The aims of the study were to quantify adherence, determine the factors that can predict adherence and identify the consequences of poorer adherence in patients with chronic inflammatory arthropathies treated with biological therapies in daily clinical practice.
MethodA descriptive, observational and retrospective study was carried out. Patients with rheumatoid arthritis, ankylosing spondylitis and psoriatic arthritis who started a biologic therapy between 1 January 2009 and 31 December 2016 were included. Variables related to socioeconomic status, the disease, the biological therapy and hospital resources were included. Adherence was calculated by using the medication possession ratio.
ResultsThree hundred and sixty-two patients and 423 lines of biological therapy were included. Mean age ± standard deviation was 50.3 ± 13.9 years, and 228 (53.9%) were women. The percentage of adherent patients was 187 out of 216 (87%) in rheumatoid arthritis, 91 out of 107 (85%) in ankylosing spondylitis and 84 out of 100 (84%) in psoriatic arthritis. Greater adherence was associated with more frequent visits to the pharmacy service (odds ratio 1.2, 95% confidence interval: 1.1-1.3 [p < 0.001]) and poorer adherence with a failure to attend scheduled appointments at the rheumatology clinic (odds ratio 0.2, 95% confidence interval: 0.1-0.9 [p = 0.030]). There were no differences between adherent and non-adherent patients in terms of the number of hospital resources used.
ConclusionsThere are no differences in adherence to biological therapies among patients with chronic inflammatory arthropathies. Adherence correlates with attendance at outpatient appointments, but this does not imply an increase in the use of hospital resources.
Los objetivos del estudio fueron cuantificar la adherencia, determinar los factores predictivos y conocer las consecuencias de una menor adherencia, en la practica clinica diaria, en pacientes con artropatias inflamatorias cronicas tratados con terapias biologicas.
MétodoEstudio descriptivo, observacional y retrospectivo. Se incluyeron pacientes con artritis reumatoide, espondilitis anquilosante y artritis psoriasica que iniciaron una terapia biologica entre el 1 de enero de 2009 y el 31 de diciembre de 2016. Se recogieron variables sociodemograficas, relacionadas con la enfermedad, sobre las terapias biologicas y los recursos hospitalarios. La adherencia se calculo mediante la ratio media de posesion.
ResultadosSe incluyeron 362 pacientes y 423 lineas de terapia biologica. La media de edad ± desviacion estandar fue de 50,3 ± 13,9 anos; 228 (53,9%) fueron mujeres. El porcentaje de adherentes fue de 187 de 216 (87%) en artritis reumatoide, 91 de 107 (85%) en espondilitis anquilosante y 84 de 100 (84%) en artritis psoriasica. La adherencia se relaciono con acudir con mas frecuencia a la consulta del servicio de farmacia (odds ratio de 1,2; intervalo de confianza 95%: 1,1-1,3 [p < 0,001]) e inversamente con no acudir a las consultas de reumatologia en la fecha prevista (odds ratio de 0,2; intervalo de confianza 95%: 0,1-0,9 [p = 0,030]). No hubo diferencias en el número de recursos hospitalarios utilizados por pacientes adherentes y no adherentes.
ConclusionesLa adherencia a las terapias biológicas entre las artropatías inflamatorias crónicas es similar. Dicha adherencia se correlaciona con la frecuentación a consultas externas, pero no implica un aumento del consumo de recursos.
The introduction of biological therapies (BT) in the treatment of chronic inflammatory arthropathies (CIA) such as rheumatoid arthritis (RA), ankylosing spondylitis (AS) and psoriatic arthritis (PsA) has led to a pharmacotherapeutic revolution that has brought about considerable improvements in the prognosis of CIA and in patients’ quality of life1. However, the healthcare system bears a high economic burden because CIA are chronic diseases and the cost of BT is very high2–5. The lack of adherence in chronic treatments is a genuine universal problem that compromises their effectiveness and can result in the worsening of the disease, death and rising healthcare costs6,7.
There are publications on adherence in CIA7–10, although most address patients with RA. Few data are available on the factors that predict adherence to BT in patients with CIA11 and the consequences for the healthcare system11,12.
The objectives of this study were:
- 1.
