In the context of the advancement of antiretroviral therapy and as the characteristics of people living with HIV progress toward an ageing population, understanding the causes of treatment interruption becomes crucial. The aim of the study was to determine the change in reasons for antiretroviral treatment discontinuation for 12 years. Secondarily, compare annual antiretroviral regimen discontinuation rate and factors associated.
MethodsWe conducted an analysis using data from people living with HIV who were receiving antiretroviral therapy and discontinued it for any reason. The study included people with HIV infection who visited an outpatient hospital pharmacy clinic from January 2010 to December 2021. Two periods were differentiated for the analysis: 2010–2015 and 2016–2021. The reasons for antiretroviral treatment discontinuation followed classification described by Swiss cohort. In the context of this study, it is pertinent to note that the term “discontinuation” is employed synonymously with “interruption”. The term “discontinuation” will be consistently used in this article to refer to the act of switching or stopping antiretroviral treatment. To examine factors associated with antiretroviral therapy discontinuation, we utilised Kaplan–Meier methods and Cox proportional models.
ResultsWe included 789 people living with HIV, predominantly male (81.5%). The main reason for discontinuation was clinical decision (50.2%) followed by adverse effects (37.9%). Focusing on clinical decision, we observed a trend change that went from antiretroviral treatment simplification regimen (56.1%) in the first part of the period analysed to the therapeutic optimisation (53.6%) in the second half. Furthermore, factors that were statistically significantly associated with antiretroviral treatment discontinuation were people with HIV≥50 years (HR 1.60; 95%CI 1.25–2.04), post-discontinuation single-tablet regimen (HR 1.49; 95%CI 1.06–2.11) and antiretroviral drug classes.
ConclusionOver the 12 years, there has been a change in the main cause of antiretroviral treatment discontinuation, currently therapeutic optimisation being the main reason. Integrase inhibitors-based regimens and single-tablet regimen strategies were less likely to be discontinued than others antiretroviral drug classes, allowing for better clinical management due to the efficacy profile, especially in people living with HIV≥50 years with comorbidities.
la optimización del tratamiento antirretroviral y a medida que las personas que viven con el VIH envejecen, comprender las causas de la interrupción del tratamiento, se vuelve fundamental. El objetivo del estudio fue determinar el cambio en las razones de discontinuación del tratamiento antirretroviral durante 12 años. Secundariamente, comparar la tasa anual de discontinuación del régimen antirretroviral y factores asociados.
Métodorealizamos un análisis con datos de personas con infección VIH que recibían terapia antirretroviral y la interrumpieron por cualquier motivo. El estudio incluyó a personas VIH que visitaron la consulta de atención farmacéutica desde enero-2010 hasta diciembre-2021. Se diferenciaron 2 períodos para el análisis: 2010–2015 y 2016–2021. Las razones para la discontinuación siguieron la clasificación descrita por la cohorte suiza. En este estudio el término «discontinuación» es sinónimo de «interrupción». El término «discontinuación» se empleó para cambios del tratamiento e interrupciones definitivas. Para analizar los factores asociados a la discontinuación, utilizamos métodos de Kaplan–Meier y modelos proporcionales de Cox.
Resultadosincluimos a 789 personas con infección por VIH, mayoritariamente hombres (81,5%). La principal razón de discontinuación fue decisión clínica (50,2%), seguida de efectos adversos (37,9%). Centrándonos en la decisión clínica, observamos un cambio de tendencia que pasó de la simplificación del régimen (56,1%) en la primera parte del período analizado a la optimización terapéutica (53,6%) en la segunda mitad. Además, los factores estadísticamente significativos asociados con la discontinuación del fueron edad ≥ 50 años (HR 1,60; IC del 95% 1,25–2,04), régimen de un único comprimido/día posdiscontinuación (HR 1,49; IC del 95% 1,06–2,11) y clases de medicamentos antirretrovirales.
Conclusiónen los últimos 12 años, ha habido un cambio en la principal causa de discontinuación del tratamiento antirretroviral, siendo la optimización terapéutica la razón principal. Los regímenes basados en inhibidores de integrasa y las estrategias de regímenes de un único comprimido/día tenían menos probabilidad de ser discontinuados que otras clases de medicamentos antirretrovirales, lo que permite un mejor manejo clínico debido al perfil de eficacia, especialmente en personas que viven con VIH, mayores de 50 años y con comorbilidades.
Antiretroviral therapy (ARV) has significantly improved the life expectancy of people living with HIV (PLWH) by reducing morbidity and mortality rates.1 Gradual ageing of PLWH results in increased polypharmacy, which subsequently increases the risk of adverse effects (EAs), drug interactions, and potentially inappropriate prescriptions.2
In the last decade, new drugs have emerged that offer enhanced efficacy, improved tolerability and toxicity profiles, and more convenient dosing and formulations compared to traditional drugs. As a result, rates of virological failure with the initial ARV regimens have declined significantly in observational cohorts.3,4 The main reason for discontinuation of treatment in the past has been the intolerance and toxicity associated with the drugs used in ARV.5 However, optimisation of the safety profile of these new drugs has allowed a reduction in the commonly linked AEs.
