This article describes a study protocol for the implementation of quality and traceability control in the hazardous medication circuit through an analysis of risks and the development and introduction of a Big Data-based software application aimed at performing a continuous and dynamic audit of the whole system.
MethodA standardized graphical modeling tool called Business Process Model Notation will be used to generate a detailed description of each of the stages in the hazardous medication circuit with a view to ensuring full traceability of the system. The information on each stage will be collected in a flowchart, which will be used —together with each event's likelihood of occurrence and severity— as a basis to calculate the criticality index of the different control points established and to determine any control measures that may be required. The flowcharts will also be used to develop the technological support needed to capture all such data as may be relevant to the model. Proper quality control of the process will be ensured by client software agents intended to allow automatic application of efficient data processing tools at the different phases. In addition, Big Data methodologies, in particular machine learning, will be used to develop algorithms based on the repository of generated data to come up with patterns capable of improving the protocols to be applied. Lastly, proper operation of the process will be ensured by means of clinical-pharmaceutical verification and a full technical-documentary review of control and registration systems.
ConclusionsThe development of a risk management system based on mobile technology will allow integration of hazardous drugs into a standardized system, ensuring the safety, quality, and traceability of the hazardous medication handling process.
Describir el protocolo del estudio para la instauración del control del proceso de los medicamentos peligrosos que asegure la calidad y su trazabilidad, mediante el análisis de riesgos, desarrollando e implantando una herramienta informatizada que, gracias a la utilización de técnicas de big data, permita conocer y auditar el conjunto del sistema de forma continua y dinámica.
MétodoMediante los procesos de notación gráfica normalizada Business Process Model Notation se desarrollarán los flujogramas específicos que permitan conocer las etapas del proceso de los medicamentos peligrosos que determinen la trazabilidad total del sistema. Cada una de las etapas será recogida en los cuadros de gestión, donde a través de la probabilidad del suceso y su gravedad se calculará el índice de criticidad de cada punto de control que se determine, y se establecerán las medidas de control. A partir de los cuadros de gestión se desarrollará el soporte tecnológico para la captura de todos los datos que sean pertinentes al modelo. Para asegurar el control de la calidad del proceso se optará por agentes software cliente, que permitan en fases posteriores aplicar herramientas eficientes en el procesamiento de datos de modo automático. A partir de aproximaciones metodológicas del big data, y en particular del ámbito de machine learning, se desarrollarán algoritmos sobre el repositorio de datos generado para poder obtener patrones que permitan mejorar los protocolos de aplicación. Por último, para asegurar el funcionamiento del proceso se realizará la verificación clínico-farmacéutica y la revisión completa, técnico-documental, de los sistemas de control y registro.
ConclusionesLa generación del sistema de gestión de riesgos mediante tecnología móvil permitirá integrar los medicamentos peligrosos en un sistema normalizado, con el fin de mejorar la seguridad, calidad y trazabilidad del proceso de manipulación de los medicamentos peligrosos.
Current management systems prioritize quality-driven healthcare and the suppression or mitigation of unsafe practices. In this respect, the World Health Organization1 has proposed a series of validation procedures to determine the appropriateness of specific healthcare processes. These procedures comprise a series of techniques aimed at finding documentary evidence that the results of a given process consistently comply with the predetermined specifications and the required quality standards.
Drug administration is undoubtedly one of the most important and complex processes that take place in any health center. However, it is often forgotten that before reaching the patient's bedside, the drug goes through a lengthy circuit that often involves more or less sophisticated manipulation. Guaranteeing the safety of the personnel handling those medications, especially when such medications are hazardous drugs (HDs), is a key concern for any hospital2.
It must be taken into consideration that healthcare providers may be exposed to risks related to drug manipulation both within the hospital and at the patients’ homes, where conditions tend to be poorly controlled and direct supervision nonexistent3.
Clinical guidelines and recommendations have been produced to minimize the incidence of errors involved in drug handling in those environments. A well-defined protocol goes a long way in ensuring the theoretical feasibility, quality, and safety of the process for which it has been designed. The factor that determines whether the desired goals can be achieved is adherence by the healthcare providers to such protocols when performing the various steps of the drug handling process.