To quantify adherence to BT in a cohort of patients diagnosed with CIA in daily clinical practice.
- 2.
To determine the factors that can predict adherence to BT.
- 3.
To identify the consequences, in terms of hospital resources, of poor adherence to BT.
A retrospective, descriptive and observational study was carried out. The study was conducted at a third-level hospital that provides healthcare to 564,452 citizens. The Pharmacy Service (PS) has a specialized clinic for patients with BT and CIA, with a workload of 3,000 consultations per year (7.5% of the total outpatient activity of the PS). All patients who attend this clinic are looked after by a pharmacist specializing in hospital pharmacy. Intravenous BT are administered at the Day Hospital (DH), part of the same hospital.
The study included adult patients diagnosed with RA, AS or PsA who were being treated by the Rheumatology Clinic, who fulfilled the 1987 American College of Rheumatology classification criteria for RA13, the modified New York criteria for classification of AS14,15 or the CASPAR classification criteria for PsA16, and who had started a BT with abatacept, adalimumab, certolizumab, etanercept, golimumab, infliximab, tocilizumab or ustekinumab between 1 January 2009 and six months before the study end date (31 December 2016), with a minimum BT duration of 180 days.
In order to obtain clinical information, each patient's electronic medical records were consulted. The data collected on the diseases and the use of drugs were consistent with the pattern of routine clinical practice. At the beginning of the BT, demographic variables (age, sex), sociocultural variables (employment status, educational level, smoking habits, size of home town, distance between home and hospital), clinical variables (years since diagnosis, comorbidities according to the Charlson Index17), and analytical parameters, such as C-reactive protein, erythrocyte sedimentation rate and haemoglobin levels at the start of the BT, were collected.
For the purposes of assessing the potential consequences of non-adherence, the health resources used by patients in Specialized Care during the adherence-measurement period were taken into account: number of hospital admissions, visits to the hospital's emergency department, visits to the Rheumatology Clinic, visits to other clinics, visits to the PS outpatient clinic and the Day Hospital, and imaging tests (X-rays, nuclear magnetic resonance and nuclear medicine). In order to make proper comparisons, the average consumption per patient and year of BT was calculated.
In relation to BT, concomitant treatments at the start of the BT (methotrexate, leflunomide and glucocorticoids), dose regimen, route of administration and the therapy line number were recorded. Any patients concomitantly using any psychotropic drugs of the groups NO5B, N05C, N06A, N06B, N06C and N06D, according to the Anatomical Therapeutic Chemical (ATC) Classification System18, were recorded due to the possible relationship between lack of adherence and psychoactive treatment19.
Adherence was calculated by using the medication possession ratio (MPR), which is defined as the number of dispensed medication doses divided by the total number of days in the period analysed. Data on the number of BT dispensations given to the patient were obtained from electronic records in the Silicon® program. In the case of treatments administered in the DH, the days on which the patient attended the unit, which were recorded in the Oncofarm® program, were taken into account. Interruptions due to hospital admissions or pregnancy were deducted.
To calculate the number of days in the period analysed, the dose prescribed by the rheumatologist, rather than the dose indicated in the data sheet, was taken into account. Dose optimization and intensification were therefore taken into consideration.
To assess possible factors that could predict better adherence to BT and the consequences of poor adherence, the sample was divided into two groups: lines of BT in which patients were adherent (MPR ≥ 0.8) and lines of BT in which patients were non-adherent (MPR < 0.8).
The statistical analysis was carried out by means of the SPSS program. A descriptive analysis of the study sample was performed. Quantitative variables were expressed as mean ± standard deviation (SD) if they had a normal distribution and as the median and interquartile range (IQR) if they did not have a normal distribution. Categorical variables were expressed as absolute values and percentages. To establish differences between quantitative variables, the Student's t-test (for two variables) or Mann-Whitney U test was used. In the case of qualitative variables, the chi-square test was performed. Values were considered statistically significant when p < 0.05.
To assess possible factors that could affect adherence, a multivariate logistic regression analysis was performed with variables that were significant in the univariate study.
The study complied with Law 15/1999 of 13 December on the Protection of Personal Data. The data were used exclusively for the research conducted as part of this study, and were kept anonymous and confidential. The study was approved by the Healthcare Research Ethics Committee, under code 2014/187.