Several studies have identified factors contributing to discontinuation, including complex regimens, multiple daily administrations, treatment with a protease inhibitor (PI), or a high baseline viral load.6,7 Beyond these factors, significant advancements and changes have occurred both clinically and in pharmaceutical care. Therefore, it can be said that there have been changes toward new causes of abandonment that imply a pharmacotherapeutic optimisation. The concept of optimising pharmacotherapy revolves around ensuring that each person receives the most appropriate pharmacotherapeutic alternatives for their clinical conditions. The activities encompassed by pharmacotherapeutic optimisation imply simplification of treatments, such as the transition from triple therapy to conformed dual therapy or change of treatment to avoid possible interactions with other essential drugs in the patient's pharmacotherapeutic regimen.8
However, most studies investigating the causes of discontinuation were conducted prior to or during the initial years of the widespread adoption of integrase strand transfer inhibitors (INSTI) regimens. Randomised controlled trials employing innovative regimens, primarily composed of various INSTIs, have consistently demonstrated favourable efficacy, tolerability, and ease of administration.9 These attributes promote discontinuation in pursuit of pharmacotherapeutic optimisation. Nevertheless, there is a paucity of studies that compare regimens containing older drugs with contemporary regimens predominantly based on INSTIs, especially concerning their durability and reasons for regimen discontinuation.10,11
The aim of this study was to determine the change in reasons for antiretroviral treatment (ARV) discontinuation for 12 years. Secondarily, to compare the annual ARV discontinuation rate and factors associated.
Material & methodsStudy design and participantsThis was a single-centre prospective observational study. PLWH who attended from January 2010 to December 2021 in hospital pharmacy outpatient service were included in our analysis if they were older than 18 years and were on active ARV. Patients who discontinued ARV for any reason discontinuation were eligible for analysis.
Variables were collected during outpatient hospital pharmacy visits when the patient discontinued ARV, both the first and subsequent discontinuations. The following variables were analysed: demographic (age, sex); analytical data, plasma viral load (copies/ml), CD4 cell count (cells/μL); and clinical variables related to comorbidities and pharmacotherapeutics, such as type of ARV (before/after discontinuation), number of drugs of ARV scheme, reason for ARV discontinuation, concomitant medications, polypharmacy, polypharmacy patterns, comorbidities, multimorbidity patterns, and medication regimen complexity index (MRCI). Only patients with all completed variables were included in the analysis.
Definition of the endpointThe outcome was defined as the duration to discontinuation (either through switching or stopping) of ARV therapy. In this study, it's important to highlight that the term “discontinuation” is used synonymously with “interruption”. The term “discontinuation” will be consistently used in this article to refer to the act of switching or stopping antiretroviral treatment. Modification of treatment was characterised as any alteration in at least one antiretroviral drug within the regimen, excluding dosage adjustments. Stoppage was considered when all drugs within the regimen were discontinued for a minimum period of 30 days.
Reasons for discontinuation were classified using the classification described by the Swiss cohort, which orders them into AEs, virological failure, clinical decision (including interactions or optimisation of pharmacotherapy), patient decision (including voluntary abandonment or lack of adherence), or others (including pregnancy).12
To find out if this cause of discontinuation was associated with treatment optimisation, a subclassification was carried out within the cause of discontinuation associated with clinical decision in the following categories: simplification, optimisation, or change due to the presence of drug interactions. ARV simplification is defined as a reduction in the number of drugs that make up the regimen or a switch to a combination that offers the ability to deliver an entire regimen in a single, once-daily pill. Optimisation of ARV can be understood as a process aimed at improving the long-term efficacy, adherence, tolerability, safety, convenience, and affordability of combination ARV.
The ARV discontinuation period was classified as short period (<3 months), medium (between 3 months and 1 year), and long period (>1 year) depending on the duration of the prescribed ARV that patients discontinued. Furthermore, we conducted a comparison between 2 distinct time periods: an early period spanning from 2010 to 2015, and a later period covering 2016 to 2021. A defined temporal cut-off was applied within the overall 6-year period for both intervals to facilitate a comparable temporal analysis. Furthermore, the second period (2016–2021) corresponds specifically to the timeframe during which the latest treatment regimens incorporating integrase strand transfer inhibitors (INSTI) and single-tablet regimens (STR) became accessible.
