Preterm infants with total parenteral nutrition are at particular risk of developing carnitine deficiency with impaired tolerance of parenteral lipids. The objective was to review the scientific literature on potencial benefits of prophylactic L-carnitine administration in parenteral nutrition of preterm newborns.
MethodsSelected scientific articles in MEDLINE/PubMed, Scopus, The Cochrane Library, British Library EThOS and TESEO databases were assessed for this systematic review. The terms used as descriptors were «Total Parenteral Nutrition» and «Carnitine». Jadad scale was chosen to evaluate the quality of them.
Results18 out of the 93 references retrieved were selected for reviewing after applying the inclusion and exclusion criteria, 4 of them were discarded for being considered of low quality. Almost all studies agreed on the analytical variables measured (free carnitine and acylcarnitine, triglycerides, free fatty acids and ketone bodies). Other clinical variables such as weight gain, apnea, or lenght of stay at hospital were also considered.
ConclusionsThe present results prove that routine supplementation in the parenteral nutrition of preterm newborns may help to increase carnitine levels, but neither a relevant improvement in the lipid profile, or an increase in weight gain, or a decrease in morbimortality or reduction of hospital stay could be demonstrated. More studies are needed in preterm infants to know whether routine supplementation of L-carnitine in neonates requiring total parenteral nutrition for a long time would provide any clinical benefit.
Los recién nacidos pretérmino con nutrición parenteral total tienen tanto una reducción de la ingesta de L-carnitina como de las reservas tisulares, lo que podría suponer una peor tolerancia de los lípidos parenterales. El objetivo fue revisar la literatura científica en busca de los posibles beneficios clínicos de su administración en la nutrición parenteral.
MétodosRevisión sistemática de los documentos recuperados en las bases de datos MEDLINE/Pubmed, Scopus, The Cochrane Library, British Library EThOS y TESEO. Los términos utilizados como descriptores fueron «Total Parenteral Nutrition» y «Carnitine». La calidad de los artículos se evaluó mediante la escala de Jadad.
ResultadosTras aplicar los criterios de inclusión y exclusión, se seleccionaron para la revisión 18 artículos de las 93 referencias recuperadas, de los cuales 4 fueron descartados al no ser considerados de alta calidad. Casi la totalidad de los estudios coincidían en las variables analíticas medidas (carnitina libre y acilcarnitina, triglicéridos, ácidos grasos libres y cuerpos cetónicos). Además, en algunos se tenían en cuenta otras variables clínicas, como la ganancia ponderal o la apnea.
ConclusionesLa suplementación rutinaria en la nutrición parenteral de recién nacidos pretérmino sí parece mejorar los niveles plasmáticos de carnitina, pero sin llegar a demostrar una mejoría significativa en el perfil lipídico, ni aumento de la ganancia ponderal, ni disminución de la morbimortalidad o reducción de la estancia hospitalaria. Son necesarios más estudios para demostrar si la suplementación sistemática a recién nacidos pretérmino que requieren nutrición parenteral total durante más de un mes aportaría beneficios clínicos.
Carnitine (4-trimethyl-amino-3-hydroxybutyrate) is a dipeptide widely distributed in all mammal tissues, and particularly abundant in muscle tissue1, which is synthesized in the liver, kidneys and brain from two essential aminoacids, lysine and methionine. It appears as D and L isomer, and the latter is its biologically active form found in certain foods; and even though D-isomer is not, it is able to compete with the former for binding sites, which increases the risk of L-carnitine deficiency. It acts as a shuttle for long-chain fatty acids, facilitating their entry in the mitochondrial matrix for lipid β-oxidation and the subsequent production of energy2. To this aim, it binds with the activated fatty acid molecule (Acyl-CoA), generating acylcarnitine, and through a transporter enzyme of the internal mitochondrial membrane, it allows this molecule to get inside the mitochondria, where it is separated again so that the fatty acid will continue on its way and obtain adenosine triphosphate (ATP). Intracellular carnitine deficiency will deteriorate the ability to use fat as fuel. Specifically, it seems to limit lipid metabolism, leading to an increase in plasmatic triglycerides, fatty acids and ketone bodies (acetoacetic and β-hydroxybutyric acids), and therefore aminoacids would be used to satisfy the endogenous energy needs, as there would be an impact on the availability of energy non-originated in proteins, and this would affect new tissue growth formation3. There is no need in healthy children and adults for carnitine intake from food, as long as their liver, kidneys and brain are generating quantities enough to meet their daily needs. Some foods rich in this product are: red meat (particularly mutton), whey, fish, chicken, rice, bread, asparagus and avocados.
