Acquired haemophilia is an uncommon condition caused by the development of clotting factor inhibitors. To eliminate them, immunosuppressive therapy with corticosteroids and cytotoxic drugs is required.
MethodsWe describe a case of rituximab use in acquired haemophilia refractory to conventional therapy in a 63 year old male patient with chronic hepatitis C virus infection who was receiving treatment with pegylated-interferon-α-2a plus ribavirin.
ResultsAfter 21 weeks of antiviral therapy, the patient was admitted to hospital with a large haematoma in the abdominal muscles. Factor VIII level was zero and inhibitor titer was 345 Bethesda units. Oral immunosuppressive therapy with methylprednisolone and cyclophosphamide was administered for 1 month, with limited improvement. Therefore, cyclophosphamide was replaced by a four once-weekly dose of intravenous rituximab. Two months later, factor VIII level was normal and inhibitor titer was undetectable.
ConclusionsRituximab may be useful for the treatment of acquired haemophilia resistant to standard therapy.
la hemofilia adquirida es un trastorno infrecuente causado por el desarrollo de inhibidores del factor de coagulación. Para eliminarlos, se requiere tratamiento con corticoides y fármacos citotóxicos. Métodos: describimos el caso del uso de rituximab en hemofilia adquirida refractaria al tratamiento convencional en un hombre de 63 años con infección crónica por el virus de la hepatitis C y que estaba recibiendo tratamiento con interferón pegilado a-2a y ribarivina.
Resultadostras 21 semanas de tratamiento antivírico, el paciente fue ingresado en el hospital por un gran hematoma en la musculatura abdominal. La concentración de factor VIII era nula y el título de inhibidor fue de 345 unidades Bethesda. Se administró tratamiento inmunosupresor oral con metilprednisolona y ciclofosfamida durante 1 mes, con escasa mejoría. Así pues, se sustituyó la ciclofosfamida por una dosis semanal de rituximab intravenoso. Dos meses después, la concentración de factor VIII se normalizó y el título de inhibidor era indetectable.
ConclusiónRituximab puede ser útil en el tratamiento de la hemofilia adquirida resistente al tratamiento estándar.
Acquired haemophilia A (AHA) is an unusual condition that results from the spontaneous development of autoantibodies against clotting factor VIII (FVIII), leading to inhibition of FVIII binding to von Willebrand factor, to activated factor IX, or to negatively charged phospholipids, and resulting in haemorrhage, principally in soft tissues and systemic bleeding episodes.1 The underlying mechanisms leading to the production of FVIII autoantibodies are not completely understood but their appearance during interferon (IFN) therapy for chronic hepatitis C virus (HCV) infection has been reported.2 Prompt diagnosis of this acquired bleeding disorder is essential for appropriate management aimed at controlling haemorrhage and suppressing the inhibitor, both of which are necessary to restore normal haemostasis and to prevent risk of future bleeding episodes.