DefinitionsComorbidity was all chronic disease that was present in the patient at the beginning or that appeared during the study. Together with the presence or absence of comorbidity, the type of comorbidity was collected. Comorbidity patterns were also classified according to the study published by De Francesco et al.13
Polypharmacy was defined as the use of 6 or more different drugs, including antiretroviral medication; major polypharmacy was restricted to the use of 11 different drugs. To describe the patterns of polypharmacy, we employed the categorisation proposed by Calderón-Larrañaga et al. which classifies patterns based on the intended treatment for specific diseases. A patient was classified under a particular polypharmacy pattern if prescribed at least 3 drugs included in that pattern.14
The MRCI index is a validated 65-item tool assessing treatment regimen complexity, considering factors such as the number of medications, dosage form, dosage frequency, and additional instructions. The index score ranges from 1.5 (for individuals taking a single tablet or capsule once daily) to an undefined maximum, as the score escalates with the number of medications. Higher scores signify increased regimen complexity.15 Additionally, according to Morillo-Verdugo et al., a cut-off value of 11.25 for MRCI index score was employed for considering complex patient.16
ARV regimens were categorised based on their classes as follows: a combination of 2 nucleoside analogue reverse transcriptase inhibitors (NRTIs) along with a third agent, which could be a non-nucleoside reverse transcriptase inhibitor (NNRTI), a protease inhibitor (PI), or an INSTI. ARV regimens that incorporate alternative approaches that do not follow the triple therapy schemes referenced above are included within the broader category of “others”. ARV regimens also were classified based on the number of drugs that make up the ARV schemes: triple therapy, bitherapy, or monotherapy.
Statistical analysesBaseline characteristics of included patients were reported as absolute numbers with percentage and median with interquartile range (IQR), as appropriate. Patients were subjected to comparison between the periods of 2010–2015 and 2016–2021, employing the chi-square test or Mann–Whitney U test, as deemed appropriate.
First, we assessed the durability of ARV regimen, which was defined as the discontinuation (switching or stopping) of the ARV drug class. The durability of ARV was analysed using the Kaplan–Meier method to identify independent factors associated. Differences among subgroups were evaluated using the log-rank test. To identify factors associated with the durability of ARV regimen, a multivariable Cox regression model was employed. All variables that demonstrated an association with discontinuation in the bivariate analysis using the Cox model (p<.20) were included in the multivariate Cox analysis. Backward selection was utilised to eliminate variables until reaching the final Cox model. Hazard ratios (HRs) with 95% confidence intervals (CIs) were reported to assess the strength and association between the variables.
P values of <0.05 were considered statistically significant. Data analysis was performed using SPSS for MacOS version 28.0 (SPSS, Chicago, IL, USA).
Ethical considerationsData collected from the study cohort is generated during usual care. The study fulfilled all the ethical requirements and was approved by the Clinical Research Ethics Committee of Sevilla-Sur (C.I. 0174-N-20). This study was carried out in accordance with the Declaration of Helsinki guidelines for biomedical research.
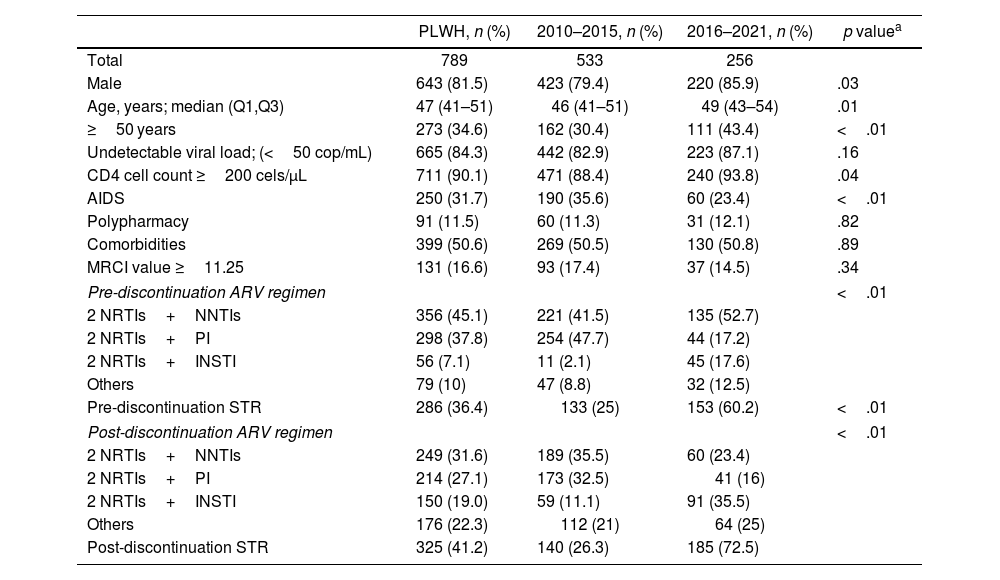
ResultsIn the period January 2010–December 2021, a total of 789 PLWH met the inclusion criteria and were eligible for our analyses. Patients were predominantly male (88.2%) and the median age at the time of discontinuation of ARV was 47 years (IQR, 40–52 years). At the ARV discontinuation moment, most PLWH were virologically suppressed (84.3%) and 90.1% of the patients had a CD4 cell count ≥200 cells/μL. Baseline patient characteristics at the first discontinuation are shown in Table 1. The baseline characteristics of patients in successive discontinuations are presented in the supplementary material (Table 1, second discontinuation; Table 2, third discontinuation).