Digestive tract immaturity and frequent complications appearing during the first weeks of life will make it difficult to implement an enteral nutrition enough to meet the metabolic needs of the preterm newborn (PTNB); it will be required to adapt their energy and metabolic balance through parenteral nutrition (PN), which will often be required even for >1 month (prolonged PN: PPN). The L-carnitine reserves in a full-term newborn (FTN) are approximately 25-50% of those in adults4, and the reserves in PTNBs are even lower than those in FTNs5. Both breast milk and baby formula contain carnitine, though it is not usually added to PN solutions. For this reason, PTNBs on total parenteral nutrition (TPN) have both a reduction in carnitine intake and in tissue reserves; and given that they tend to be more demanding due to their rapid growth, it is not surprising that newborns fed with PN without supplements will reach very low carnitine levels after two weeks of life6.
In the past, PTNBs were usually kept on a strict diet and received calorie intakes quite below their energy requirements for a prolonged time, during some days or weeks, for fear of presenting metabolic complications derived of an early enteral nutrition or a rapid increase in macronutrients. Currently, enteral nutrition practices with more accelerated advancement are considered safe7, as well as an administration of parenteral nutrients earlier than in the past, and with a higher volume of lipids since the first day of life8. Lack of carnitine can be an etiological factor in the limited ability of preterm newborns to use parenteral lipids. In vitro studies have suggested that fatty acid oxidation will be irregular when levels of tissue carnitine are below 10% of their normal level9. The objective of this study was to review literature in search of the potential benefits of the prophylactic administration of L-carnitine in the PN of PTNBs, such as a potential improvement in lipid profile, increase in weight gain, reduction in morbimortality, reduction in hospital stay, or development of apnea of prematurity.
MethodsA descriptive study and critical analysis of the articles retrieved, through a systematic technique, from the following databases: MEDLINE/ Pubmed, Scopus, The Cochrane Library, British Library EThOS and TESEO (Doctoral Thesis Database of the Ministry of Education, Culture and Sport). It was decided to select for analysis those articles that met the following inclusion criteria: original documents, adequate to the search objectives (relationship between serum L-carnitine levels and an improvement in clinical parameters, such as a significant progress in the lipid profile, an increase in weight gain, or a reduction in hospital stay, among others), published in any country, by any institution or individual researcher, and in English or Spanish. The Medical Subject Headings (MeSH) developed by the U.S. National Library of Medicine was used to define the search terms. «Total Parenteral Nutrition» and «Carnitine» were considered adequate as descriptors (MeSH). The final search equation was developed through the use of boolean connectors for their use in the MEDLINE/Pubmed database, as follows: (“Parenteral Nutrition, Total”[Mesh] OR “Parenteral Nutrition Solutions”[Mesh]) AND “Carnitine”[Mesh] (English [lang] OR Spanish [lang]).
The same strategy was subsequently adapted to the characteristics of the remaining databases previously mentioned. The search was conducted from the first date available and until December, 2017. Besides, the bibliographic list of the articles selected was reviewed, in order to identify any studies undetected during the database review. Those articles with a study population other than PTNBs were excluded, as well as any articles that were not original (exclusion criteria).