In these high-risk contexts it is essential to ensure that all pre-established protocols are followed during drug handling and that the expected results are achieved. To do this, certain points of time and/or events, known as control points (CPs), must be defined at which the actions carried out must (should) be monitored. CPs allow verification of the requirements established.
Now, not all CPs are equally critical. For every hazardous process there will be CPs that require specific monitoring; these are known as critical control points (CCPs)4. Both CPs and CCPs are used as a basis to generate data records containing information on the monitoring exercise. Such data records, known as traces, allow for an explicit statement to be made about the states and/or interactions between the different elements involved in the various processes carried out. A thorough and complete log of traces makes it possible to trace the history, usage, and location of an entity. This ability is known as traceability.
It is essential to ensure that all operations are performed in the expected manner and to take the steps required to carry out an exhaustive assessment of the HD process. However, current monitoring systems tend to be retrospective and, although they are capable of identifying potential errors, i.e., potential areas for improvement, they are unable to avoid deficiencies in the follow-up process5.
The availability of rapid-response systems that allow avoidance, or at least prevention, of potential risks associated to HDs would significantly contribute to bolstering healthcare providers’ safety. In this regard, this project proposes introducing Semantic Web and Big Data technologies which, through the implementation of tools capable of processing large amounts of data within a short period of time, may produce patterns that could guide decision-making.
Hypothesis and purposeAlthough the clinical significance of the risks associated with continuous exposure of low levels of HDs has not been firmly established, enough signs exist indicating that such an exposure may result in long-term adverse events warranting the adoption of prevention measures. A better understanding and an evaluation of the risks inherent in the HD handling process could contribute to minimizing the hazards associated with these drugs.
The purpose of this study is to describe a study protocol aimed at developing and implementing an integrated Big Data-based IT risk analysis system capable of monitoring/verifying the HP handling process, which allows continuous and dynamic quality and traceability control of HDs by hospitalat-home units (HHUs).
MethodsScopeThe controls and techniques developed on the basis of this study will be applied to the chemical risks associated to the storage, transport, administration and management of HD residues by the pharmacy departments and HHUs of the two participating (third-level) hospitals.
DesignA standardized graphical modeling tool called Business Process Model Notation will be used to design processes based on the previously implemented methodology5, which will allow representation of the different phases in one single workflow.
Drugs to be analyzedThe study cohort will be made of the HDs included in the List of Hazardous Drugs of the National Institute for Occupational Safety and Health6.
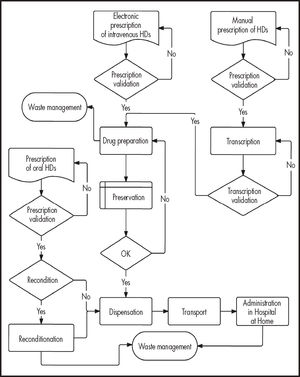
Stages and traceability controlUsing a general flowchart (Figure 1) as a basis, specific flowcharts will be designed by an expert group with details of the different stages of the process and the operations they comprise. The stages of the process were obtained from the systematic review carried out by Bernabeu et al.7 Consensus among the experts will be obtained through the nominal group technique and a series of documentary techniques. This combined method will comprise two face-to-face rounds (meeting of participants and approval of proposals) and three masked rounds (individualized review).
Criticality analysisStandardized hazard identification techniques will be used to create a dashboard that will allow detection of significant hazards and application of appropriate control measures to prevent or mitigate risks.
Sequencing of decisions geared towards identifying CCPs will be carried out in accordance with the Recommended International Code of Practice: General Principles of Food Hygiene (CAC/RCP 1-1969, Rev. 5-2020)8.
The variables considered for characterizing each hazard will be as follows: [1] existence of a hazard; [2] incorporation and contamination; [3] inception or escalation of the hazard; and [4] persistence of the hazard (continuity).