Baseline characteristics at the first discontinuation.
| PLWH, n (%) | 2010–2015, n (%) | 2016–2021, n (%) | p valuea | |
|---|---|---|---|---|
| Total | 789 | 533 | 256 | |
| Male | 643 (81.5) | 423 (79.4) | 220 (85.9) | .03 |
| Age, years; median (Q1,Q3) | 47 (41–51) | 46 (41–51) | 49 (43–54) | .01 |
| ≥50 years | 273 (34.6) | 162 (30.4) | 111 (43.4) | <.01 |
| Undetectable viral load; (<50 cop/mL) | 665 (84.3) | 442 (82.9) | 223 (87.1) | .16 |
| CD4 cell count ≥200 cels/μL | 711 (90.1) | 471 (88.4) | 240 (93.8) | .04 |
| AIDS | 250 (31.7) | 190 (35.6) | 60 (23.4) | <.01 |
| Polypharmacy | 91 (11.5) | 60 (11.3) | 31 (12.1) | .82 |
| Comorbidities | 399 (50.6) | 269 (50.5) | 130 (50.8) | .89 |
| MRCI value ≥11.25 | 131 (16.6) | 93 (17.4) | 37 (14.5) | .34 |
| Pre-discontinuation ARV regimen | <.01 | |||
| 2 NRTIs+NNTIs | 356 (45.1) | 221 (41.5) | 135 (52.7) | |
| 2 NRTIs+PI | 298 (37.8) | 254 (47.7) | 44 (17.2) | |
| 2 NRTIs+INSTI | 56 (7.1) | 11 (2.1) | 45 (17.6) | |
| Others | 79 (10) | 47 (8.8) | 32 (12.5) | |
| Pre-discontinuation STR | 286 (36.4) | 133 (25) | 153 (60.2) | <.01 |
| Post-discontinuation ARV regimen | <.01 | |||
| 2 NRTIs+NNTIs | 249 (31.6) | 189 (35.5) | 60 (23.4) | |
| 2 NRTIs+PI | 214 (27.1) | 173 (32.5) | 41 (16) | |
| 2 NRTIs+INSTI | 150 (19.0) | 59 (11.1) | 91 (35.5) | |
| Others | 176 (22.3) | 112 (21) | 64 (25) | |
| Post-discontinuation STR | 325 (41.2) | 140 (26.3) | 185 (72.5) | |
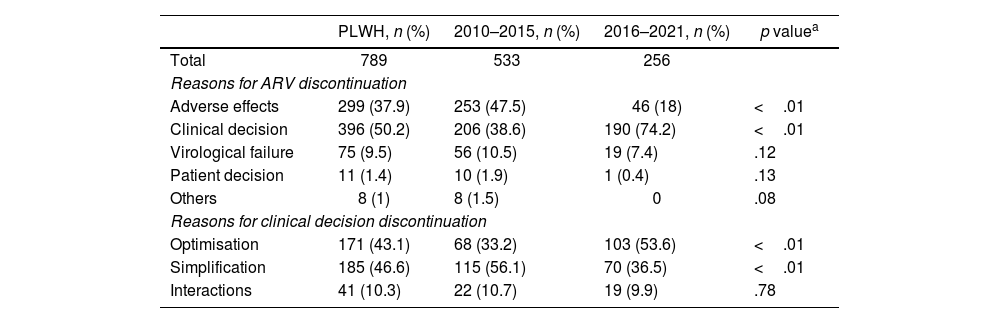
Reasons for ARV discontinuation.
| PLWH, n (%) | 2010–2015, n (%) | 2016–2021, n (%) | p valuea | |
|---|---|---|---|---|
| Total | 789 | 533 | 256 | |
| Reasons for ARV discontinuation | ||||
| Adverse effects | 299 (37.9) | 253 (47.5) | 46 (18) | <.01 |
| Clinical decision | 396 (50.2) | 206 (38.6) | 190 (74.2) | <.01 |
| Virological failure | 75 (9.5) | 56 (10.5) | 19 (7.4) | .12 |
| Patient decision | 11 (1.4) | 10 (1.9) | 1 (0.4) | .13 |
| Others | 8 (1) | 8 (1.5) | 0 | .08 |
| Reasons for clinical decision discontinuation | ||||
| Optimisation | 171 (43.1) | 68 (33.2) | 103 (53.6) | <.01 |
| Simplification | 185 (46.6) | 115 (56.1) | 70 (36.5) | <.01 |
| Interactions | 41 (10.3) | 22 (10.7) | 19 (9.9) | .78 |
Over time, there have been significant changes in the composition of ARV regimens, primarily driven by the introduction of new drugs such as INSTI or co-formulated medications that make it easier to prescribe single-tablet regimens (STRs). Initially, from 2010–2015, the most frequently prescribed ARV regimens before discontinuation consisted of 2 NRTIs and a PI (47.7%), followed by NNTI-based regimen (41.5%). However, in the subsequent period, ARV regimens composed of 2 NRTIs and a NNTI became more common (52.7%).