The diagnosis of AHA is based on the demonstration of an isolated prolongation of activated partial thromboplastin time which is not corrected by mixing patient plasma with normal plasma, associated with reduced FVIII levels and the presence of FVIII inhibitor. Treatment strategies in patients with acquired FVIII inhibitors have two main objectives. During the acute stage, effective control of bleeding is the primary aim which can be achieved with plasma exchange, the administration of human or porcine FVIII, desmopressin, activated prothrombin complex concentrate (APCC) and recombinant human activated factor VII (rFVIIa). Both APCC and rFVIIa have FVIII bypassing activity and are used for the treatment of patients with high titers of inhibitors. The long-term therapeutic goal is the eradication of the inhibitor, thereby curing the condition.3-5
Although up to 36% of patients experience a spontaneous disappearance of their autoantibodies within a few months of drug discontinuation, this is unpredictable. To eliminate FVIII inhibitors, immunosuppressive therapy with corticosteroids and cytotoxic drugs, alone or in combination, is regarded as the mainstay of therapy.1
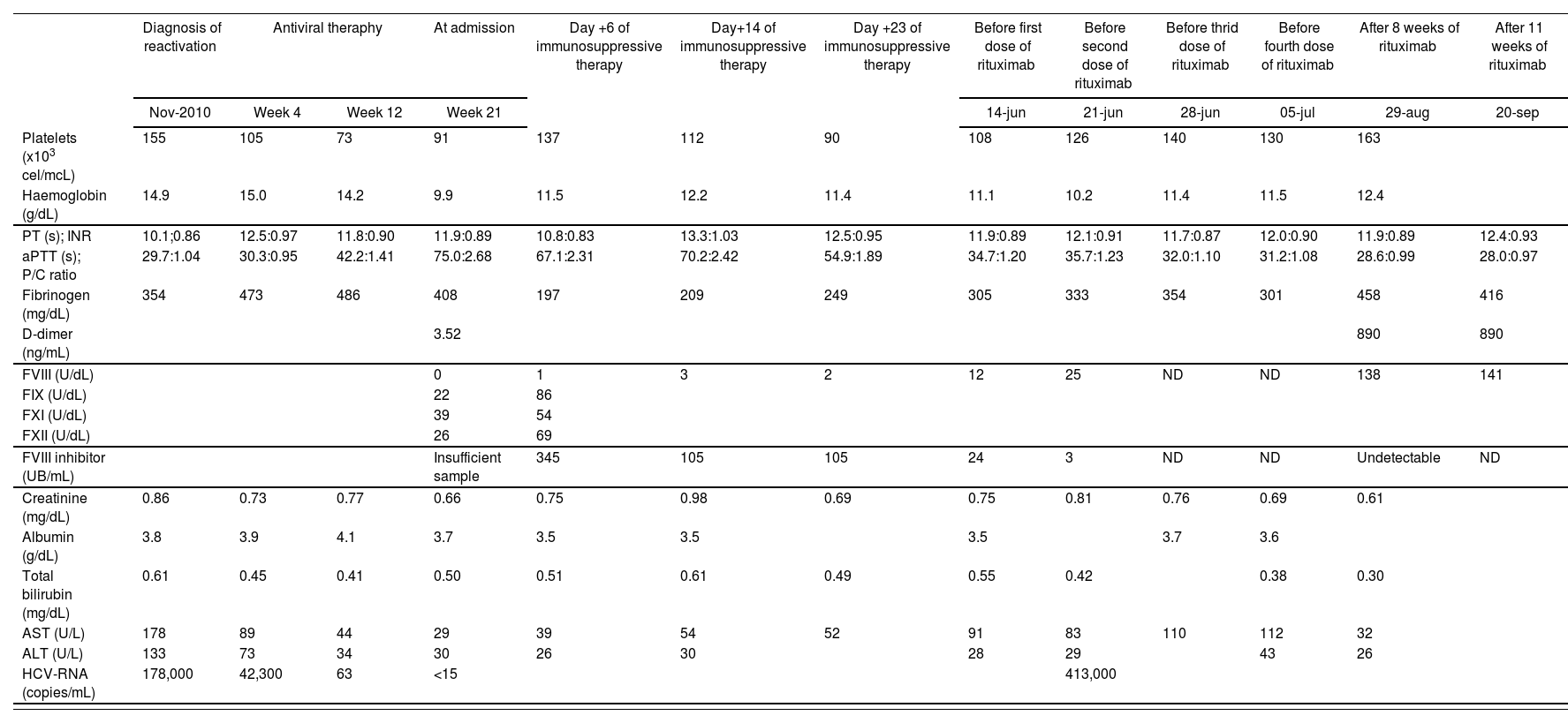
Case ReportA single, non-haemophilic, 63 year old male patient with chronic HCV infection was started on treatment with pegylated-interferon-α-2a (peg-INF-α-2a), administered subcutaneously at a total dose of 180 mg weekly, plus ribavirin, administered orally at a dose of 800 mg/day in divided doses (400 mg twice daily). It was the first reactivation but he was diagnosed on HCV infection in 1980. Normal levels of albumin, total bilirubin, creatinine, prothrombin time and platelets count indicated compensated chronic liver disease without echographic signs of portal hypertension. For 10 months, patient clinical history was reviewed and the following laboratory investigations were collected: platelets count, haemoglobin, prothrombin time (PT), international normalized ratio (INR), activated partial thromboplastin time (aPTT), patient/control plasma ratio (P/C ratio), fibrinogen, D-dimer, clotting factors levels (FVIII, IX, XI and XII), FVIII inhibitor titers, creatinine, albumin, total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and viral load as HCV-RNA. Analytical data are shown in Table 1.