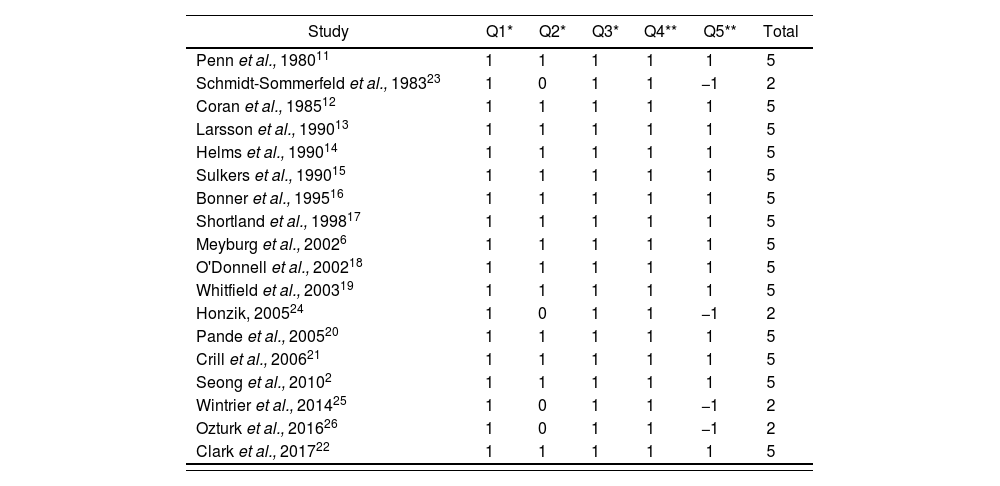
Article selection was conducted independently by two of the authors of this present review. Any discrepancies detected were solved through discussion, and in case that consensus was not reached, a third evaluator was asked to participate. The methodological quality of the studies was analyzed through the Jadad Scale or Oxford Quality Scoring System, a critical reading tool with 5 questions associated with clinical trial analysis, which classifies the study as of low quality if its score is below 3, and considers rigorous a randomized clinical trial with a score of 510.
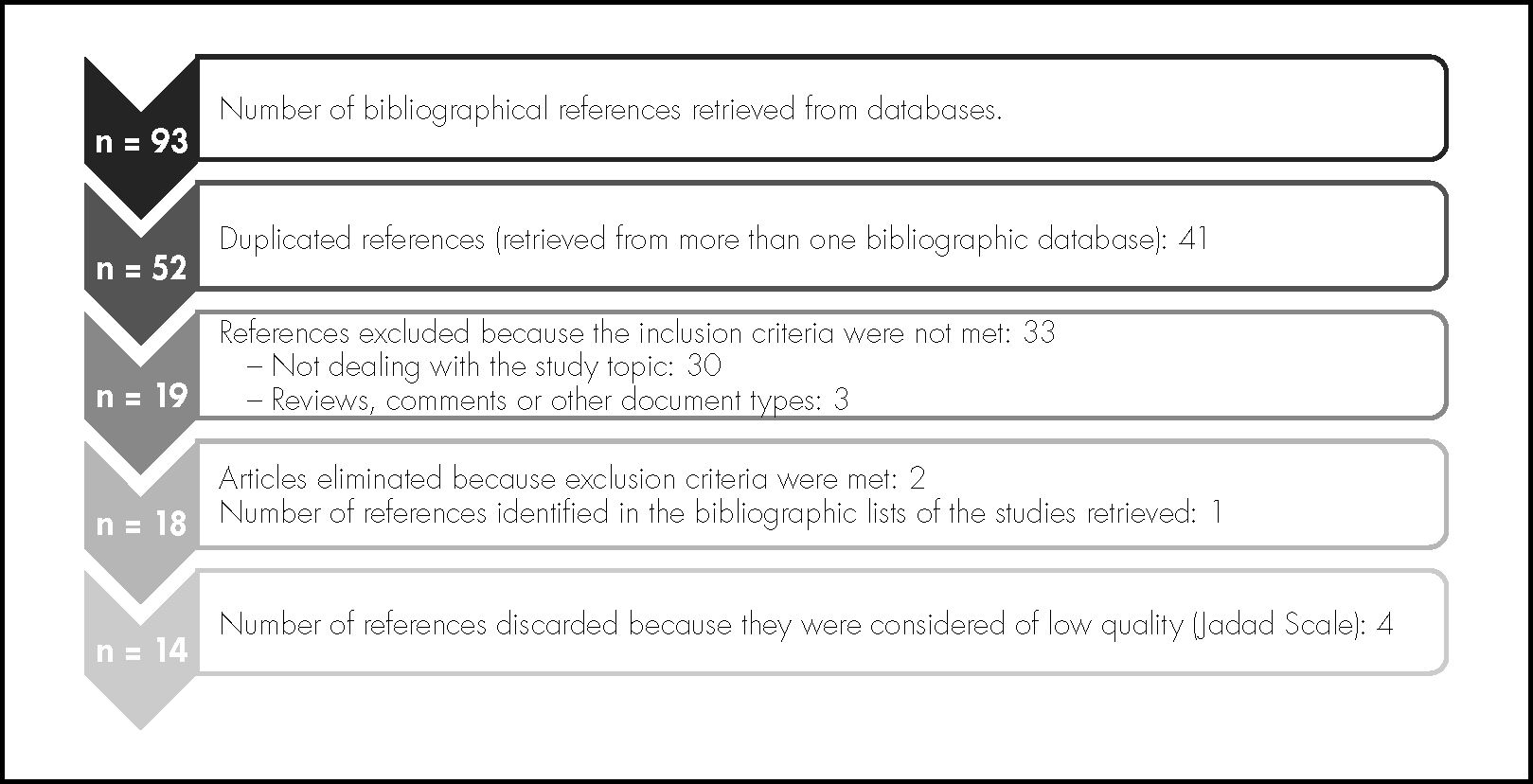
ResultsThe strategy for search in different databases reported 93 references in total. After the first duplicity review, 52 studies were obtained; and after applying the inclusion and exclusion criteria (figure 1), 30 of these were rejected because they did not adjust to the topic of our review, 3 of them were reviews, comments, or other document types, and therefore did not meet the inclusion criteria, and another 2 because the study population were not PTNBs (exclusion criteria). When evaluating the quality of the 18 articles2,6,11–26 selected through the Jadad Scale, their scores ranged between 2 and 5, with a median score of 5 (Table 1). Those articles with a score <3 were rejected, and therefore 14 articles were left for our review2,6,11–22, as shown in Figure 1. These articles had been published by international institutions and were written in English.