However, the ARV regimens after discontinuation in the first period resembled the pre-continuation regimens, with a higher prevalence of combinations based on NNTI (35.5%) and PI-based (32.5%) combinations. On the contrary, during 2016–2021 period, INSTI-based ARV regimens began to dominate (35.5%). Of the 789 PLWH who discontinued their ARV, 41.2% switched to a STR ARV regimen. The percentage of STR schemes witnessed a significant increase, rising from 26.3% during the 2010–2015 period to a substantial 72.5% between 2016 and 2021 (p<.01).
Considering the defined time periods of duration of the prescribed ARV that patients discontinue, 103 of 789 patients discontinued (13.1%) in an early period, 12.1% in the medium, and 74.9% in a later period. Of all PLWH who discontinued the ARV regimen, 485 had a second interruption (69.4% in the late period) and 243 a third discontinuation (71.8% in the late period). The median duration of ARV to discontinuation was 45 months (IQR: 13–69). Considering the 2 periods analysed, this median duration to discontinuation was longer in the 2016–2021 period (77 months; IQR: 22–111) than in the earlier period (38 months; IQR: 13–69).
The main reason for discontinuing the ARV regimen was the clinical decision (50.2%), followed by AEs (37.9%), virological failure (9.5%), patient decision (1.4%), and others (1%). Focusing on the reasons associated with the clinical decision, treatment simplification (46.6%) followed by optimisation (43.1%) and avoiding drug–drug interactions (10.3%) predominates. The full description of the reasons associated with the discontinuation of ARV by period studied is shown in Table 2.
The discontinuation rate for AEs decreased over time (47.5% for 2010–2015 vs 18% for 2016–2021), while the modification of the clinical decision showed an increasing trend (38.6% and 74.2% during 2010–2015 and 2016–2021, respectively). Virologic failure was not commonly reported as a reason for the discontinuation of ARV, and its percentage did not vary significantly between the periods studied. In the period 2016–2021, the change in regimen according to clinical decision associated with therapeutic optimisation increased significantly compared to the period 2010–2015 (53.6% vs 33.2%). However, the clinical decision associated with the simplification of treatment was reduced and the prevention of interactions remained constant throughout the study period.