The variables for managing the process will be: [1] stage; [2] likelihood of occurrence (L); [3] severity of the damage (S); [4] criticality index (CI): L x S; [5] Steps of the decision tree: S1, S2, S3 and S4; [6] CP; [7] CCP; [8] Control measure.
Calculating the criticality index for each hazardEach hazard identified will be evaluated using the criteria established in the IFS Global Markets HPC program9.
The criticality index (CI) is the value obtained by multiplying the likelihood of occurrence of an undesired event by the severity of its potential consequences (CI = L x S).
Calculation of the CI helps determine which points will be CPs and which must be subject to sequencing of decision-making to decide whether they constitute CCPs and, if appropriate, establish a control protocol to address any potential hazards.
Initial quantification of the L and S of undesired events and their potential consequences to determine the CI of each stage in the process is carried out by consensus among the experts (nominal group and documentary techniques). These will be healthcare providers (physicians, nurses, and pharmacists) adept at managing HDs as a result of either their work experience (at a hospital, healthcare center or HHU) or because of their technical expertise in the area. Recruitment of such experts will be carried out with the assistance of the Spanish Group for the Development of Hospital Pharmacy (GEDEFO), the Spanish Society of Hospital Pharmacists (SEFH), the Management and Quality Working Group of the Spanish Hospital at Home Society (SEHAD), and occupational hazard and preventive medicine practitioners from the Spanish School of Occupational Medicine (ENMT).
Development of the technological supportA digital Hazard Analysis and Critical Control Points (HACCP) system will be generated to capture all relevant data (all the models proposed for the analysis of risks and their traceability will also have been tested in the hospital setting)10-11. A series of client software agents will be used to this effect which can be executed on adapted devices (tablets and smartphones) to centralize all the data in a common data server.
After this first phase, all data will be processed using data analytics techniques. The Big Data analysis will be carried out using mathematical models that allow monitorization of both CPs and CCPs. These models will be applied to the data stored in the project's server and will allow real-time determination of the values corresponding to each specific parameter.
Data collectionManagement, implementation, and piloting data will be collected using Android-supported next-generation optical scanning devices (tablets or smartphones) and stored in a repository located in the project's server.
Documentary and technological support data will be generated following the development of the HACCP system.
Data monitoring and registration systemEvents will be registered on the basis of a formalized knowledge model in accordance with the standards laid out in the Semantic Web (the SPARQL language that allows operations for the maintenance, creation, erasure and modification of data). In addition, a cross-domain ontology will be constructed that will be supplemented by an upper layer dedicated to HD modeling. This information will be stored in a Linked Open Data (LOD) repository overlaid with a SPARQL endpoint. This technology facilitates flowchart design and makes it possible for such flowcharts to be shared across different environments and users.
Technical-documentary verificationOnce the system has been designed, it will be subjected to a verification process that will encompass all documents generated and all techniques implemented.
Tests will include local emulations for the different areas of interest. This will require a series of tests/retests aimed at detecting potential errors or conflicting situations in the different contexts and control processes across the whole HD lifecycle11. This will allow monitoring the interval validity of the process.
Clinical-pharmaceutical validation of the processThe HD control system will be independently verified at each participating hospital. Once the appropriateness of the whole system is ascertained, a validity period will be established, together with review and verification milestones (external validity).
Compatibility with surveillance measuresThe HACCP system will be compatible with any surveillance measures that the prevention services of participating hospitals may decide to enforce.
Ethical considerationsThe project proposed is only based on the monitoring of the HD-related phases, without any consideration of the patients’ personal data. At any event, the confidentiality of the participating healthcare providers’ data will be guaranteed in accordance with the stipulations of the 1975 Helsinki Declaration and its subsequent amendments.
The project was approved by the Research Ethics Committee of the Alicante General University Hospital (Ref. CEIC PI2017/93).
DiscussionArticle 87 of the spanish Law 29/2006, on the guarantees and rational use of medicines and medical products refers to the importance of drug traceability for ensuring and reinforcing their safety. To that effect, a transparent, reliable, and agile system is indispensable, which makes it possible to react rapidly and appropriately whenever risks are detected regarding the quality and safety of a certain drug12.