Evaluation of methodological quality and risk of bias with the Jadad Scale
| Study | Q1* | Q2* | Q3* | Q4** | Q5** | Total |
|---|---|---|---|---|---|---|
| Penn et al., 198011 | 1 | 1 | 1 | 1 | 1 | 5 |
| Schmidt-Sommerfeld et al., 198323 | 1 | 0 | 1 | 1 | −1 | 2 |
| Coran et al., 198512 | 1 | 1 | 1 | 1 | 1 | 5 |
| Larsson et al., 199013 | 1 | 1 | 1 | 1 | 1 | 5 |
| Helms et al., 199014 | 1 | 1 | 1 | 1 | 1 | 5 |
| Sulkers et al., 199015 | 1 | 1 | 1 | 1 | 1 | 5 |
| Bonner et al., 199516 | 1 | 1 | 1 | 1 | 1 | 5 |
| Shortland et al., 199817 | 1 | 1 | 1 | 1 | 1 | 5 |
| Meyburg et al., 20026 | 1 | 1 | 1 | 1 | 1 | 5 |
| O'Donnell et al., 200218 | 1 | 1 | 1 | 1 | 1 | 5 |
| Whitfield et al., 200319 | 1 | 1 | 1 | 1 | 1 | 5 |
| Honzik, 200524 | 1 | 0 | 1 | 1 | −1 | 2 |
| Pande et al., 200520 | 1 | 1 | 1 | 1 | 1 | 5 |
| Crill et al., 200621 | 1 | 1 | 1 | 1 | 1 | 5 |
| Seong et al., 20102 | 1 | 1 | 1 | 1 | 1 | 5 |
| Wintrier et al., 201425 | 1 | 0 | 1 | 1 | −1 | 2 |
| Ozturk et al., 201626 | 1 | 0 | 1 | 1 | −1 | 2 |
| Clark et al., 201722 | 1 | 1 | 1 | 1 | 1 | 5 |
Score = *0: no; 1: yes; **-1: no; 1: yes; Q1C33: regarding the manner of patient randomization; Q2C35: regarding the use of double blinding; Q3: regarding the loss of individuals during the study. If the score is <3, the clinical trial is considered to have low quality.
