The off-label use of drugs requires scientific support to balance risk/benefit, being limited to exceptional cases in which there are no therapeutic alternatives.
MethodsRetrospective descriptive study of the reports of the Pharmacy and Therapeutics Committee between 2018 and 2022 in a third level hospital; analyzing the requests, drugs, final opinion, reasons, level of evidence and economic impact.
ResultsA total of 124 reports were analyzed, highlighting oncohematological (41.9%) and autoimmune (27.4%) diseases as main indications. Oncology (37.1%) and Pediatrics (18.5%) were the main applicants, with 87.9% for antineoplastic and immunomodulatory drugs. A total of 74.2% of the applications were approved due to lack of alternatives and solid evidence (phase II-III trials), while 25.8% were denied due to the availability of therapeutic options or insufficient evidence. In terms of cost, 53% of oncohematological drugs cost between €10,000–50,000/treatment and 62.1% of non-oncohematological drugs cost between €1,000–10,000/year. Approval of the rejected treatments would have generated an additional expenditure of €2,272,603.
ConclusionAn increase of up to four times in the evaluation of off-label use drugs was evidenced, with a high approval rate.
el uso off-label de medicamentos requiere respaldo científico para equilibrar el balance riesgo/beneficio, limitándose a casos excepcionales en los que no existen alternativas terapéuticas.
Métodosestudio descriptivo retrospectivo de los informes de la Comisión de Farmacia y Terapéutica, entre 2018 y 2022, en un hospital de tercer nivel. Se analizaron las solicitudes, los medicamentos, el dictamen final y los motivos, el nivel de evidencia y el impacto económico.
Resultadosse analizaron 124 informes, destacando enfermedades oncohematológicas (41,9%) y autoinmunes (27,4%) como principales indicaciones. Oncología (37,1%) y Pediatría (18,5%) fueron los principales solicitantes, con un 87,9% de medicamentos antineoplásicos e inmunomoduladores. El 74,2% de las solicitudes fueron aprobadas por falta de alternativas y evidencia sólida (ensayos fase II-III), mientras que el 25,8% fueron denegadas por disponibilidad de opciones terapéuticas o evidencia insuficiente. En cuanto al coste, el 53% de los fármacos oncohematológicos tenían un precio comprendido entre 10.000 y 50.000 €/tratamiento, y el 62,1% de los no oncohematológicos entre 1.000 y 10.000 €/año. La aprobación de los tratamientos rechazados habría generado un gasto adicional de 2.272.603 €.
Conclusiónse evidenció un aumento de hasta 4 veces en la evaluación de medicamentos de uso off-label, con una alta tasa de aprobación.
Off-label drugs are those used in populations, at dosages or for indications other than those specified in the Summary of Product Characteristics. The latter is the most common form of off-label use.1,2 This clinical practice is considered a therapeutic alternative that must be supported by scientific evidence demonstrating a favourable benefit/risk ratio3,4. One cause of off-label use is the underrepresentation or exclusion of certain minorities, such as paediatric, geriatric, or pregnant patients, in clinical trials (CTs)5. Moreover, there are delays in publishing CT results and in authorisation by regulatory agencies1. Laboratories are also less likely to apply for authorisation for new indications due to the low cost of drugs already marketed for these indications or the small target patient population1,3. Finally, terminal situations may prompt the off-label prescription of a drug5.
Consequently, off-label use is most prevalent in paediatrics, psychiatry, and oncohaematology6. The paediatric population is under-represented in CTs due to high costs, limited results, and ethical implications7. The inclusion of psychiatric patients in CTs is limited due to ethical issues6,8. Oncohaematological patients often exhaust standard treatment lines. However, due to the severity of their condition, they have easier access to drugs with clinical benefit, even if they have not been approved4.
In Spain, Royal Decree 1015/2009 sets out the regulations for drugs used in special situations. It states that the use of drugs for purposes other than those authorised is exceptional and restricted to situations in which there are no authorised alternatives available2,6. In accordance with Law 41/2002, the physician must justify their use, inform the patient of the risk/benefit ratio, and obtain their consent9. The Pharmacy and Therapeutics Committee (PTC) is responsible for evaluating each request.2 The PTC pharmacists prepare a report containing a proposed recommendation based on the patient's clinical situation, scientific evidence, the availability of authorised or unauthorised alternatives, cost, and convenience. The final decision is made by a vote among the other members of the PTC.