ResultsThe sample consisted of 362 patients, who accounted for 423 lines of BT. The median duration of BT was 823 days (IQR 419-1,459) in the adherent group (MPR ≥ 0.8) and 891 days (IQR 608-1,443) in the non-adherent group (MPR < 0.8), with no differences between the two groups.
The clinical, sociodemographic and pharmacological characteristics of the initial patient sample are shown in Table 1.
General characteristics of patients
| Age in years | n=423 |
|---|---|
| Mean ± SD | 50.3 ± 13.9 |
| Sex, n (%) | n=423 |
| Females | 228 (53.9) |
| Males | 195 (46.1) |
| Education level, n (%) | n = 229 |
| University | 35 (15.3) |
| Upper secondary/Vocational education | 65 (28.4) |
| Basic | 124 (54.1) |
| No schooling | 5 (2.2) |
| Employment status, n (%) | n=349 |
| Homemaker/employed | 169 (48.4) |
| Unemployed/on sick leave/studying | 79 (22.6) |
| Retired | 101 (28.9) |
| Smoker1 | n=283 |
| 86 (30.4) | |
| Comorbidities (Charlson index)2 | n=422 |
| 0-3 | 154 (36.5) |
| 4-9 | 201 (47.6) |
| > 10 | 67 (15.9) |
| Undergoing treatment with a psychoactive drug3, n (%) | n=420 |
| 141 (33.6) | |
| Size of the patient's home town, n (%) | n = 423 |
| < 5,000 residents | 24 (5.7) |
| 5,000-20,000 residents | 127 (30.0) |
| > 20,000 residents | 272 (64.3) |
| Distance between home and hospital, n (%) | n=423 |
| < 10 km | 238 (56.3) |
| ≥ 10 km | 185 (43.7) |
| Disease | n=423 |
| Rheumatoid arthritis | 216 (51.1) |
| Ankylosing spondylitis | 107 (25.3) |
| Psoriatic arthritis | 100 (23.6) |
| Years since diagnosis, mean ± SD | n=423 |
| 8.3 ± 8.1 | |
| Erythrocyte sedimentation rate (mm/h), median (IQR) | n=396 |
| 23.0 (1-140) | |
| C-reactive protein (mg/L), median (IQR) | n=391 |
| 9.0 (0-120) | |
| Haemoglobin (mg/dL), mean ± SD | n=406 |
| 13.4 ± 1.6 | |
| Concomitant methotrexate, n (%) | n=423 |
| 166 (39.2) | |
| Concomitant leflunomide, n (%) | n=423 |
| 29 (6.9) | |
| Concomitant glucocorticoids, n (%) | n=411 |
| 247 (60.1) | |
| Daily glucocorticoids dose (mg), mean ± SD | n=411 |
| 4.9±5.3 | |
| Biological therapy, n (%) | n=423 |
| Adalimumab | 180 (42.5) |
| Etanercept | 121 (28.6) |
| Golimumab | 35 (8.3) |
| Abatacept | 25 (5.9) |
| Tocilizumab | 29 (6.8) |
| Certolizumab | 20 (4.7) |
| Infliximab | 11 (2.6) |
| Ustekinumab | 2 (0.5) |
BT: biological therapy; IQR: interquartile range; n: number of lines of biological therapy; SD: standard deviation.
The total number of lines of BT analysed was 423. The values presented in this
Table refer to the number of lines for which data were available on the variables analysed.
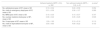
The mean adherence ± SD measured according to the MPR was 0.89 ± 0.16. There were no differences between the pathologies: the mean ± SD was 0.90 ± 0.17 in RA, 0.89 ± 0.16 in AS and 0.89 ± 0.15 in PsA. The percentage of patients with an MPR ≥ 0.8 was similar across all three diseases: 187 in RA (87%), 91 in AS (85%) and 84 in PsA (84%).
Table 2 shows the MPR data separately for each BT. Given the difference in the number of lines between the different BT, it was not possible to perform a statistical analysis that showed statistically significant differences between them.