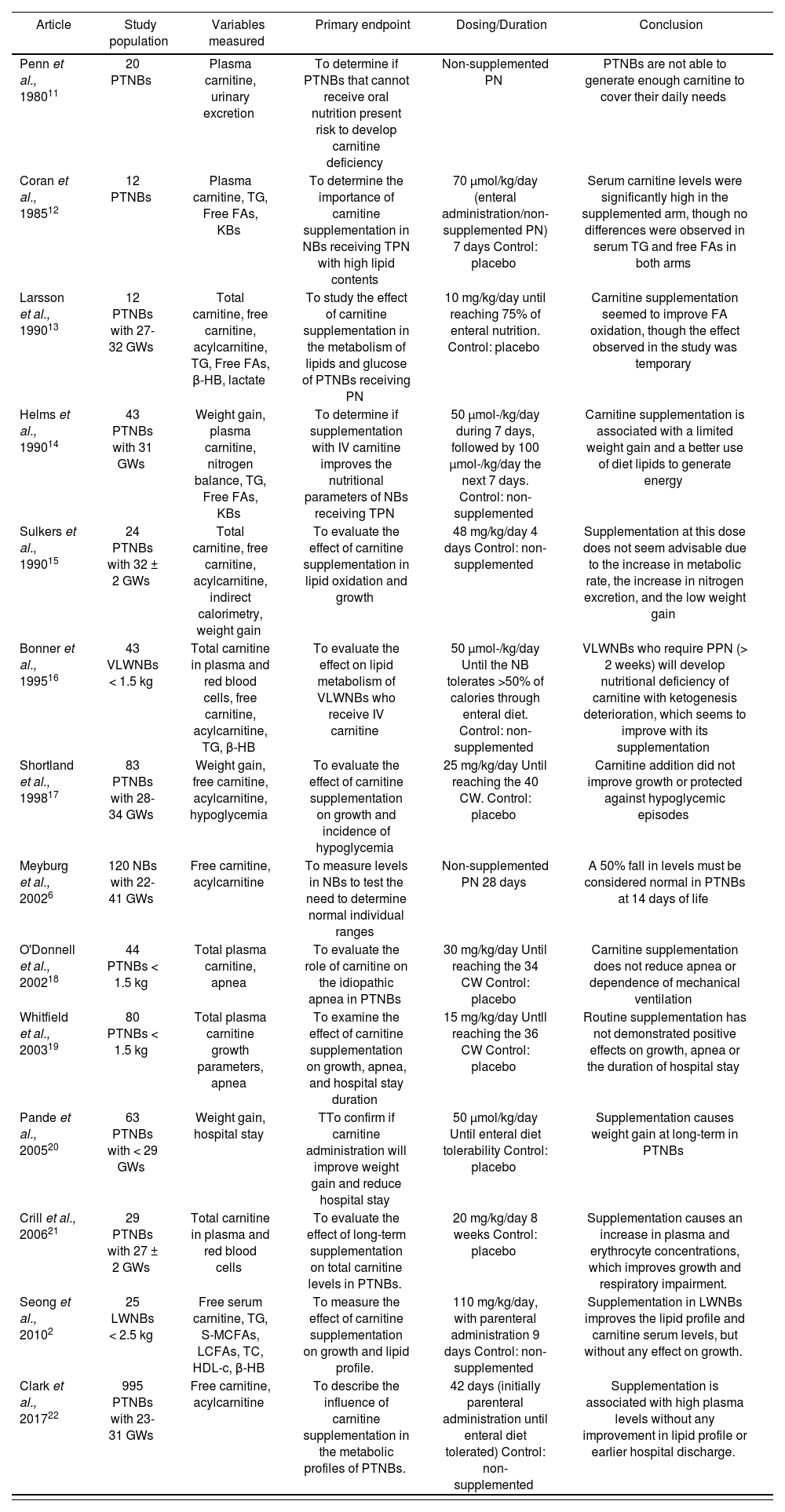
All relevant data from each article were summarized in one table (Table 2); specifically, these were coded according to the first author of the bibliographic reference and year of publication, population who received carnitine, variables measured, both clinical and analytical, primary endpoint, dosing and time during which the supplement was administered, as well as the final conclusion of the study.
