen with radiopharmaceuticals. Notably, almost one in four HPDs reported participation in advanced therapies—a rapidly expanding area. Marzal-Alfaro et al. underscore the pharmacist's key role in CAR-T therapy, ensuring regulatory compliance, treatment efficacy, and patient safety within multidisciplinary teams.15 Although the pharmacist-to-100-bed ratio is still used as a reference indicator, it is not the most accurate measure of HPD activity. Similarly, metrics like the number of outpatients treated or compounded preparations fall short, as what truly matters are health outcomes and patient satisfaction. The data from our survey, analyzed in this study, reveal an increase in the number of specialist pharmacists per HPD, with the current average standing at 7, representing a 32% increase compared to 2019. There has been an increase across all types of hospitals, regardless of ownership or size. Notably, hospitals with 501 to 999 beds have experienced the most significant growth with their specialist pharmacist workforce doubling.6 Despite these improvements, a considerable gap remains compared to the U.S., where in 2022 the average was 16.9 full-time pharmacists per 100 occupied beds. However, 4.7% of pharmacist positions and 12.3% of pharmacy technician posts remained unfilled due to workforce shortages.12 Regarding non-pharmacist staff in HPD, pharmacy technicians are the most common profile, with a nearly 75% increase. This contrasts with 2019, when nursing care assistants were the largest group. The American survey mentioned referred to a mean of 16.1 for pharmacy technicians.16 In 2022, the total number of professionals working in HPD nationwide, including resident pharmacists, reached 9936, marking 28% compared to 2019.6
The integration of pharmacists into multidisciplinary hospital teams is a strategic priority for Spanish HPD and professional societies due to its impact on optimizing medication management.17 This approach has also been highlighted by the Independent Authority for Fiscal Responsibility (AIReF), which recommends strengthening the role of hospital pharmacists in clinical teams to enhance the efficiency and safety of pharmacological treatments and to ensure patient-centered pharmaceutical care.18 The current survey shows a notable increase – over 50% - with an average of 3.8 pharmacists per HPD spending at least part of their time in clinical units. They are primarily assigned to oncology, hematology, infectious diseases, emergency, and intensive care, in that order. However, there has also been notable integration into other areas, such as cardiology, and, especially, home hospitalization, with 35 pharmacists joining the latter in 2022.
A 2021 study assessing clinical pharmacy services in Germany, with a 34% response rate, found that 63% of responding HPD provided some clinical care. On average, these hospitals had 2.4 full-time equivalent (FTE) clinical pharmacists, ranging from 0.2 to 22.0 FTE. However, when extrapolated to all surveyed hospitals, the integration rate fell to 22%. Unlike in Spain, where pharmacist is more involved in oncology/hematology, in Germany, they primarily work in surgical and intensive care units.19 In Spain, we are gradually moving to U.S. model, with increasing pharmacists' involvement increasingly not only in oncology and hematology, but also across other medical and surgical specialties.13
According to our survey, 66% of pharmacists are between 30 and 50 years old, and only 6% are over 60. In contrast to the aging trend among European healthcare professionals—40% of whom are over 50, with one-third retiring by 2025—Spanish hospital pharmacists show a notably younger profile. This reflection aligns with the findings of Negro-Vega et al., who describe the coexistence of four generations in hospital pharmacy services, with Generation Y being the most represented.20
A key focus for HPD over the past two decades has been the automation of dispensing, compounding, traceability, and pharmaceutical care for both inpatients and outpatients. A major advancement is the widespread adoption of semi-automatic carousels, especially vertical ones, now present in nearly all HPD. The number of beds managed through ADS has also grown, covering one in five beds overall—and one in two in larger hospitals. Most centers have also implemented ADS in non-hospitalization units. Robotic systems for outpatient dispensing have doubled in presence since 2019, now reaching 70% of HPD with over 1000 beds.6 In comparison, the latest ASHP survey reports ADS as the primary distribution method in 74.5% of U.S. hospitals, a notably higher implementation than in Spain.21
One of the main functions of HPD is the preparation and centralization of sterile compounds within the hospital pharmacy. There has been an increase of 30% in clean rooms compared to 2019, particularly for chemotherapy or other intravenous drugs. Sterile preparations increased by over 80% since 2019, with growth across all categories. Chemotherapy preparations have doubled in the past four years.6 This trend highlights the need for drug compounding and preparation in day hospitals, and the shift toward centralization within HPD, with medications delivered ready-to-use to nursing units and patients.