Adherence measured in accordance with the medication possession ratio of the lines of biological therapy
| Biological therapy, n=423 | Medication possession ratio: mean ± SD |
|---|---|
| Abatacept1, n=25 | 0.86 ± 0.19 |
| Adalimumab, n = 180 | 0.88 ± 0.17 |
| Certolizumab, n=20 | 0.92 ± 0.15 |
| Etanercept, n = 121 | 0.88 ± 0.17 |
| Golimumab, n=35 | 0.94 ± 0.11 |
| Infliximab, n = 11 | 0.91 ± 0.15 |
| Tocilizumab1, n=29 | 0.93 ± 0.11 |
| Ustekinumab, n = 2 | 0.89 ± 0.16 |
SD: standard deviation.
The sample was divided into two groups: adherent patients (MPR ≥ 0.8; n = 362) and non-adherent patients (MPR < 0.8; n = 61). Table 3 shows the factors analysed in the univariate study.
Factors that may influence non-adherence to biological therapy. Univariate study
| Lines of BT with MPR ≥ 0.8, n= 362 | Lines of BT with MPR < 0.8, n= 61 | p-value1 | |
|---|---|---|---|
| Age in years | |||
| Mean ± SD | 49.7 ± 13.8 | 49.8 ± 14.3 | 0.968 |
| Sex, n (%) | |||
| Females | 201 (55.5) | 27 (44.3) | 0.068 |
| Males | 161 (44.5) | 34 (55.7) | |
| Years since diagnosis | |||
| Mean ± SD | 8.2 ± 8.0 | 8.5 ± 9.4 | 0.938 |
| Education level, n (%) | |||
| University | 35 (16.9) | 0 (0.0) | 0.172 |
| Upper secondary/Vocational education | 57 (27.5) | 8 (36.4) | |
| Basic | 111 (53.6) | 13 (59.1) | |
| No schooling | 4 (1.9) | 1 (4.5) | |
| Employment status, n (%) | |||
| Unemployed/on sick leave/studying | 64 (21.2) | 15 (31.3) | 0.192 |
| Retired | 86 (28.6) | 15 (31.3) | |
| Employed/homemaker | 151 (50.2) | 18 (37.5) | |
| Size of the patient's home town, n (%) | |||
| < 5,000 residents | 24 (6.6) | 0 (0.0) | |
| 5,000-20,000 residents | 109 (30.1) | 18 (29.5) | |
| > 20,000 residents | 229 (63.3) | 43 (70.5) | 0.107 |
| Distance between home and hospital, n (%) | |||
| < 10 km | 207 (57.2) | 31 (50.8) | 0.215 |
| ≥ 10 km | 155 (42.8) | 30 (49.2) | |
| Comorbidities (Charlson index), n (%)2 | |||
| 0-3 | 135 (37.4) | 19 (31.1) | |
| 4-9 | 174 (48.2) | 27 (44.3) | |
| ≥ 10 | 52 (14.4) | 15 (24.6) | 0.126 |
| Smoker3, n (%) | |||
| Yes | 67 (27.6) | 19 (47.5) | 0.011 |
| No | 176 (72.4) | 21 (52.5) | |
| Undergoing treatment with a psychoactive drug4, n (%) | |||
| No | 120 (33.4) | 21 (34.4) | |
| 239 (66.6) | 40 (65.6) | 0.493 | |
| Disease | |||
| Rheumatoid arthritis | 187 (51.7) | 29 (47.5) | |
| Ankylosing spondylitis | 91 (25.1) | 16 (26.2) | |
| Psoriatic arthritis | 84 (23.2) | 16 (26.2) | 0.819 |
| Haemoglobin (mg/dL), mean ± SD | 13.3 ± 1.6 | 13.3 ± 1.6 | 0.758 |
| C-reactive protein (mg/L), median (IQR) | 8 (0-120) | 8 (0-105) | 0.293 |
| Erythrocyte sedimentation rate (mm/h), median (IQR) | 21 (1-140) | 31 (5-96) | 0.060 |
| Therapy line number, n (%) | |||
| 1st line | 217 (59.9) | 34 (55.7) | 0.315 |
| Subsequent lines | 145 (40.1) | 27 (44.3) | |
| Type of BT, n (%) | |||
| Anti-TNF-α | 315 (87.0) | 52 (85.2) | |
| Non-anti-TNF-α | 47 (13.0) | 9 (14.8) | 0.418 |
| Concomitant methotrexate at the start, n (%) | |||
| Yes | 148 (45.0) | 18 (35.3) | |
| No | 181 (55.0) | 33 (64.7) | 0.125 |
| Concomitant glucocorticoids at the start, n (%) | |||
| Yes | 214 (60.6) | 33 (56.9) | |