Characteristics of the studies evaluated about L-carnitine supplementation in PTNBs
| Article | Study population | Variables measured | Primary endpoint | Dosing/Duration | Conclusion |
|---|---|---|---|---|---|
| Penn et al., 198011 | 20 PTNBs | Plasma carnitine, urinary excretion | To determine if PTNBs that cannot receive oral nutrition present risk to develop carnitine deficiency | Non-supplemented PN | PTNBs are not able to generate enough carnitine to cover their daily needs |
| Coran et al., 198512 | 12 PTNBs | Plasma carnitine, TG, Free FAs, KBs | To determine the importance of carnitine supplementation in NBs receiving TPN with high lipid contents | 70 μmol/kg/day (enteral administration/non-supplemented PN) 7 days Control: placebo | Serum carnitine levels were significantly high in the supplemented arm, though no differences were observed in serum TG and free FAs in both arms |
| Larsson et al., 199013 | 12 PTNBs with 27-32 GWs | Total carnitine, free carnitine, acylcarnitine, TG, Free FAs, β-HB, lactate | To study the effect of carnitine supplementation in the metabolism of lipids and glucose of PTNBs receiving PN | 10 mg/kg/day until reaching 75% of enteral nutrition. Control: placebo | Carnitine supplementation seemed to improve FA oxidation, though the effect observed in the study was temporary |
| Helms et al., 199014 | 43 PTNBs with 31 GWs | Weight gain, plasma carnitine, nitrogen balance, TG, Free FAs, KBs | To determine if supplementation with IV carnitine improves the nutritional parameters of NBs receiving TPN | 50 μmol-/kg/day during 7 days, followed by 100 μmol-/kg/day the next 7 days. Control: non-supplemented | Carnitine supplementation is associated with a limited weight gain and a better use of diet lipids to generate energy |
| Sulkers et al., 199015 | 24 PTNBs with 32 ± 2 GWs | Total carnitine, free carnitine, acylcarnitine, indirect calorimetry, weight gain | To evaluate the effect of carnitine supplementation in lipid oxidation and growth | 48 mg/kg/day 4 days Control: non-supplemented | Supplementation at this dose does not seem advisable due to the increase in metabolic rate, the increase in nitrogen excretion, and the low weight gain |
| Bonner et al., 199516 | 43 VLWNBs < 1.5 kg | Total carnitine in plasma and red blood cells, free carnitine, acylcarnitine, TG, β-HB | To evaluate the effect on lipid metabolism of VLWNBs who receive IV carnitine | 50 μmol-/kg/day Until the NB tolerates >50% of calories through enteral diet. Control: non-supplemented | VLWNBs who require PPN (> 2 weeks) will develop nutritional deficiency of carnitine with ketogenesis deterioration, which seems to improve with its supplementation |
| Shortland et al., 199817 | 83 PTNBs with 28-34 GWs | Weight gain, free carnitine, acylcarnitine, hypoglycemia | To evaluate the effect of carnitine supplementation on growth and incidence of hypoglycemia | 25 mg/kg/day Until reaching the 40 CW. Control: placebo | Carnitine addition did not improve growth or protected against hypoglycemic episodes |
| Meyburg et al., 20026 | 120 NBs with 22-41 GWs | Free carnitine, acylcarnitine | To measure levels in NBs to test the need to determine normal individual ranges | Non-supplemented PN 28 days | A 50% fall in levels must be considered normal in PTNBs at 14 days of life |
| O'Donnell et al., 200218 | 44 PTNBs < 1.5 kg | Total plasma carnitine, apnea | To evaluate the role of carnitine on the idiopathic apnea in PTNBs | 30 mg/kg/day Until reaching the 34 CW Control: placebo | Carnitine supplementation does not reduce apnea or dependence of mechanical ventilation |
| Whitfield et al., 200319 | 80 PTNBs < 1.5 kg | Total plasma carnitine growth parameters, apnea | To examine the effect of carnitine supplementation on growth, apnea, and hospital stay duration | 15 mg/kg/day Untll reaching the 36 CW Control: placebo | Routine supplementation has not demonstrated positive effects on growth, apnea or the duration of hospital stay |
| Pande et al., 200520 | 63 PTNBs with < 29 GWs | Weight gain, hospital stay | TTo confirm if carnitine administration will improve weight gain and reduce hospital stay | 50 μmol/kg/day Until enteral diet tolerability Control: placebo | Supplementation causes weight gain at long-term in PTNBs |
| Crill et al., 200621 | 29 PTNBs with 27 ± 2 GWs | Total carnitine in plasma and red blood cells | To evaluate the effect of long-term supplementation on total carnitine levels in PTNBs. | 20 mg/kg/day 8 weeks Control: placebo | Supplementation causes an increase in plasma and erythrocyte concentrations, which improves growth and respiratory impairment. |
| Seong et al., 20102 | 25 LWNBs < 2.5 kg | Free serum carnitine, TG, S-MCFAs, LCFAs, TC, HDL-c, β-HB | To measure the effect of carnitine supplementation on growth and lipid profile. | 110 mg/kg/day, with parenteral administration 9 days Control: non-supplemented | Supplementation in LWNBs improves the lipid profile and carnitine serum levels, but without any effect on growth. |
| Clark et al., 201722 | 995 PTNBs with 23-31 GWs | Free carnitine, acylcarnitine | To describe the influence of carnitine supplementation in the metabolic profiles of PTNBs. | 42 days (initially parenteral administration until enteral diet tolerated) Control: non-supplemented | Supplementation is associated with high plasma levels without any improvement in lipid profile or earlier hospital discharge. |
β-HB: β-hydroxybutirate; CWs: corrected weeks; FAs: fatty acids; FTN: full-term newborn; GW: gestation weeks; IV: intravenous; KBs: ketonic bodies; LCFAs: long chain fatty acids; LWNB: low-weight newborn; NB: newborn; PN: parenteral nutrition; PPN: prolonged parenteral nutrition; PTNB: preterm new born; RDS: respiratory distress syndrome; S-MCFAs: short-medium chain fatty acids; TC: total cholesterol; TG: triglycerides; TPN: total parenteral nutrition; VLWNB: very-low-weight newborn.