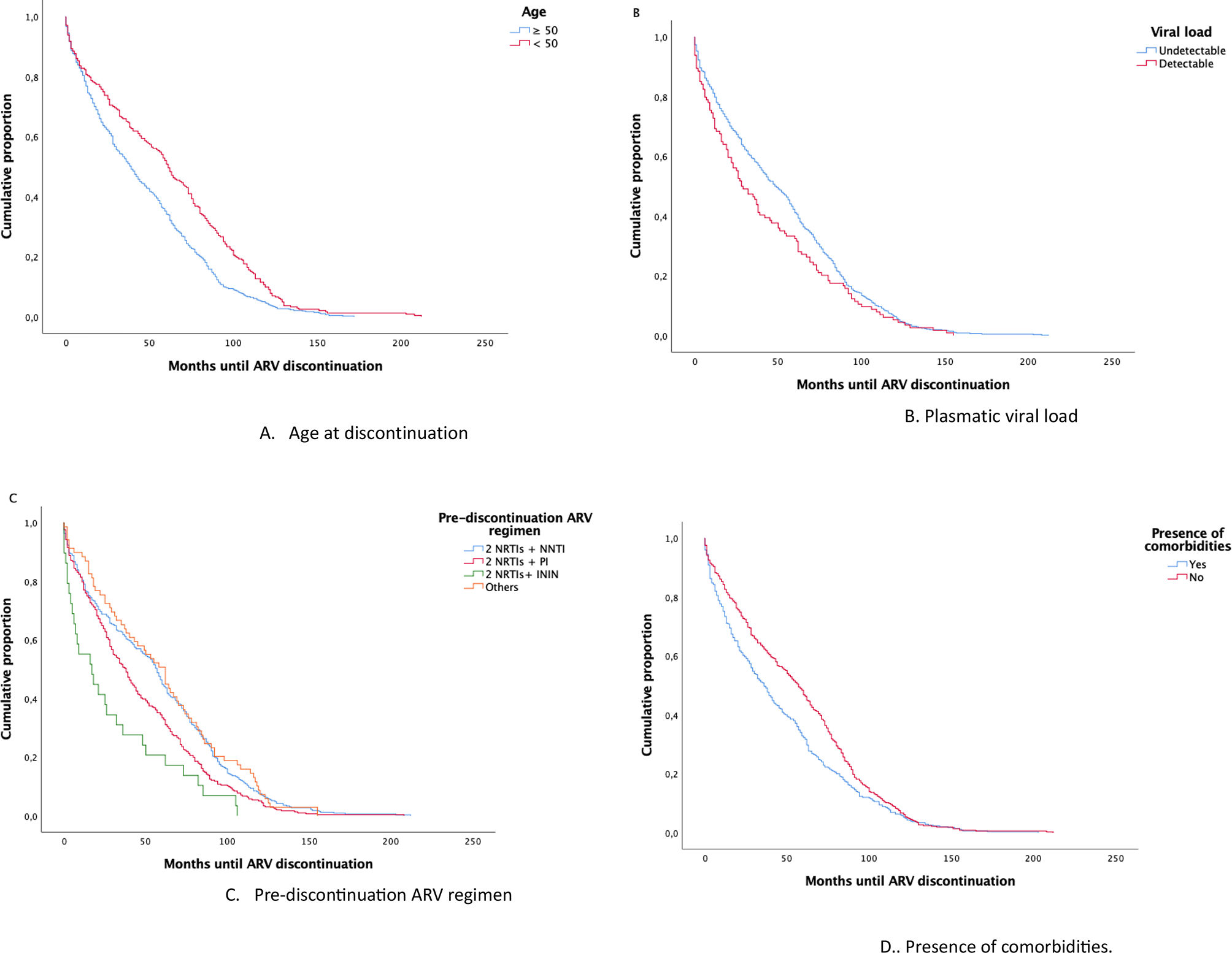
Focusing on factors associated with discontinuation of ARV, statistically significantly associated were identified using the Long-Rank test between the age of PLWH age at discontinuation (p<.01), detectable plasmatic viral load (p=0.04), pre-discontinuation ARV regimen (p<.01), comorbidities (p<.01) and the year of ARV initiation (p<.01). Time-to-event analyses were performed to identify prognostic factors associated with discontinuation of the ARV regimen. These factors, except for the year of ARV initiation, were illustrated in the the Kaplan–Meier plots depicted in Fig. 1 (A, age at discontinuation; B, plasmatic viral load; C, pre-discontinuation ARV regimen; D, presence of comorbidities). An included supplementary figure illustrates the cumulative proportion of pre-discontinuation antiretroviral (ARV) durability based on the pre-discontinuation ARV regimen (Fig. 1) during the 2 analysed periods (A, 2010–2015; B, 2016–2021) to differentiate drug patterns across the studied time frames.
PLWH ≥50 years were more likely to discontinue ARV regimen than less than 50 years (HR 1.45; 95%CI 1.24–1.69). The presence of comorbidities was significantly more likely to discontinue ARV (HR 1.25; 95%CI 1.08–1.45). Furthermore, patients who had any concomitant treatment with ARV had a higher risk of discontinuation (HR 1.17; 95%CI 1.01–1.37). However, no differences were found with polypharmacy. Considering pre-discontinuation ARV regimens, we found that being on a PI-based (HR 1.37; 95% CI 1.06–1.78) or an INSTI-based (HR 2.22; 95%CI 1.44–3.34) pre-discontinuation ARV regimen was associated with higher rates of modification, compared to others schemes. The discontinuation of ARV regimens increased in the 2010–2015 period compared to 2016–2021 (HR 2.31; 95%CI 1.92–2.76).
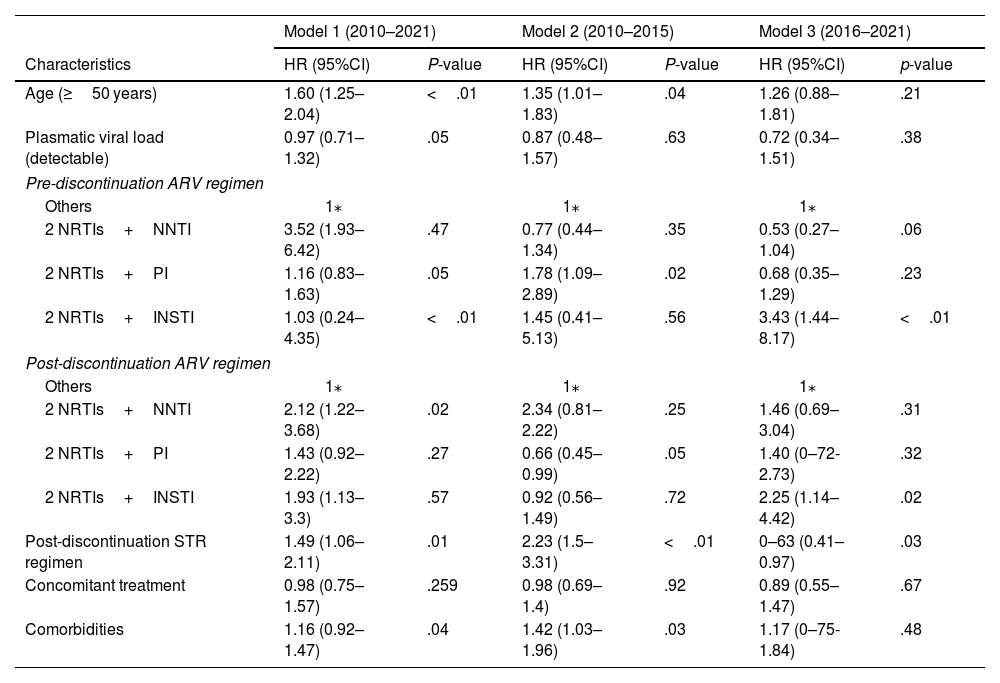
Three multivariate models were carried out to see the differences that exist between the time periods defined for the study. Table 3 presents the results of multivariate Cox proportional-hazard models of factors associated with ARV discontinuation.