| No | 139 (39.4) | 25 (43.1) | 0.345 |
| Glucocorticoids dose, mg, mean ± SD | 5.2 ± 5.5 | 5.2 ± 6.2 | 0.567 |
| Concomitant leflunomide, n (%) | |||
| Yes | 27 (8.3) | 2 (3.9) | 0.216 |
| No | 298 (91.7) | 49 (96.1) | |
| BT dose regimen at the start, n (%) | |||
| Every 7 days | 120 (33.1) | 23 (37.7) | |
|
|
| 0.615 |
| Optimization of the BT dose regimen, n (%) | |||
| Yes | 108 (29.8) | 14 (23.0) | 0.173 |
| No | 254 (70.2) | 47 (77.0) | |
| Place where the BT was administered, n (%) | |||
| Away from the hospital | 324 (89.5) | 55 (90.2) | |
| In the Day Hospital | 38 (10.5) | 6 (9.8) | 0.545 |
| No. visits to the RC per patient/year of BT, mean ± SD | 2.50 ± 1.40 | 2.40 ± 1.90 | 0.168 |
| No. no-shows to RC appointments, per patient/year of BT mean ± SD | 0.05 ± 0.15 | 0.17 ± 0.38 | 0.004 |
| No. visits to PS, per patient/year of BT, mean ± SD | 7.97 ± 3.19 | 5.5 ± 3.02 | < 0.001 |
To calculate the percentages, the number of events was divided by the number of adherent or non-adherent patients.
Anti-TNF-α: anti-tumour necrosis factor alpha; BT: biological therapy; IQR: interquartile range; MPR: medication possession ratio; n: number of patients; RC: Rheumatology Clinic; PS: Pharmacy Service; SD: standard deviation.
The logistic regression analysis showed that better adherence to BT correlated with more frequent visits to the PS (odds ratio [OR] 1.2; 95% confidence interval [CI]: 1.1-1.3; p < 0.001) and inversely correlated with a failure to attend scheduled Rheumatology Clinic appointments (OR 0.2; 95% CI: 0.1-0.9; p < 0.001).
With respect to the consequences of poor adherence to BT, no statistically significant differences were detected between the adherent and non-adherent groups. The results are outlined in Table 4.
Consequences of non-adherence to biological therapy
| Adherent patients (MPR ≥ 0.8), n= 362 | Non-adherent patients (MPR < 0.8), n= 61 | p-value1 | |
|---|---|---|---|
| No. admissions/year of BT, mean ± SD | 0.13 ± 0.34 | 0.24 ± 0.56 | 0.054 |
| No. visits to emergency dept/year of BT, mean ± SD | 0.31 ± 0.56 | 0.56 ± 1.07 | 0.069 |
| No. MRIs/year of BT, mean ± SD | 0.15 ± 0.33 | 0.15 ± 0.37 | 0.707 |
| No. nuclear medicine tests/year of BT, mean ± SD | 0.06 ± 0.42 | 0.05 ± 0.29 | 0.535 |
| No. X-rays/year of BT, mean ± SD | 1.50 ± 1.93 | 2.10 ± 3.19 | 0.110 |
| No. visits to Specialized Care/year of BT, mean ± SD | 3.50 ± 4.63 | 4.80 ± 6.22 | 0.153 |
To calculate the percentages, the number of events was divided by the number of adherent or non-adherent patients.
BT: biological therapy; MPR: medication possession ratio; MRI: magnetic resonance imaging; SD: standard deviation.
The data obtained on the percentage of adherent patients were similar to those published in studies on patients with RA, and ranged from 85.7% to 88.8%8,9. The percentage of adherent patients with PsA and AS (89% for both diseases) was similar to the percentage of adherent patients with RA. Arturi P et al.10 reported similar findings in their publication, which found that patients with AS presented a similar degree of adherence to patients with RA.