The study population in different articles was very heterogeneous, though they were all PTNBs. Almost all studies coincided in the analytical variables measured (free carnitine and acylcarnitine, triglycerides, free fatty acids and ketonic bodies). Besides, some studies such as those by Whitfield et al.19 and Pande et al.20, took into account other clinical variables, such as weight gain or apnea. In the majority of the studies, carnitine was added to the PN solution as long as there was tolerability to enteral administration, and at this time supplementation became oral. Only some studies had no supplements administered, such as the one by Meyburg et al.6, where only plasma levels were measured in order to compare them with those in FTNs. Supplement administration was conducted for a short term (<4 weeks), except in the study by Crill et al.21, with an 8-week duration.
DiscussionL-carnitine facilitates the entry of long-chain fatty acids into the mitochondrial matrix for their oxidation and subsequent energy generation; therefore, its lack could limit the lipid metabolism and increase triglycerides, fatty acids and ketonic bodies in plasma. Likewise, there could be a reduction in weight gain, by an increase in protein metabolism for energy generation, mostly in PTNBs, with L-carnitine levels well below usual levels, due to a lower tissue reserve and a difficult nutrient intake. However, the evidence available is still controversial in terms of the clinical relevance of low tissue levels and therefore, regarding the need for prophylactic supplementation.
The first studies on serum L-carnitine levels on PTNBs are dated in the 80s; their conclusion was that those who did not receive supplements presented major deficiencies11–12. Subsequent experimental studies, such as the one conducted by Larsson et al.13, demonstrated a higher tolerability to parenteral lipids in patients who received L-carnitine, with a positive effect on fatty acid metabolism. However, no data were collected regarding variables with higher clinical relevance, such as weight gain. In many Neonatal Intensive Care Units, prophylactic L-carnitine administration was initiated routinely as a supplement in the PN of PTNBs, based on these publications.
After this trend, the studies conducted in the 90s already started to include not only biochemical but also clinical variables among those measured, such as weight gain or hospital stay. Helms et al.14 and Sulkers et al.15 observed some limited weight gain in their studies, though their patient samples were not very large. Afterwards, and with a larger sample size, Shortland et al.17 concluded in 1998 that adding carnitine to PN did not improve PTNB growth. It was only in 2005 when Pande et al.20 designed a specific study with the sole objective to demonstrate weight increase in this type of patients who received prolonged supplement administration (until Gestation Week 36 or hospital discharge) of L-carnitine; their results did not show statistically significant differences in the primary efficacy variable, weight gain: the mean weight increase in the arm receiving the supplement was 18.9±4.7 grams/day vs. 18.5±4.6 grams/day in the control arm (p>0.05); there were no statistically significant differences found in the rest of secondary variables measured in both groups.
In accordance with this, it is worth highlighting the study conducted by Clark et al.22, including 995 very-low-weight newborns (VLWNBs), which demonstrated that adding L-carnitine to the PN solution generated an increase in plasma levels without any improvement in the lipid profile (measured as free carnitine, acylcarnitine and free fatty acids), weight gain during the first 28 days of life, or specific mortality or morbidity. During the PN stage, free carnitine levels were consistently higher in those babies who received the supplement, and the recovery in plasma levels occurred faster in those babies with higher gestational age; this was probably associated with an early initiation of enteral nutrition, supplemented with carnitine. Despite these results, carnitine supplementation did not alter the metabolism of long-chain fatty acids. This study also collected the hospital stay variable, like Whitfield et al.19 had already done in 2003; both reached the conclusion that L-carnitine supplementation does not translate into hospital stay reduction.