Repackaging activity in 2021 reached over 100 million units, doubling the 2019 figure.7 It is essential to urge both the pharmaceutical industry and health authorities to promote unit-dose packaging—not only to address this challenge but also as a key strategy to optimize resources and enhance the efficiency, safety, and sustainability of pharmaceutical processes.
The adoption of robotic systems for compounding cytotoxic drugs, nutritional solutions, and other intravenous admixtures has grown from 3–4% to 10% over the last four years. Similarly, sterile workflow management technology in HPDs has increased significantly, nearly doubling the figures reported in 2019. However, traceability systems for preparing parenteral nutrition and other intravenous admixtures remained less widespread, although their implementation has also doubled in recent years.6,7 The latest ASHP survey indicates that 21.3% of hospitals use a workflow system, a lower percentage than in Spain. Implementation varies significantly by hospital size, like the trend observed in Spain, where 73% of larger hospitals have a traceability system, and 21.9% of hospitals with more than 600 beds in the U.S. do not use a workflow system. The use of smart infusion pumps has grown significantly, now reaching over 62% of hospitals, approaching the U.S. adoption rate.21
By 2019, remote pharmaceutical care was available in 15% of hospitals. However, partly due to the impact of the SARS-CoV-2 pandemic and the publication of a practical guide for the implementation and use of Pharmaceutical Care through telepharmacy-targeted at healthcare professionals and patients across different areas of application, its adoption has significantly increased.22 According to our study, 58% of the HPD have implemented telepharmacy, with approximately 10% of outpatients receiving treatment through this method.
The hospital pharmacy service portfolio includes actions focused on medications and patients, ensuring treatments are tailored to clinical needs. To achieve this, an HPD must provide pharmaceutical care services alongside drug selection, procurement, dispensing, and compounding. Among the activities related to medicine evaluation in the HPD, those involving special situations—mostly off-label use—stand out. This was carried out in nearly all HPD within our healthcare system, with increases exceeding 70% over four years.7 Off-label use, due to its frequency, is sometimes seen as a routine concept. Although present for many years, it remains relevant due to unmet clinical needs, posing challenges for professionals.
In the field of pharmaceutical dispensing, our latest survey highlights the involvement of HPD and pharmacists in home hospitalization units, with more than half of HPD participating. In over one-third of the centers, this involvement included PC, particularly in larger hospitals. Studies have demonstrated the benefits of multidisciplinary home hospitalization units, including reduced hospital stays and shorter treatment durations. In these models, pharmacists are fully integrated and play a key role in the pharmacotherapeutic process.23 A recent meta-analysis evaluated the safety and effectiveness of home hospitalization units based on the type of care model: early discharge with home follow-up, or admission avoidance through direct home care. The first model showed similar mortality and readmission rates to conventional hospitalization, but with shorter stays. The second indicated a trend toward lower mortality and costs, with similar or slightly reduced readmission rates.24 In the U.S., only 8.7% of hospitals reported offering hospital-at-home services.12
Although monitoring serum drug levels is a common practice for individualizing the dosing of certain medications, it did not appear widely implemented across most HPD. However, the survey showed progress in clinical pharmacokinetics, with increases of over 30% in HPD performing pharmacokinetic level determinations and over 45% in the dosage adjustment reports, compared to 2019.6,7 Compared with ASHP survey data,24 notable differences emerged: in more than half of U.S. hospitals, pharmacists have the authority to order drug level tests and other laboratory analyses. Regarding pharmacogenetics, the results showed that the number of HPDs issuing pharmacogenetic reports had doubled. In the case of our American counterparts, although progress was also noted, only 12% of hospitals carried out any pharmacogenetics-related activity from their HPD.25
Various organizations and institutions recognize the leadership of HPD as a key to the success of patient safety programs. In our context, Challenge No. 10 of the SEFH 2030 Project emphasizes the crucial role of the pharmacist in managing medication safety.17 As in previous surveys, the involvement of hospital pharmacists in medication safety activities has remained consistent, including the detection and reporting of medication errors and the communication of suspected adverse drug reactions. Furthermore, in 76% of public hospitals, pharmacists were members of the Clinical Safety Committee, compared to 55% in 2019.7 According to the 2022 report on safe medication use practices in Spanish hospitals, 56.2% of centers had systems for reporting medication errors and analyzing them through multidisciplinary teams, with the aim of improving processes and support professionals in performing their duties more safely.26
The survey also reflected a strong interest in gaining insight into all aspects of education and training within HPD, including undergraduate, postgraduate, and continuing education. Regarding undergraduate training, there was an increase in the number of agreements with universities to participate in the supervised internship program, as well as a rise in the number of students and associate faculty members, with the latter increasing by 10%.7