Laboratory investigations. Normal values are indicated below
| Diagnosis of reactivation | Antiviral theraphy | At admission | Day +6 of immunosuppressive therapy | Day+14 of immunosuppressive therapy | Day +23 of immunosuppressive therapy | Before first dose of rituximab | Before second dose of rituximab | Before thrid dose of rituximab | Before fourth dose of rituximab | After 8 weeks of rituximab | After 11 weeks of rituximab | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nov-2010 | Week 4 | Week 12 | Week 21 | 14-jun | 21-jun | 28-jun | 05-jul | 29-aug | 20-sep | ||||
| Platelets (x103 cel/mcL) | 155 | 105 | 73 | 91 | 137 | 112 | 90 | 108 | 126 | 140 | 130 | 163 | |
| Haemoglobin (g/dL) | 14.9 | 15.0 | 14.2 | 9.9 | 11.5 | 12.2 | 11.4 | 11.1 | 10.2 | 11.4 | 11.5 | 12.4 | |
| PT (s); INR | 10.1;0.86 | 12.5:0.97 | 11.8:0.90 | 11.9:0.89 | 10.8:0.83 | 13.3:1.03 | 12.5:0.95 | 11.9:0.89 | 12.1:0.91 | 11.7:0.87 | 12.0:0.90 | 11.9:0.89 | 12.4:0.93 |
| aPTT (s); P/C ratio | 29.7:1.04 | 30.3:0.95 | 42.2:1.41 | 75.0:2.68 | 67.1:2.31 | 70.2:2.42 | 54.9:1.89 | 34.7:1.20 | 35.7:1.23 | 32.0:1.10 | 31.2:1.08 | 28.6:0.99 | 28.0:0.97 |
| Fibrinogen (mg/dL) | 354 | 473 | 486 | 408 | 197 | 209 | 249 | 305 | 333 | 354 | 301 | 458 | 416 |
| D-dimer (ng/mL) | 3.52 | 890 | 890 | ||||||||||
| FVIII (U/dL) | 0 | 1 | 3 | 2 | 12 | 25 | ND | ND | 138 | 141 | |||
| FIX (U/dL) | 22 | 86 | |||||||||||
| FXI (U/dL) | 39 | 54 | |||||||||||
| FXII (U/dL) | 26 | 69 | |||||||||||
| FVIII inhibitor (UB/mL) | Insufficient sample | 345 | 105 | 105 | 24 | 3 | ND | ND | Undetectable | ND | |||
| Creatinine (mg/dL) | 0.86 | 0.73 | 0.77 | 0.66 | 0.75 | 0.98 | 0.69 | 0.75 | 0.81 | 0.76 | 0.69 | 0.61 | |
| Albumin (g/dL) | 3.8 | 3.9 | 4.1 | 3.7 | 3.5 | 3.5 | 3.5 | 3.7 | 3.6 | ||||
| Total bilirubin (mg/dL) | 0.61 | 0.45 | 0.41 | 0.50 | 0.51 | 0.61 | 0.49 | 0.55 | 0.42 | 0.38 | 0.30 | ||
| AST (U/L) | 178 | 89 | 44 | 29 | 39 | 54 | 52 | 91 | 83 | 110 | 112 | 32 | |
| ALT (U/L) | 133 | 73 | 34 | 30 | 26 | 30 | 28 | 29 | 43 | 26 | |||
| HCV-RNA (copies/mL) | 178,000 | 42,300 | 63 | <15 | 413,000 | ||||||||
Platelets (150-350 x 103 cel/mcL), haemoglobin (13.50-17.50 g/dL), prothrombin time (PT, 10.50-15.80 s), international normalized ratio (INR), activated partial thromboplastin time (aPTT, 21-35 s), patient/control plasma ratio (P/C ratio), fibrinogen (150-560 mg/dL), D-dimer (0-275 ng/mL), clotting factors levels (F) (FVIII (50-150 U/dL), FIX (60-140 U/dL), FXI (60-140 U/dL) and FXII (60-140 U/dL)), FVIII inhibitor titers, creatinine (0.70-1.20 mg/dL), albumin (3.50-5.20 g/dL), total bilirubin (0.05-1.20 mg/dL), aspartate aminotransferase (AST, 5-41 U/L), alanine aminotransferase (ALT, 5-40 U/L) and viral load as HCV-RNA. ND: no data.
After 15 days of antiviral therapy, he developed a spontaneous haematoma in his left lower eyelid, which resolved without treatment. At week 8 of antiviral therapy, he had an eczematous dermatologic reaction with pruritus and petechias, treated for a week with oral dexametasone, topic methylprednisolone and oral ebastine, with complete resolution. At week 20 of antiviral therapy, patient showed several haematomas in both thighs, glutei and upper limbs, predominantly in the right forearm, secondary to thrombocytopenia and coagulopathy, and he was discharged and made an appointment for analytical control in a week due to he had no previous personal or family history of bleeding. But after four days, he was admitted to hospital for a large haematoma in right lateral abdominal muscles (oblique, external, internal and transversal), coagulopathy (normal PT and prolonged aPTT not corrected with normal plasma after a 2 hour incubation) and acquired haemophilia in high suspicion. Lupus anticoagulant and other autoimmune diseases were excluded. Patient had received 21 weeks of antiviral therapy, which was stopped.