The study by Clark et al.22 was designed with preterm babies who did not require nutritional supplements administered parenterally for >28 days, so that plasma carnitine levels were normalized as the transition from parenteral to enteral nutrition took place (days 7-28), supplemented with L-carnitine. However, in usual clinical practice we find VLWNBs who need prolonged PN, for >1 month. Further studies would be required to demonstrate if this patient population would benefit of a routine L-carnitine supplementation in their PN solution, as their plasma levels are highly lower than usual due to a lower tissue reserve and the impossibility to make a transition to enteral nutrition due to their digestive immaturity.
It is worth highlighting that O'Donnell et al.18 in 2002, and Whitfield et al.19 in 2003 included in their study designs some parameters associated with idiopathic apnea in PTNBs. Apnea of prematurity can be due to an alteration in the brain center which controls breathing, in the so called central apnea, or to a mechanical process, in obstructive apnea, where breathing is stopped due to a blockage in respiratory airways. Problems in other organs can also affect the respiratory control center. Apnea of prematurity is likely to have no other identifiable cause but the immaturity of the central nervous system. The potential improvement to this condition caused by carnitine can be explained by the fact that its lack causes a reduction in energy generation at muscle level. In both studies, no positive effects were observed with its supplementation in the PN solution. Subsequently, in 2006 Crill et al.21 published their results, and reached the conclusion that supplementation in preterm children could improve periodical breathing in this patient group, but without any statistically significant differences between patient arms in terms of the need for mechanical ventilation and duration, and incidence of bronchopulmonary dysplasia. In the study by Ozturk et al.26, there was a lower incidence of bronchopulmonary incidence in the arm treated with carnitine, though there was no statistical significance. The reason for this discrepancy could lie in the different dose of carnitine used in each study: 20 mg/ kg/d in the first and 30 mg/kg/d in the second. The study designs were also different, because Ozturk et al.26 intended to demonstrate the clinical benefit of carnitine supplementation in preterm babies with Respiratory Distress Syndrome (RDS). Many studies have associated the presence of low plasma carnitine levels in PTNBs with RDS27. Treatment with carnitine for preterm children with RDS can reduce the duration of mechanic ventilation, the use of pulmonary surfactant and the development of bronchopulmonary dysplasia26.
Although there is a high risk of carnitine deficiency, there are no standards for the administration of supplements in PTNBs receiving TPN. Practically all studies seem to confirm that the deficiency of carnitine in plasma levels at short term is not associated with clinically relevant symptoms in those patients presenting it: neither lower weight gain nor hypertriglyceridemia or shorter hospital stay. Therefore, there is no recommendation for the prophylactic supplementation of L-cartinine in PN, also because this is not free from risks. Sulkers et al.15 observed an increase in the metabolic rate and nitrogen excretion; however, it is true that they were using a dose (48 mg/kg/day) far above those currently recommended (10-20 mg/kg/day). Clark et al.28 have recently published the outcomes of their study, conducted with the objective of assessment the knowledge, beliefs, and clinical practice regarding carnitine deficiency and supplement administration among neonatologists, through an on-line survey. In total, 492 professionals participated in this survey, and only 5% determined this deficiency in preterm babies through lab tests; 40% of them administered L-carnitine supplements routinely, though 60% believed that its deficiency could have severe consequences. These outcomes showed the lack of consensus among healthcare professionals about the potential benefit of supplementation, as well as about the potential risks for PTNBs caused by its deficiency.
The limitations of this review lie in the different designs of different studies, as well as in the carnitine doses used (a range of 8 to 48 mg/kg/day), or duration of the supplementation. It would be convenient to conduct studies that are more homogeneous in terms of design, as well as to analyze the need for supplementation in those newborns requiring long-term PN.
In conclusion, and according to the bibliographic review available, almost all authors demonstrate that routine L-carnitine supplementation in the PN of PTNBs can improve plasma levels, but not reaching a significant improvement in lipid profile; and what is most important, without any increase in weight gain, reduction in morbidity and mortality, or reduction in hospital stay. Further studies will be required to demonstrate whether the systematic L-carnitine supplementation in PTNBs who require TPN over one month would offer any benefit with clinical relevance to such vulnerable patients.
FundingNo funding.
Conflict of interestsNo conflict of interest.