In healthcare professions, continuing education and professional development are essential to maintaining competencies and keeping knowledge up to date. The SEFH 2030 Project includes 20 challenges, one of which is to foster a culture of training and professional growth as key pillars of development. Among the many initiatives related to education and training, support for BPS certification stands out. Over the past four years,7 the number of certified pharmacists has increased by 25% with certification expanding into areas beyond oncology and nutrition, although it still represents only 16% of all specialist pharmacists. Spain leads Europe in the number of BPS-certified pharmacists and is an international benchmark in oncology pharmacy certification.27 The growth of subspecialties and the high number of certified professionals reflect the strong commitment of pharmacists to advancing their clinical expertise. BPS certification is expected to play a key role in enhancing pharmaceutical care and, ultimately, patient outcomes.28 In the United States, 30% of pharmacists maintained their BPS credentials unchanged between 2019 and 2022.12
Regarding the four-year specialized health training program, one in three HPD was accredited to offer this training. In 2022, nearly 20% of departments provided three residency positions per year. While in Spain, it is mandatory to be a board-certified hospital pharmacist to work in a public or private hospital pharmacy, in the U.S., only 32.8% of pharmacists have completed a PGY1 residency, and just 9.7% have completed a PGY2.12
Finally, the growing interest of hospital pharmacists in research was reflected in the 75% increase in the number of PhD holders in hospital pharmacists compared to 2019, with growth observed across all hospital types, regardless of size or ownership.7 Hospitals with more than 1000 beds now have an average of over seven PhD-trained professionals. This encouraging trend highlights the increasing academic and scientific engagement of hospital pharmacists. Although the overall impact factor remains modest, the steady rise in publications demonstrates a strong and sustained commitment to research.
The survey results in this study have certain limitations, including its voluntary nature and the complexity and length of the questionnaire. Some questions were difficult to interpret, requiring respondents to deduce their meaning. Comparisons with the 2019 survey should be made cautiously, as the HPD sample differed from that of the 2019 survey.6,7
In short, the data from the 2022 SEFH survey suggest that the number of specialist pharmacists in Spanish hospital pharmacy departments has increased, with a very positive trend in their integration into multidisciplinary teams. However, this integration is still insufficient, given the broad scope of medication management and the demonstrated value of hospital pharmacists. There has been continued progress in the automation of medication dispensing logistics, as well as in the implementation of traceability systems for the preparation of sterile medications—particularly in the field of chemotherapy—aimed at making the compounding process safer and more traceable.
Access to these results can be highly valuable for HPD and for SEFH, helping to define strategic action plans that address the key issues identified in the survey. SEFH remains committed to regularly updating this information as part of its ongoing efforts to monitor and promote the advancement of hospital pharmacy in Spain.
We used artificial intelligence (ChatGPT) to help improve the English language and grammar in this text. We reviewed and approved the final version.
Contribution to scientific literatureThis study provides updated data on the organization and development of Spanish hospital pharmacy departments in 2022, covering healthcare services, staffing, technology, education, and research. Stratification by hospital size and ownership offers relevant comparisons, highlighting key advances and persistent disparities. These results contribute to the scientific literature by serving as a benchmarking reference and a strategic planning tool for enhancing the quality, efficiency, and equity of hospital pharmacy services.
CRediT authorship contribution statementMontserrat Pérez-Encinas: Writing – review & editing, Writing – original draft, Validation, Supervision, Formal analysis, Data curation, Conceptualization. Eva Negro-Vega: Validation, Supervision, Methodology, Formal analysis, Data curation, Conceptualization. Cecilia M. Fernández-Llamazares: Writing – review & editing, Validation, Formal analysis, Data curation, Conceptualization.
Ethical considerationsEthics committee approval was not required, as no personal or clinical data were collected. Department heads were informed in advance about the study's purpose. Participation was voluntary and responses were submitted via a secure key-based system, ensuring confidentiality. Data were analyzed and presented in aggregate form.
FundingThis study was promoted by the Spanish Society of Hospital Pharmacists.
The authors declare no conflict of interest related to this work.
The authors would like to express our sincere gratitude to all the hospital pharmacy departments and professionals who participated in the survey, as well as SEFH's regional representatives for their assistance in spreading the word about the project and the SEFH staff as a whole for their support and encouragement. Their time, dedication, and valuable input made this study possible.