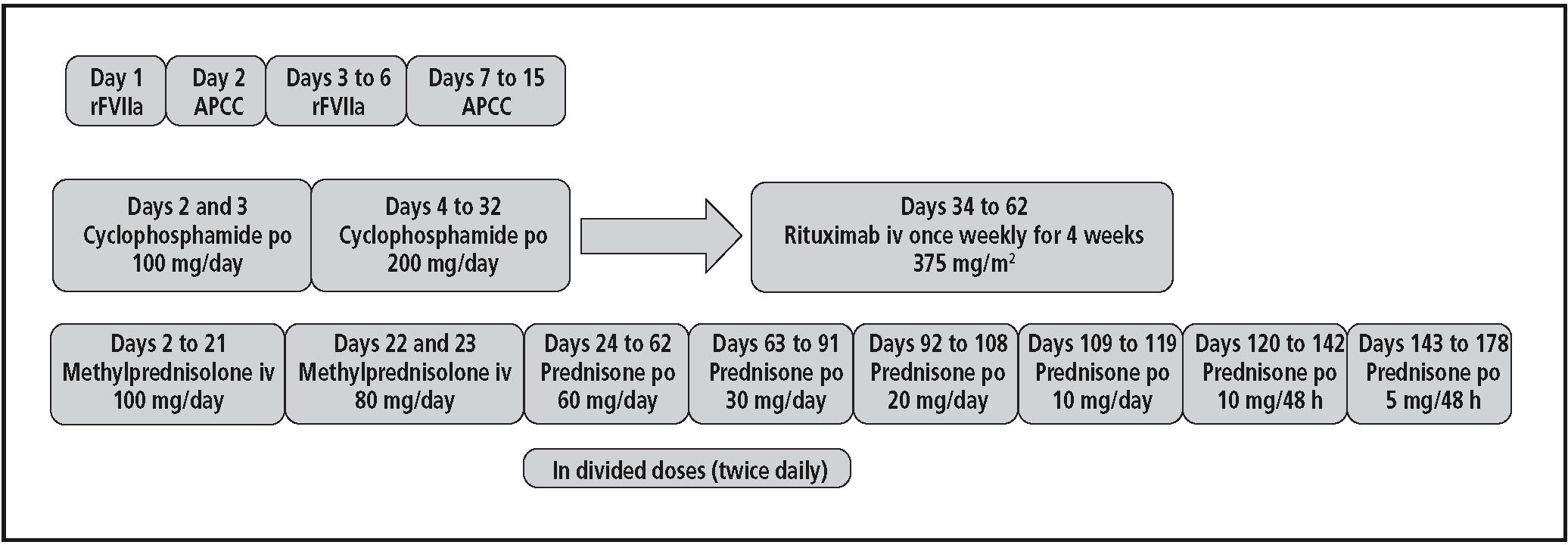
For the acute bleeding stage, treatment with rFVIIa (Novoseven®) was started at a dose of 90 mcg/Kg every 2 hours (patient weight = 100 Kg) during 4 doses, and then, every 4 hours for 4 doses; treatment in the second day was continued with APCC (Feiba®) at a dose of 5,000 U every 8 hours; from third to sixth day, treatment was changed again to rFVIIa, administered at a dose of 9 mg with different dosing interval, receiving a total dose of 166 mg. Due to the lack of new bleeding, treatment was replaced to APCC again for 9 days, at a total dose of 120,000 U, obtaining a good response.
At the same time, immunosuppressive therapy6 was started in the second day with methylprednisolone and cyclophosphamide. Treatment algorithm is summarized in figure 1. After a month, a slight improvement in FVIII levels and a small reduction in inhibitors were obtained. For this reason, oral cyclophosphamide was replaced by intravenous rituximab, which was used outwit its licensed indications. He received rituximab at 375 mg /m2 once weekly for 4 weeks and oral prednisone at a dose of 60 mg/day in divided doses (30 mg twice daily) up to the last dose of rituximab; then the dose was gradually tapered over a 16-week period and the agent discontinued. Two months from the start of rituximab therapy, the FVIII level was normal and inhibitor titer undetectable. After resolution, this severe adverse event was reported to our Regional Pharmacovigilance Centre. The patient has remained completely asymptomatic for the last year. However, he has not received HCV therapy and, therefore, his viral load has relapsed to 17 million copies/mL (last measurement on December 2011) while his ALT and AST levels have remained normal or slightly increased.