The factor that correlated most with adherence to BT was frequent attendance at PS appointments. Furthermore, a failure to attend rheumatology appointments on the scheduled date was found to be a predictor of non-adherence. We were not able to find any studies on patients with CIA and BT that reported a correlation between these aspects, although the relationship has been contemplated in other conditions such as HIV20. Therefore, the fact that patients with more involvement in the healthcare system and greater trust in healthcare professionals have a higher likelihood of adhering to biological therapies represents a novel finding.
In line with our results, studies published on the Spanish population have reported no differences with respect to age, sex or biological therapy line number and adherence to BT8,9. However, Calip et al.11 conducted a study in 2018 that related increased age, female gender and presence of comorbidities with poorer adherence, although the adherence data in that study, which was conducted in the United States, differed greatly from ours; just 37% of the patients were considered adherent.
With respect to BT-related aspects, the use of subcutaneously administered BT could be a predictor of non-adherence9 with respect to intravenously administered BT. However, our study found no differences in terms of whether the BT was administered at the DH or during a home visit (subcutaneous). This difference between our study and the published data may be due to the low number of BT that were administered intravenously in our study. Moreover, we found no differences with respect to the different dosing intervals, unlike other studies on RA, which reported that weekly administration as opposed to monthly administration was a predictor of poor adherence to BT8. This inconsistency with the results of our study could be attributed to the fact that we performed a joint analysis of patients with RA, PsA and AS. No differences were found in adherence between patients with optimized and non-optimized dosage regimens, which could explain the lack of influence of the dosing interval type on adherence.
Our work presented significant differences between the number of patients with adalimumab or etanercept with respect to other BT, a factor that ruled out a comparative analysis between the different BT. When BT were grouped according to their mechanism of action (those with an anti-tumour necrosis factor alpha mechanism of action versus those with another mechanism of action), no differences were found between the two groups, although in a publication by Smolen et al. (2019)7, the use of anti-tumour necrosis factor alpha was a predictor of adherence, not compared to other BT but compared to synthetic disease-modifying drugs.
According to our results, poorer adherence to BT does not translate into a higher number of emergency department visits, hospital appointments or hospital admissions. However, these data are not consistent with other studies on patients with CIA, in which non-adherent patients made significantly greater use of resources compared to adherent patients11,12. One possible explanation for this finding is that non-adherent patients reduce their dosage independently when they feel well, much like when healthcare professionals optimize BT in a more regulated way when a patient is stable21.
One of the limitations of our study was its retrospective nature; however, the ability to conduct an eight-year follow-up study represented an advantage. Another potential limitation was the single method used to assess adherence. However, the application of a method such as the Morisky-Green test in such patients does not seem to be as useful as in other pathologies8. Moreover, given the retrospective nature of the study, the use of a questionnaire would not be valid for prior therapies.
ConclusionsAccording to the data obtained, patients with RA, AS and PsA present no differences in terms of their adherence to BT. It would seem that adherence to BT is not influenced by sociodemographic or pharmacological factors. However, a correlation was detected between a patient's level of cooperation with the pharmacist or doctor and his or her adherence. The use of BT at lower doses due to a lack of adherence does not translate into a reduction in the survival of the BT or a rise in the use of healthcare resources.
FundingThis study was funded by an unrestricted grant from Pfizer.
AcknowledgementsThanks to the Statistics Unit of our Hospital, and specifically to Cristina Martínez Reglero for all her statistical support.
Conflict of interestsNo conflict of interest.
Presentation in CongressesNational Congress of the Spanish Society of Hospital Pharmacy, Valencia, November 15, 2015.
Contribution to the scientific literature
In the case of patients undergoing treatment with biological therapies, there are no differences in the adherence of patients diagnosed with rheumatoid arthritis, ankylosing spondylitis and psoriatic arthritis.
Sociodemographic and medication-related factors were not found to influence adherence. However, patients with greater involvement in the healthcare system have a higher probability of adhering to biological therapies.
Our study found that poor adherence to biological therapies by patients with chronic inflammatory arthropathies does not imply a greater use of hospital resources by these patients, in contrast to patients with other diseases.