The aim of this study was to analyse the cases of off-label use requested from the PTC between 2018 and 2022, how these were resolved, and the level of evidence on which the final decisions were based.
MethodsA retrospective descriptive study of the reports produced by the PTC, focusing on the characteristics of requests, the requested drugs, and the final decision. The following information was collected for each request: total number of requests, year of request, the patient's sex and age, type of therapeutic indication, and the requesting department. The following information was recorded for the requested drugs: ATC classification, type of dispensing, additional monitoring status, orphan medicine, and year of authorisation. Treatment costs were also calculated on an annual basis for both non-oncohaematological drugs and complete oncohaematological drug treatment. The final decision and its justification were both recorded. Approved applications were categorised as follows: a) due to the absence of authorised therapies; or b) due to the absence of both authorised and unauthorised therapies. In the case of rejected applications: a) the availability of alternatives in the therapeutic arsenal (authorised or off-label); b) a lack of evidence to support a favourable benefit/risk ratio; or c) an unfavourable cost/benefit ratio. Finally, the level of evidence that supported the decision was analysed.
ResultsA total of 124 reports relating to 50 drugs for 74 different indications were located. The distribution over time was as follows: 13 reports in 2018; 12 in 2019; 16 in 2020; 32 in 2021; and 51 in 2022.
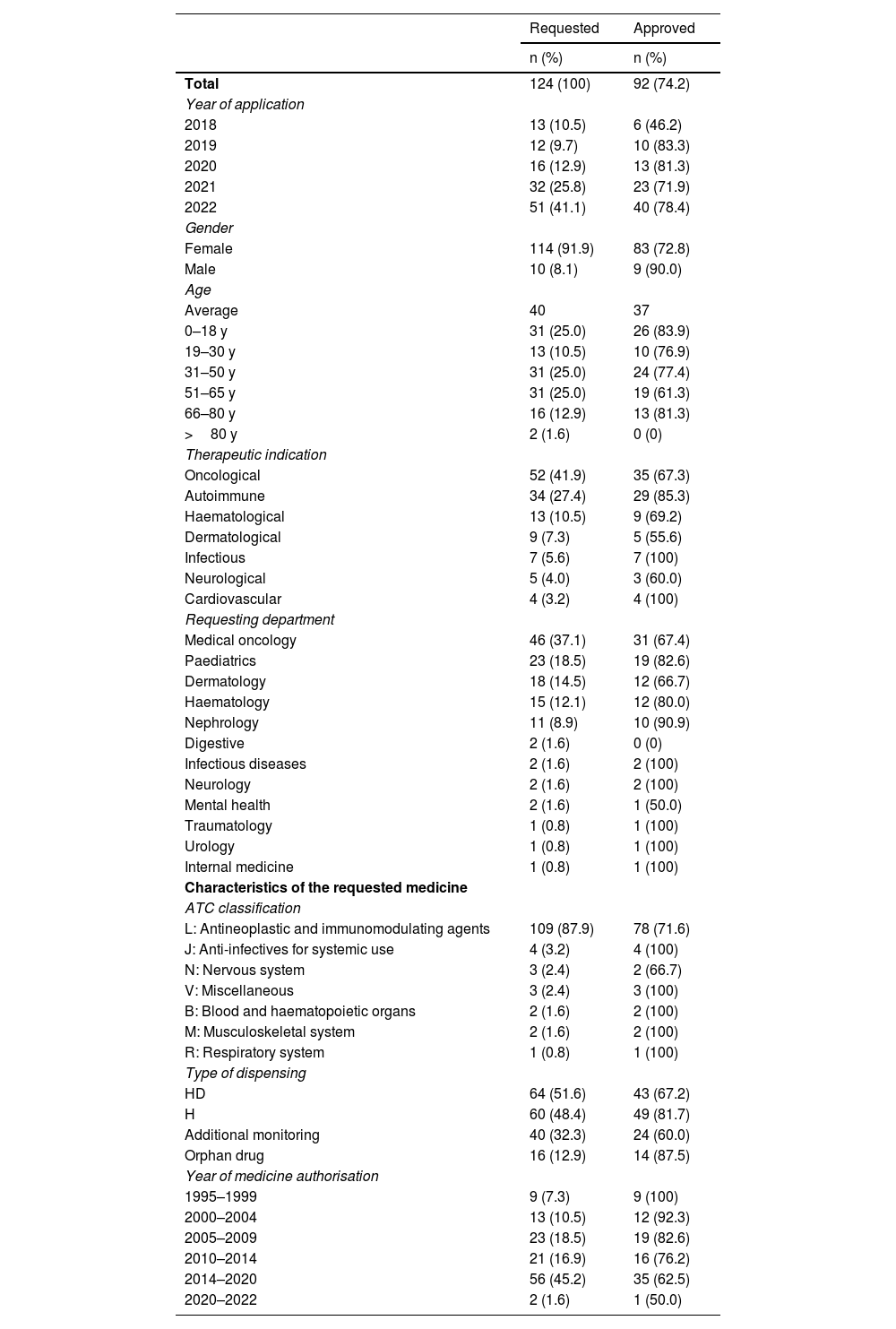
Table 1 shows the characteristics of the requests and the drugs requested. The most common indications were oncohaematological (41.9%), autoimmune (27.4%), and haematological (10.5%) diseases. The main requesting departments were oncology (37.1%) and paediatrics (18.5%). Most of the drugs (87.9%) belonged to ATC classification group L (antineoplastic and immunomodulating agents).
Characteristics of requests for off-label drugs evaluated (2018–2022).
| Requested | Approved | |
|---|---|---|
| n (%) | n (%) | |
| Total | 124 (100) | 92 (74.2) |
| Year of application | ||
| 2018 | 13 (10.5) | 6 (46.2) |
| 2019 | 12 (9.7) | 10 (83.3) |
| 2020 | 16 (12.9) | 13 (81.3) |
| 2021 | 32 (25.8) | 23 (71.9) |
| 2022 | 51 (41.1) | 40 (78.4) |
| Gender | ||
| Female | 114 (91.9) | 83 (72.8) |
| Male | 10 (8.1) | 9 (90.0) |
| Age | ||
| Average | 40 | 37 |
| 0–18 y | 31 (25.0) | 26 (83.9) |
| 19–30 y | 13 (10.5) | 10 (76.9) |
| 31–50 y | 31 (25.0) | 24 (77.4) |
| 51–65 y | 31 (25.0) | 19 (61.3) |
| 66–80 y | 16 (12.9) | 13 (81.3) |
| >80 y | 2 (1.6) | 0 (0) |
| Therapeutic indication | ||
| Oncological | 52 (41.9) | 35 (67.3) |
| Autoimmune | 34 (27.4) | 29 (85.3) |
| Haematological | 13 (10.5) | 9 (69.2) |
| Dermatological | 9 (7.3) | 5 (55.6) |
| Infectious | 7 (5.6) | 7 (100) |
| Neurological | 5 (4.0) | 3 (60.0) |
| Cardiovascular | 4 (3.2) | 4 (100) |
| Requesting department | ||
| Medical oncology | 46 (37.1) | 31 (67.4) |
| Paediatrics | 23 (18.5) | 19 (82.6) |
| Dermatology | 18 (14.5) | 12 (66.7) |
| Haematology | 15 (12.1) | 12 (80.0) |
| Nephrology | 11 (8.9) | 10 (90.9) |
| Digestive | 2 (1.6) | 0 (0) |
| Infectious diseases | 2 (1.6) | 2 (100) |
| Neurology | 2 (1.6) | 2 (100) |
| Mental health | 2 (1.6) | 1 (50.0) |
| Traumatology | 1 (0.8) | 1 (100) |
| Urology | 1 (0.8) | 1 (100) |
| Internal medicine | 1 (0.8) | 1 (100) |
| Characteristics of the requested medicine | ||
| ATC classification | ||
| L: Antineoplastic and immunomodulating agents | 109 (87.9) | 78 (71.6) |
| J: Anti-infectives for systemic use | 4 (3.2) | 4 (100) |
| N: Nervous system | 3 (2.4) | 2 (66.7) |
| V: Miscellaneous | 3 (2.4) | 3 (100) |
| B: Blood and haematopoietic organs | 2 (1.6) | 2 (100) |
| M: Musculoskeletal system | 2 (1.6) | 2 (100) |
| R: Respiratory system | 1 (0.8) | 1 (100) |
| Type of dispensing | ||
| HD | 64 (51.6) | 43 (67.2) |
| H | 60 (48.4) | 49 (81.7) |
| Additional monitoring | 40 (32.3) | 24 (60.0) |
| Orphan drug | 16 (12.9) | 14 (87.5) |
| Year of medicine authorisation | ||
| 1995–1999 | 9 (7.3) | 9 (100) |
| 2000–2004 | 13 (10.5) | 12 (92.3) |
| 2005–2009 | 23 (18.5) | 19 (82.6) |
| 2010–2014 | 21 (16.9) | 16 (76.2) |
| 2014–2020 | 56 (45.2) | 35 (62.5) |
| 2020–2022 | 2 (1.6) | 1 (50.0) |
H, hospital use; HD, hospital diagnostic use.
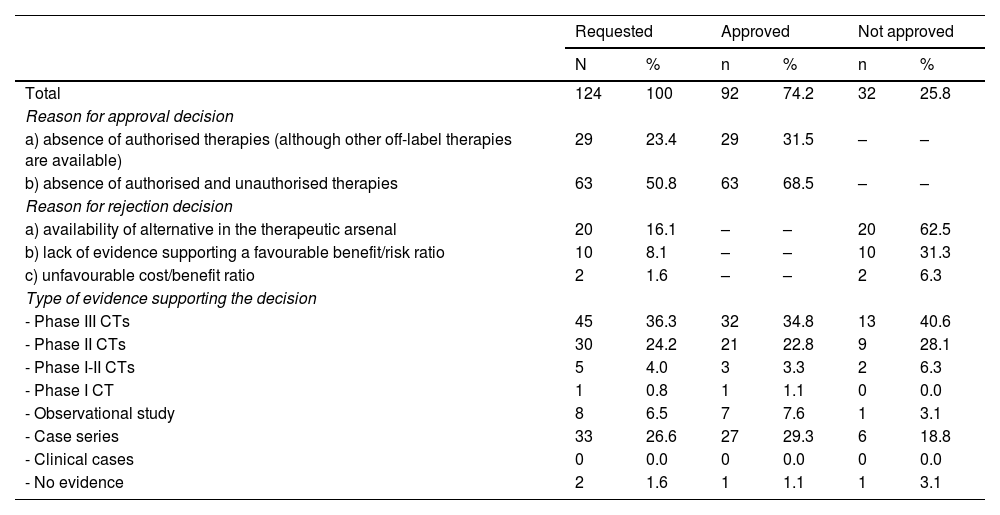
A total of 92 (74.2%) of the 124 evaluation reports were approved (Table 2). Of these, 68.5% were approved due to a lack of authorised alternatives. The remaining 31.5%, however, were approved despite the availability of other off-label therapies. A total of 34.8% of approvals were based on Phase III CTs, followed by case series (29.3%), and Phase II CTs (22.8%).
Outcomes of the requests following evaluation by the Pharmacy and Therapeutics Committee, justifications, and supporting evidence.
| Requested | Approved | Not approved | ||||
|---|---|---|---|---|---|---|
| N | % | n | % | n | % | |
| Total | 124 | 100 | 92 | 74.2 | 32 | 25.8 |
| Reason for approval decision | ||||||
| a) absence of authorised therapies (although other off-label therapies are available) | 29 | 23.4 | 29 | 31.5 | – | – |
| b) absence of authorised and unauthorised therapies | 63 | 50.8 | 63 | 68.5 | – | – |
| Reason for rejection decision | ||||||
| a) availability of alternative in the therapeutic arsenal | 20 | 16.1 | – | – | 20 | 62.5 |
| b) lack of evidence supporting a favourable benefit/risk ratio | 10 | 8.1 | – | – | 10 | 31.3 |
| c) unfavourable cost/benefit ratio | 2 | 1.6 | – | – | 2 | 6.3 |
| Type of evidence supporting the decision | ||||||
| - Phase III CTs | 45 | 36.3 | 32 | 34.8 | 13 | 40.6 |
| - Phase II CTs | 30 | 24.2 | 21 | 22.8 | 9 | 28.1 |
| - Phase I-II CTs | 5 | 4.0 | 3 | 3.3 | 2 | 6.3 |
| - Phase I CT | 1 | 0.8 | 1 | 1.1 | 0 | 0.0 |
| - Observational study | 8 | 6.5 | 7 | 7.6 | 1 | 3.1 |
| - Case series | 33 | 26.6 | 27 | 29.3 | 6 | 18.8 |
| - Clinical cases | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| - No evidence | 2 | 1.6 | 1 | 1.1 | 1 | 3.1 |
CTs, controlled trials; GVHD, graft-versus-host disease; HPT, haematopoietic precursor transplantation; CIDP, chronic idiopathic demyelinating polyneuropathy; CANDLE, atypical neutrophilic dermatosis-lipodystrophy-elevated temperature syndrome; GIST, gastrointestinal stromal tumour.
Of the 32 (25.8%) applications that were not approved, 20 (62.5%) were rejected due to available therapeutic alternatives, 10 (31.3%) due to a lack of evidence supporting a favourable benefit/risk ratio, and 2 (6.3%) due to an unfavourable cost/benefit ratio. Of the applications that were not approved, 40.6% were based on Phase III CTs, 28.1% on Phase II CTs, and 18.8% on case series.
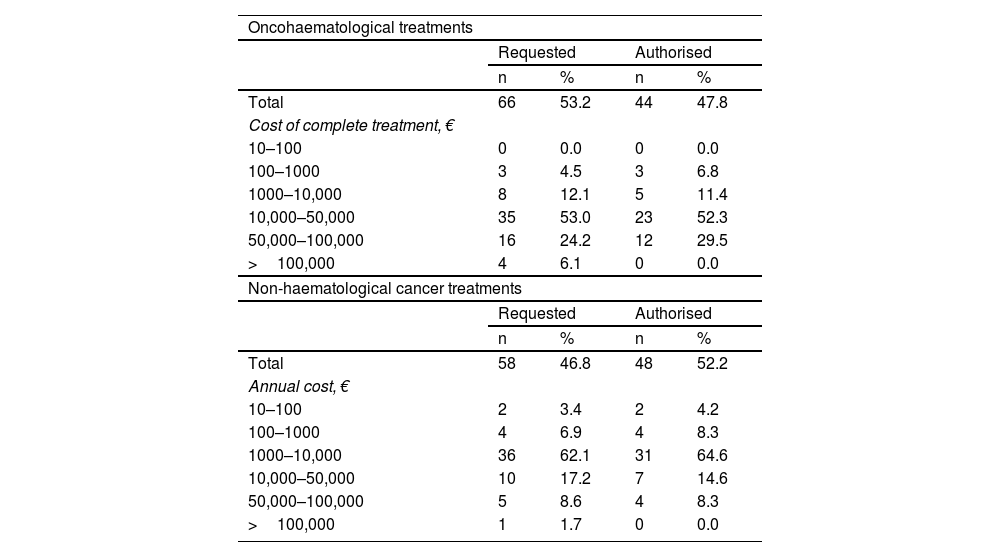
Regarding the economic impact of off-label approval (Table 3), most requests for oncohaematological drugs were for between €10,000 and €50,000 per complete treatment (53%). A total of 44 of the 66 applications were approved. Of the 58 applications for non-oncohaematological treatments, 48 were approved, the majority of which ranged in cost from €1000 to €10,000/year (62.1%). A total of 5 applications with a high economic impact, exceeding €100,000, were submitted. Of these, 4 were for oncohaematological treatments. None were authorised. The total budgetary impact of the approved drugs was €2,265,670. It was estimated that the rejected treatments, if approved, would have entailed an additional expenditure of €2,272,603, increasing the total expenditure to €4,538,274.
Economic impact of off-label drug applications and approval rate.
| Oncohaematological treatments | ||||
| Requested | Authorised | |||
| n | % | n | % | |
| Total | 66 | 53.2 | 44 | 47.8 |
| Cost of complete treatment, € | ||||
| 10–100 | 0 | 0.0 | 0 | 0.0 |
| 100–1000 | 3 | 4.5 | 3 | 6.8 |
| 1000–10,000 | 8 | 12.1 | 5 | 11.4 |
| 10,000–50,000 | 35 | 53.0 | 23 | 52.3 |
| 50,000–100,000 | 16 | 24.2 | 12 | 29.5 |
| >100,000 | 4 | 6.1 | 0 | 0.0 |
| Non-haematological cancer treatments | ||||
| Requested | Authorised | |||
| n | % | n | % | |
| Total | 58 | 46.8 | 48 | 52.2 |
| Annual cost, € | ||||
| 10–100 | 2 | 3.4 | 2 | 4.2 |
| 100–1000 | 4 | 6.9 | 4 | 8.3 |
| 1000–10,000 | 36 | 62.1 | 31 | 64.6 |
| 10,000–50,000 | 10 | 17.2 | 7 | 14.6 |
| 50,000–100,000 | 5 | 8.6 | 4 | 8.3 |
| >100,000 | 1 | 1.7 | 0 | 0.0 |
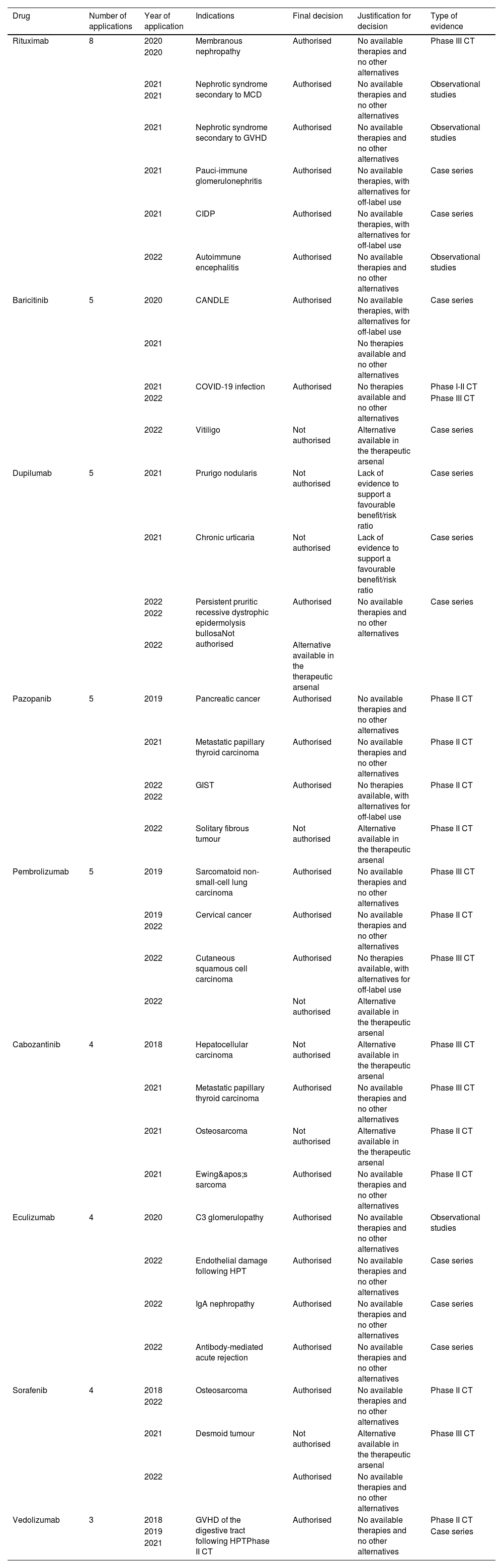
Table 4 summarises the most frequently requested drugs, their indication, and the final decision. The drugs are listed in descending order by number of applications for their use: rituximab, baricitinib, dupilumab, pazopanib, pembrolizumab, cabozantinib, eculizumab, sorafenib, and vedolizumab.
Most frequently requested drugs: indications, final decision, justification, and evidence.
| Drug | Number of applications | Year of application | Indications | Final decision | Justification for decision | Type of evidence |
|---|---|---|---|---|---|---|
| Rituximab | 8 | 2020 | Membranous nephropathy | Authorised | No available therapies and no other alternatives | Phase III CT |
| 2020 | ||||||
| 2021 | Nephrotic syndrome secondary to MCD | Authorised | No available therapies and no other alternatives | Observational studies | ||
| 2021 | ||||||
| 2021 | Nephrotic syndrome secondary to GVHD | Authorised | No available therapies and no other alternatives | Observational studies | ||
| 2021 | Pauci-immune glomerulonephritis | Authorised | No available therapies, with alternatives for off-label use | Case series | ||
| 2021 | CIDP | Authorised | No available therapies, with alternatives for off-label use | Case series | ||
| 2022 | Autoimmune encephalitis | Authorised | No available therapies and no other alternatives | Observational studies | ||
| Baricitinib | 5 | 2020 | CANDLE | Authorised | No available therapies, with alternatives for off-label use | Case series |
| 2021 | No therapies available and no other alternatives | |||||
| 2021 | COVID-19 infection | Authorised | No therapies available and no other alternatives | Phase I-II CT | ||
| 2022 | Phase III CT | |||||
| 2022 | Vitiligo | Not authorised | Alternative available in the therapeutic arsenal | Case series | ||
| Dupilumab | 5 | 2021 | Prurigo nodularis | Not authorised | Lack of evidence to support a favourable benefit/risk ratio | Case series |
| 2021 | Chronic urticaria | Not authorised | Lack of evidence to support a favourable benefit/risk ratio | Case series | ||
| 2022 | Persistent pruritic recessive dystrophic epidermolysis bullosaNot authorised | Authorised | No available therapies and no other alternatives | Case series | ||
| 2022 | ||||||
| 2022 | Alternative available in the therapeutic arsenal | |||||
| Pazopanib | 5 | 2019 | Pancreatic cancer | Authorised | No available therapies and no other alternatives | Phase II CT |
| 2021 | Metastatic papillary thyroid carcinoma | Authorised | No available therapies and no other alternatives | Phase II CT | ||
| 2022 | GIST | Authorised | No therapies available, with alternatives for off-label use | Phase II CT | ||
| 2022 | ||||||
| 2022 | Solitary fibrous tumour | Not authorised | Alternative available in the therapeutic arsenal | Phase II CT | ||
| Pembrolizumab | 5 | 2019 | Sarcomatoid non-small-cell lung carcinoma | Authorised | No available therapies and no other alternatives | Phase III CT |
| 2019 | Cervical cancer | Authorised | No available therapies and no other alternatives | Phase II CT | ||
| 2022 | ||||||
| 2022 | Cutaneous squamous cell carcinoma | Authorised | No therapies available, with alternatives for off-label use | Phase III CT | ||
| 2022 | Not authorised | Alternative available in the therapeutic arsenal | ||||
| Cabozantinib | 4 | 2018 | Hepatocellular carcinoma | Not authorised | Alternative available in the therapeutic arsenal | Phase III CT |
| 2021 | Metastatic papillary thyroid carcinoma | Authorised | No available therapies and no other alternatives | Phase III CT | ||
| 2021 | Osteosarcoma | Not authorised | Alternative available in the therapeutic arsenal | Phase II CT | ||
| 2021 | Ewing's sarcoma | Authorised | No available therapies and no other alternatives | Phase II CT | ||
| Eculizumab | 4 | 2020 | C3 glomerulopathy | Authorised | No available therapies and no other alternatives | Observational studies |
| 2022 | Endothelial damage following HPT | Authorised | No available therapies and no other alternatives | Case series | ||
| 2022 | IgA nephropathy | Authorised | No available therapies and no other alternatives | Case series | ||
| 2022 | Antibody-mediated acute rejection | Authorised | No available therapies and no other alternatives | Case series | ||
| Sorafenib | 4 | 2018 | Osteosarcoma | Authorised | No available therapies and no other alternatives | Phase II CT |
| 2022 | ||||||
| 2021 | Desmoid tumour | Not authorised | Alternative available in the therapeutic arsenal | Phase III CT | ||
| 2022 | Authorised | No available therapies and no other alternatives | ||||
| Vedolizumab | 3 | 2018 | GVHD of the digestive tract following HPTPhase II CT | Authorised | No available therapies and no other alternatives | Phase II CT |
| 2019 | Case series | |||||
| 2021 |
CTs, controlled trials; GVHD, graft-versus-host disease; HPT, haematopoietic precursor transplantation; CIDP, chronic idiopathic demyelinating polyneuropathy; CANDLE, atypical neutrophilic dermatosis-lipodystrophy-elevated temperature syndrome; GIST, gastrointestinal stromal tumour.
This study reveals a significant increase in the number of evaluations, with requests increasing fourfold. This increase could be due to the incorporation of new drugs10 and the emergence of new diseases, particularly the 2020 COVID-19 pandemic11. Most of the drugs requested were in ATC classification group L, with indications for oncohaematological and autoimmune diseases, and associated with the medical oncology and paediatrics departments. These results are consistent with those reported in the study by Pérez-Moreno et al.1, conducted in the same hospital in the period 2009 to 2011. Most of the drug requests for autoimmune diseases were issued by the paediatrics department, reflecting the hospital's role as a reference centre for paediatric autoimmune diseases.
The results show a high authorisation rate of close to 75%, demonstrating that the off-label drug request pathway remains a vital resource even a decade after the publication of RD 1015/20092. Similar results were shown in the study by Pérez-Moreno et al.1, which could be explained by the complexity of patients in a tertiary hospital. Approval decisions tend to authorise drugs for indications where there are no treatment options or limited alternatives. In contrast, requests with available alternatives or an unfavourable cost–benefit ratio tend to be rejected. On the other hand, it is notable that the approval rate is high even with low-level scientific evidence, probably because there are no alternatives for patients who have exhausted all other therapeutic options. Analysis of the evidence for the rejected applications shows that it was mainly based on Phase II-III CTs. These requests were rejected despite being supported by scientific evidence because therapeutic alternatives with an adequate cost–benefit ratio that had not yet been tested in patients were available.
Another aspect to highlight is the cost of the drugs. Lower-cost treatments have a higher approval rate, whereas none of the treatments exceeding €100,000 were approved. As might be expected, even greater priority is given to the level of evidence and the availability of alternatives in these cases. Most authorised oncohaematological drugs have an average cost of €10,000 to €50,000 per treatment. This range is consistent with the range reported by González-Morcillo et al.12. They analysed the cost-effectiveness of off-label drugs, mainly in the area of oncohaematology, and identified an average cost of €16,288 per application. In contrast, non-oncohaematological drugs have significantly lower average costs, ranging from €1000 to €10,000 per year.
Although there were more requests for oncohaematological drugs than for non-oncohaematological drugs, the authorisation rate was significantly higher for the latter (66% vs 82%, respectively). This difference could be linked to the higher costs associated with oncohaematological treatments, for which greater scientific support is required.
In terms of budgetary impact, approving 92 of the 124 requests resulted in savings of €2.2 million. Thus, drug evaluation and selection policies in hospital management minimise patient exposure to treatments with limited scientific support, while optimising available resources by prioritising the most efficient options.
Ethical responsibilitiesAll authors have accepted their responsibilities as defined by the International Committee of Medical Journal Editors (ICMJE).
Declaration of authorshipLupe Rodríguez-de Francisco drafted the manuscript; Ángela María Villalba-Moreno and Eva Rocío Alfaro-Lara critically reviewed the intellectual content of the manuscript and approved the final version to be submitted; Lupe Rodríguez-de Francisco submitted the manuscript.
CRediT authorship contribution statementLupe Rodríguez-de Francisco: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. Ángela María Villalba-Moreno: Validation, Supervision, Conceptualization. Eva Rocío Alfaro-Lara: Validation, Supervision, Conceptualization.
FundingNone declared.
None declared.