Results of multivariate Cox proportional-hazard models.
| Model 1 (2010–2021) | Model 2 (2010–2015) | Model 3 (2016–2021) | ||||
|---|---|---|---|---|---|---|
| Characteristics | HR (95%CI) | P-value | HR (95%CI) | P-value | HR (95%CI) | p-value |
| Age (≥50 years) | 1.60 (1.25–2.04) | <.01 | 1.35 (1.01–1.83) | .04 | 1.26 (0.88–1.81) | .21 |
| Plasmatic viral load (detectable) | 0.97 (0.71–1.32) | .05 | 0.87 (0.48–1.57) | .63 | 0.72 (0.34–1.51) | .38 |
| Pre-discontinuation ARV regimen | ||||||
| Others | 1⁎ | 1⁎ | 1⁎ | |||
| 2 NRTIs+NNTI | 3.52 (1.93–6.42) | .47 | 0.77 (0.44–1.34) | .35 | 0.53 (0.27–1.04) | .06 |
| 2 NRTIs+PI | 1.16 (0.83–1.63) | .05 | 1.78 (1.09–2.89) | .02 | 0.68 (0.35–1.29) | .23 |
| 2 NRTIs+INSTI | 1.03 (0.24–4.35) | <.01 | 1.45 (0.41–5.13) | .56 | 3.43 (1.44–8.17) | <.01 |
| Post-discontinuation ARV regimen | ||||||
| Others | 1⁎ | 1⁎ | 1⁎ | |||
| 2 NRTIs+NNTI | 2.12 (1.22–3.68) | .02 | 2.34 (0.81–2.22) | .25 | 1.46 (0.69–3.04) | .31 |
| 2 NRTIs+PI | 1.43 (0.92–2.22) | .27 | 0.66 (0.45–0.99) | .05 | 1.40 (0–72-2.73) | .32 |
| 2 NRTIs+INSTI | 1.93 (1.13–3.3) | .57 | 0.92 (0.56–1.49) | .72 | 2.25 (1.14–4.42) | .02 |
| Post-discontinuation STR regimen | 1.49 (1.06–2.11) | .01 | 2.23 (1.5–3.31) | <.01 | 0–63 (0.41–0.97) | .03 |
| Concomitant treatment | 0.98 (0.75–1.57) | .259 | 0.98 (0.69–1.4) | .92 | 0.89 (0.55–1.47) | .67 |
| Comorbidities | 1.16 (0.92–1.47) | .04 | 1.42 (1.03–1.96) | .03 | 1.17 (0–75-1.84) | .48 |
NRTI: nucleoside analogue reverse transcriptase inhibitors; NNTI: non-nucleoside reverse transcriptase inhibitor; PI: protease inhibitor; INSTI: integrase strand transfer inhibitor; STR: single-tablet regimen.
This cohort analysis examined the trend and factors associated with discontinuation of the ARV regimen during 2010–2021 in routine clinical care conditions. The results suggest the evolution of the main cause of discontinuation of ARV over the years, highlighting the importance that the clinical decision has acquired in recent years with the aim of optimising pharmacotherapeutics. On the other hand, we observed a reduction in discontinuation of the regimen for AEs during the study period in the cohort.
In line with what has been published by other studies, there has been a trend toward a longer duration of the ARV regimen until its discontinuation in recent years, as identified in other studies.11,17 In our cohort, the primary reasons for the modifications were predominantly attributed to AEs of ARV and clinical decisions, specifically focused on simplifying ARV regimens and optimising therapy.
As a result, we hypothesise that throughout the studied period there would be a change in the main cause of discontinuation toward therapeutic optimisation due to the development of new drugs within groups as important as INSTI that present a favourable profile of efficacy and safety, as well as the increase in the options of ARV regimens with STR strategies.18,19 Our findings confirmed a notable observation that pharmacotherapeutic optimisation regimens by physicians as a driving factor for treatment modification were significantly more prevalent during 2016–2021.