Regarding to safety, after two weeks from the last dose of rituximab, patient was readmitted to hospital for 7 seven days affected by right lower leg cellulitis and associated with neutropenia. He was treated with antibiotherapy (imipenem and vancomycin for a week, then levofloxacin and clindamycin for 10 days, and therefore ceftriaxone and cotrimoxazole for two weeks) and 3 doses of filgrastim. He had a previous neuropathic ulcer in his right foot (4th and 5th fingers), which was complicated by the immunosuppressive therapy. No other adverse effects have been reported. No other acute or delayed relevant side effect or infectious complication occurred.
DiscussionIt’s known that both chronic HCV infection and its treatment with IFN can occasionally lead to the development of FVIII autoantibodies. Several cases of acquired factor VIII inhibitors during treatment with IFN-α have been described, but most of these were in patients who had an underlying haematological malignancy.7,8 Our patient, however, developed FVIII autoantibodies after IFN treatment without having an underlying hereditary coagulopathy, similar to that reported by Schreiber and Braü.9 There are also cases of spontaneous development of FVIII inhibitors in HCV infected and HIV/HCV co-infected patients after treatment with IFN.10-12 Other haematological immune side effects of IFN-α treatment include autoimmune haemolytic anaemia, immune thrombocytopenia, thrombotic thrombocytopenic purpura and increased incidence of cardiolipin antibodies.13 It is possible that prolonged treatment with IFN increases the propensity for the development of autoantibodies to FVIII, and this can occur in two ways: autoantibodies may either appear de novo or existing antibodies may arise while patients are treated with IFN.
To our knowledge, in Spain and according to FEDRA (Farmacovigilancia Española, Datos de Reacciones Adversas), the database of Spanish Pharmacovigilance System, this is the second case of AHA in a patient treated with INF- α (the first time was in a patient with melanoma) and the first time in a patient with HCV infection treated with peg-IFN-α-2a. Karch and Lasagna modified by Naranjo algorithm14 was applied to this case and the causality assessment resulted as probable.
Rituximab is a chimaeric mouse-human anti-CD20 monoclonal antibody licensed for the treatment of CD20-positive lymphomas, chronic lymphatic leukemia and rheumatoid arthritis. Its mechanism of action is to cause B-cell depletion in lymph nodes, peripheral blood and bone marrow.15 Over the last few years, it has been used in off-label conditions in numerous autoimmune diseases including both idiopathic and non-familial thrombotic thrombocytopenic purpura, autoimmune haemolytic anemia16,17 and mixed cryoglobulinemia. Recent reports also suggest a role for rituximab in the treatment of patients with acquired FVIII inhibitors;18,19 even a treatment algorithm has been proposed by Aggarwal et al.,20 with three options according to FVIII inhibitor titer: 1) patients with minimal bleeding and inhibitor levels < 5 Bethesda Units (BU) should be treated with prednisone alone; 2) those with an inhibitor level < 30 BU but with serious bleeding should receive rituximab in combination with prednisone; and 3) those with an inhibitor level ≥ 30 BU should be treated with rituximab, prednisone and cyclophosphamide. They also suggested that patients not responding to or relapsing after these therapies may benefit from repeated courses of rituximab or rituximab maintenance therapy. Before rituximab was started, our patient belonged to type 2 regarding this algorithm.
As far as the safety is concerned, our patient suffered an infectious complication due to the immunosuppressive therapy. Recently, several types of side effects have been reported during immunosuppression by the European Acquired Haemophilia Registry (EACH2),21 such as sepsis, neutropenia, diabetes, and psychiatric illness. For instance, this study suggests that the development of less toxic immunosuppressive regimens would be an important step forward in AHA.
Rituximab treatment of AHA may be cost-effective. Bypassing haemostatic agents are extremely expensive. The estimated cost of treating this severe bleeding episode with recombinant rFVIIa and APCC was € 199,800 without taking into account other costs related to four weeks inpatient treatment. In contrast, treatment with rituximab, which cost € 7,200 for four once-weekly doses, can prevent new bleeding episodes resulting in significant savings in drugs. In conclusion, the administration of rituximab appears to be an effective option for treatment of patients with acquired haemophilia resistant to standard therapies.
Prior publicationSent as abstract to EAHP Congress 2012 (Milan). Accepted.
No financial sources.
Conflict of interestThe authors have no conflict of interest to declare.
AcknowledgementsMa José Peñalver Jara. Centro de Farmacovigilancia de la Región de Murcia. Servicio de Ordenación y Atención Farmacéutica. Consejería de Sanidad y Consumo de la Región de Murcia.
Hemofilia adquirida en un paciente con infección crónica por virus de la hepatitis que recibió tratamiento con interferón pegilado y ribavirina: papel de rituximab