In the context of HDs, traceability allows following up their progression through the different stages of their lifecycle. Several technological proposals have been made, especially in the logistics sphere, to enhance traceabilty. However, these proposed deployments are unfortunately based on automatically tracking the location of a drug and do not allow immediate compliance with all the requirements of integrated HD control management. Nowadays, mobile technologies are contributing new solutions based on integrated care models, which hold much promise for the enhancement of clinical care13.
IT systems may be of great help for improving the handling of HDs as such systems may incorporate alerts capable of detecting when the safety thresholds established in the course of the validation process have been exceeded.
In the context of HDs, technological development has centered mainly on the hemato-oncological arena, with special attention given to the compounding and administration phases (drug and patient identification systems such as radio-frequency identification, barcode tagging, data matrix code recognition; and robotized compounding systems). Nonetheless, although the purpose of these new technologies is basically to ensure traceability of the handling process and, ultimately, guarantee patient safety, they are —with the exception of automated robots— unable to mitigate the exposure risk faced by healthcare providers manipulating the drugs. To the best of our knowledge, none of the integrated management systems developed in the last few years to mitigate the risks faced by healthcare providers manipulating HDs make use of the new information technologies.
In the face of this, several technologies have recently been proposed to enhance traceability and hazard analysis in the context of parenteral nutrition mixtures14 to allow the development of simple and highly safe risk management systems. Clearly, these new applications should in the future be adapted to the specific management of HDs. However, that cannot be achieved only on the basis of data demonstrating that the process has been correctly completed, or of the verification of such data. A specific system will have to be developed that is capable of verifying and feeding back data related to the L and the S of the event, which should allow overall control of any CCPs that may be established. This means that a key lever for achieving these goals is the collection and processing of data.
It will also be necessary to develop an efficient, non-intrusive, and interoperable data capturing system that allows gathering of the documentary support needed to apply the required data processing mechanisms. Care will have to be exercised however when selecting the techniques to be used for this development. Indeed, given the high data volumes likely to be generated, not only during the piloting stage, but also at subsequent phases, “classical” statistical techniques may prove insufficient. The need to process inordinate amounts of data and ensuring its availability, mostly in real time (the data will not be known beforehand but only during the computation process itself), will make it necessary to resort to data analytics. The idea is therefore to embrace Big Data methodological approaches (related particularly to machine learning) such as Bayesian networks, neural networks, automatic clustering techniques, etc. This will provide an insight into patterns, trends, and models that will boost the effectiveness of CCPrelated data and, therefore, make it possible to dynamically manage potential hazards, monitor the system's integrity at all times, and handle HDs in a fully efficient way15,16.
StrengthsThe research team have invaluable experience of designing and implementing platforms such as the one described in this study11,17. At the same time, previous studies have demonstrated that low-cost easy-to-use mobile applications such as the one proposed in this study enjoy wide acceptance on account of their adaptability and beneficial application to clinical practice18.
Moreover, the exhaustive review made in this study of all the different stages in the process and the multicenter nature of the analysis will allow application of the tool to other centers in the future.
LimitationsThe limitations of this study are related to the unavailability of reference levels for all HDs (critical limits), which would make it possible to easily reduce exposure to HDs, which is the ultimate goal of the HACCP system. Having said this, the risk reduction achieved by controlling the quality and traceability of the handling process, and the access obtained to updated information on potential non-conformities ensures a better control of the stages that could pose the greatest hazards, thereby helping to (indirectly) minimize potential exposure.
The foregoing indicates that HDs should be integrated within a standardized management system that promotes the safety of both patients and healthcare providers, optimizes resource utilization, and minimizes procedural issues, guaranteeing the quality, traceability, and safety of HD handling in HHUs.
FundingThis research project was awarded a grant from the Carlos III Health Institute from Madrid, Spain (Grant reference number: PI16/00788).
Conflict of interestsNo conflict of interests.
Early Access date (06/10/2021).