In the 2010–2015 period, EAs related to ARV were the primary cause of discontinuation, consistent with previous studies.20,21 In the 2016–2021 period, there was a notable shift in prescription patterns, marked by the swift adoption INSTI-based regimens, replacing PI-based and NNRTI-based regimens. Additionally, there was a slight decrease in ARV discontinuations due to virologic failures (10.5% in 2010–2015 to 7.4% in 2016–2021), indicating improved adherence and lower rates of virological failure with the introduction of these well-tolerated and effective regimens, including STR.
Once-daily regimens, especially those with an STR, showed increased durability of the treatment, as also demonstrated in other studies.22,23 This effect was likely due to the potentially improved adherence seen with these treatment regimens and allows pharmacotherapeutic optimisation in polymedicated patients.24 Our data confirmed this trend, as we found a greater number of post-discontinuation STR regimens, mostly in 2016–2021.
Several European cohort studies, examining the discontinuation of initial ARV regimens with a 1-year follow-up, particularly in treatment-naïve patients, indicate that regimens incorporating INSTI and STR strategies exhibit lower rates of ARV discontinuation.25,26 Our difference from this study was found in the inclusion of both naive patients and pretreated patients. However, the results followed the trend of these published studies.
The ageing HIV population often requires concomitant ARV treatment due to age-related comorbidities.27,28 Older patients exhibit higher ARV discontinuation rates, possibly linked to comorbidities and concurrent treatment.29 PLWH ≥50 years and comorbidities were associated with ARV discontinuation in general multivariate analysis in our study. Although bivariate analysis indicated a higher likelihood of discontinuation of ARV with concomitant treatment, this difference did not reach statistical significance in multivariate analysis.
The change in the main cause of discontinuation highlighted both the improvement of the drugs that make up antiretroviral medication schemes and the better therapeutic management of PLWHs who aged and presented comorbidities. However, we should not limit ourselves to that clinical evaluation; it is necessary to incorporate a multidimensional evaluation of patients that considers other aspects such as the results reported by the patients.
Despite these results, one of its main limitations must be highlighted, which was its unicentric nature, and hence the possible influence of favouring some guidelines over others, generating a selection bias. However, this bias was reduced given that infectious disease consultations at our centre followed national and international clinical practise guidelines, which standardised care practise with the different national centres that follow these same working documents.
In conclusion, similar characteristics of ARV discontinuation were observed in our cohort compared to those in European countries. The findings of this study indicated a decrease in discontinuation due to AEs over time. This trend was likely attributed to the improved effectiveness and safety profile, as well as the increased dosing convenience of newer treatment regimens that employed STR strategies, primarily centred on INSTI-based regimens. We identified several factors that exhibited a significant association with ARV discontinuation, such as PLWH ≥50 years and presence of comorbidities. Additionally, focusing on 2016–2021 period, a higher discontinuation rate due to clinical decision was found associated with pharmacotherapeutic optimisation, predominantly schemes formed by INSTI and STR strategies.
FundingNone to declare.
Ethical considerationsThe study complied with all ethical requirements and was approved by the Clinical Research Ethics Committee of Seville-South (C.I. 0174-N-20). This study was conducted in accordance with the guidelines of the Declaration of Helsinki for biomedical research.
Transparency statementThe author for the correspondence, on behalf of the other signatories, guarantees the accuracy, transparency and honesty of the data and information contained in the study; that no relevant information has been omitted; and that all discrepancies between authors have been adequately resolved and described.
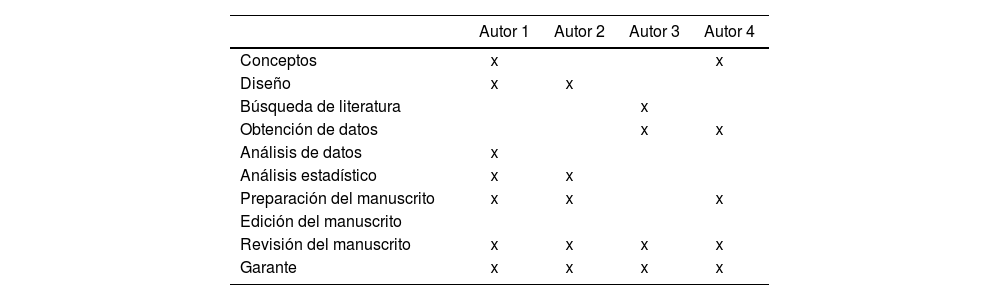
Contribuciones de los autoresCRediT authorship contribution statementEnrique Contreras Macías: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing, Project administration, Validation, Visualization. Antonio Gutiérrez-Pizarraya: Data curation, Methodology, Project administration, Validation, Writing – review & editing. Juan Antonio Pineda Vergara: Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization. Ramón Morillo Verdugo: Conceptualization, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing.