To assess the economic impact following the inclusion of an intravitreal implant of dexamethasone for the treatment of diabetic macular oedema in a healthcare area in Spain.
MethodA 3-year budget impact model was designed to estimate healthcare direct costs for adult patients with diabetic macular oedema from the National Health System perspective. The approved therapies in use (aflibercept/ranibizumab/dexamethasone) were considered. The target population was estimated from published diabetic macular oedema prevalence (6.41%) and incidence (0.82%) for a population of 25,000 adults. Dexamethasone was assumed to be used annually in 20%, 30% and 40% of patients, respectively. Annual total costs included: drug acquisition (based on frequency of injections per every year, considering ex-factory prices with mandatory deduction and split of vials), intravitreal administration, patient monitoring, management of cardiovascular and ocular adverse events (cataracts, increased intraocular pressure, endophthalmitis, vitreous haemorrhage and retinal detachment). Detailed resource consumption reflecting clinical practice was provided from local experts in retina and vitreous. Unitary costs (€, 2016) were obtained from national databases and literature. Sensitivity analyses were performed to assess model robustness.
ResultsThe inclusion of intravitreal dexamethasone implant would lead to annual cost savings of €35,030 (–4.2%), €10,743 (–1.8%) and €5,051 (–0.9%), years 1-3 respectively. Total costs were reduced mainly by the fewer annual injections required by dexamethasone. The average annual incremental costs were –€350, –€96 and –€41 per patient.
ConclusionsThe inclusion of an intravitreal dexamethasone implant for the treatment of diabetic macular oedema would lead to cost-savings for the considered health area, mainly by reducing the administration costs.
Determinar el impacto económico tras la inclusión del implante intravítreo de dexametasona para el tratamiento del edema macular diabético en un área sanitaria en España.
MétodoSe diseñó un modelo de impacto presupuestario a tres años para estimar los costes directos en pacientes adultos con edema macular diabético, desde la perspectiva del Sistema Nacional de Salud, considerando terapias intravítreas actualmente utilizadas (aflibercept/ranibizumab/dexametasona). La población diana se obtuvo a partir de la prevalencia (6,41%) e incidencia (0,82%) del edema macular diabético publicadas para una población de 25.000 pacientes adultos. Se asumió un 20%, 30% y 40% anual de pacientes tratados con dexametasona, respectivamente. El coste total incluyó: coste farmacológico (precio de venta del laboratorio con deducción obligatoria y fraccionamiento de viales, según frecuencia de inyecciones necesarias cada año de tratamiento), administración intravítrea, seguimiento de pacientes y manejo de eventos oculares (cataratas, hipertensión ocular, endoftalmitis, hemorragia intravítrea y desprendimiento de retina) y cardiovasculares. El consumo de recursos según la práctica habitual fue estimado por expertos en retina y vítreo. Los costes unitarios (€, 2016) se obtuvieron de la literatura y de bases de datos nacionales. Los análisis de sensibilidad evaluaron la robustez del modelo.
ResultadosLa inclusión del implante intravítreo de dexametasona supondría reducciones de 35.030 € (-4,2%), 10.743 € (-1,8%) y 5.051 € (-0,9%) cada año, respectivamente, disminuyendo principalmente por el menor número anual de inyecciones requeridas con dexametasona. La reducción anual promedio supondría 350 €, 96 € y 41 € por paciente.
ConclusionesLa inclusión del implante intravítreo de dexametasona para el tratamiento del edema macular diabético supone ahorros para el área sanitaria considerada, fundamentalmente por la reducción de costes de administración.
Diabetic macular oedema (DME) is the leading cause of vision loss in diabetic retinopathy and, consequently, of blindness in patients with diabetes mellitus1. As such, it has a strong negative impact on patients.
This inflammatory disorder is a consequence of metabolic changes secondary to hyperglycaemia caused by diabetic retinopathy in the macula. Vasogenic changes that induce rupture of the blood-retinal barrier together with inflammatory activation lead to severe retinal damage and chronic macular changes2.
The clinical objectives of the treatment of DME are to reduce the severity of oedema and prevent loss of vision3. Treatment is based on effective metabolic control (glycaemia, hypertension, lipid profile, renal function), but requires further measures to prevent vision loss, such as laser photocoagulation and drug treatments4. Approved treatments for DME include two groups of medications which are delivered by intravitreal injection: vascular endothelial growth factor inhibitors (anti-VEGF), such as ranibizumab and aflibercept, and corticosteroids, such as dexamethasone in a sterile sustained-release implant. Posology is variable because the response to treatment over time determines the frequency of injections. Several clinical trials have found that these therapies are effective5–7. They are indicated for patients with DME, regardless of the recommendations or protocols that establish specific treatment algorithms.
Blindness, diabetic retinopathy, and DME are very frequent in diabetic patients. Blindness, diabetic retinopathy, and DME have prevalences of between 4% and 11%, of 40%, and between 1.4% and 7.9%, respectively8. DME and loss of visual acuity negatively affect patients’ health-related quality of life and affects their ability to perform everyday tasks, including the self-management of diabetes9,10.
DME and diabetic retinopathy have a high economic impact because of their direct costs as well as their indirect costs, such as reduced income or an increased need for social support as vision worsens11. It has been estimated that the annual resource utilisation and direct health cost per patient with DME are approximately double those of patients without DME12. Bilateral DME is associated with higher direct health costs and with higher indirect costs caused by its impact on working life13.
The aim of this study was to determine the budget impact on the Spanish National Health System of the inclusion of dexamethasone intravitreal implant (IVI) in the treatment of DME in a specific healthcare region in Spain.
MethodsThe budget impact analysis was developed based on national and international recommendations14,15. The analysis was conducted using Microsoft Excel. An expert panel comprising three vitreoretinal specialists was consulted to validate the values of the parameters obtained from the literature and to reach a consensus on resource utilisation in standard clinical practice. This study assessed the incremental budget impact of the inclusion of dexamethasone IVI as a new therapeutic alternative for DME.
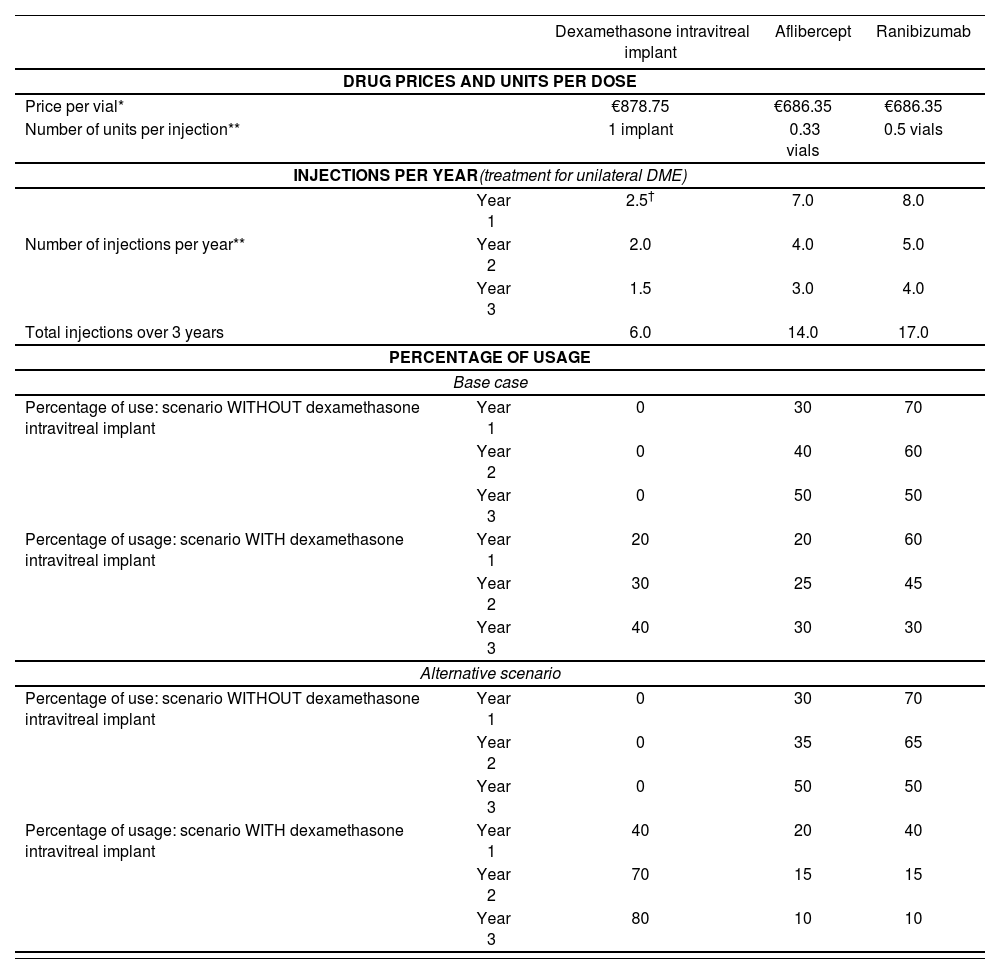
Therapeutic alternatives and scenariosThe analysis considered the therapeutic options currently funded by the Spanish National Health System with approved indication and currently in use for the treatment of DME: aflibercept 40 mg/mL injectable solution; ranibizumab 10 mg/mL injectable solution; and 700 µg dexamethasone IVI in applicator. Two different scenarios were compared: in scenario 1 dexamethasone IVI is not available; in scenario 2 dexamethasone IVI is included in the therapeutic armamentarium for DME. For each scenario, the costs associated with the management of DME were calculated for each of the treatment options. The costs of each of the selected drugs were weighted according to the percentage of usage to obtain the total costs of each scenario. The budget impact of introducing the new treatment was calculated by comparing the total costs generated in each scenario (without dexamethasone IVI vs with dexamethasone IVI). Table 1 shows the percentage of usage of each drug in each scenario (internal estimate [base case] and the expert panel estimate [alternative analysis]).
Number of injections per year, percentage of usage, and unitary price of the therapies
| Dexamethasone intravitreal implant | Aflibercept | Ranibizumab | ||
|---|---|---|---|---|
| DRUG PRICES AND UNITS PER DOSE | ||||
| Price per vial* | €878.75 | €686.35 | €686.35 | |
| Number of units per injection** | 1 implant | 0.33 vials | 0.5 vials | |
| INJECTIONS PER YEAR(treatment for unilateral DME) | ||||
| Year 1 | 2.5† | 7.0 | 8.0 | |
| Number of injections per year** | Year 2 | 2.0 | 4.0 | 5.0 |
| Year 3 | 1.5 | 3.0 | 4.0 | |
| Total injections over 3 years | 6.0 | 14.0 | 17.0 | |
| PERCENTAGE OF USAGE | ||||
| Base case | ||||
| Percentage of use: scenario WITHOUT dexamethasone intravitreal implant | Year 1 | 0 | 30 | 70 |
| Year 2 | 0 | 40 | 60 | |
| Year 3 | 0 | 50 | 50 | |
| Percentage of usage: scenario WITH dexamethasone intravitreal implant | Year 1 | 20 | 20 | 60 |
| Year 2 | 30 | 25 | 45 | |
| Year 3 | 40 | 30 | 30 | |
| Alternative scenario | ||||
| Percentage of use: scenario WITHOUT dexamethasone intravitreal implant | Year 1 | 0 | 30 | 70 |
| Year 2 | 0 | 35 | 65 | |
| Year 3 | 0 | 50 | 50 | |
| Percentage of usage: scenario WITH dexamethasone intravitreal implant | Year 1 | 40 | 20 | 40 |
| Year 2 | 70 | 15 | 15 | |
| Year 3 | 80 | 10 | 10 | |
DME: diabetic macular oedema.
The target population considered for treatment comprised diabetic patients with macular oedema undergoing treatment with intravitreal ophthalmologic therapies. Epidemiological data were used to calculate the target population in an area with a population of 25,000 adults. We considered the prevalence of diagnosed diabetes mellitus (7.8%)16 and of DME (5.73% in type 1 diabetes and 6.44% in type 2 diabetes)17, as well as the incidence of DME (6.36% during 8-year follow-up in diabetic patients)18 over the total study period. The percentages of patients diagnosed with DME receiving treatment (80%) and patients with bilateral DME (60%) were determined by the expert panel according to standard clinical practice.
Perspective, time horizon, and discount rateDirect health costs alone were considered because the analysis was conducted from the perspective of the Spanish National Health System. No discount rate was applied because inter-annual costs were not compared. The time horizon used was 3 years.
Resources and costsGiven the chosen perspective, the following costs were considered: drug costs, administration costs, monitoring/follow-up costs (including tests and follow-up visits), and the costs of managing adverse events. Resource costs were based on the estimated utilisation of each resource and their unitary costs
Drug costs were estimated using the ex-factory price (EFP), which corresponds to the official price19. The deductions established by Royal Decree-Law 8/2010 were applied to the EFP. The estimated drug costs of each therapy were based on the number of injections required per year and the number of vials of each drug used per injection. In line with standard clinical practice, it was assumed that the contents of the vials would be divided (aflibercept divided into 3 intravitreal injections, and ranibizumab divided into 2 intravitreal injections). We excluded the additional cost of the division process to the pharmacy services. The estimated drug administration costs of each drug were based on the number of annual intravitreal injections needed for each therapeutic alternative and on whether DME was unilateral or bilateral as determined by the expert panel (Table 1).
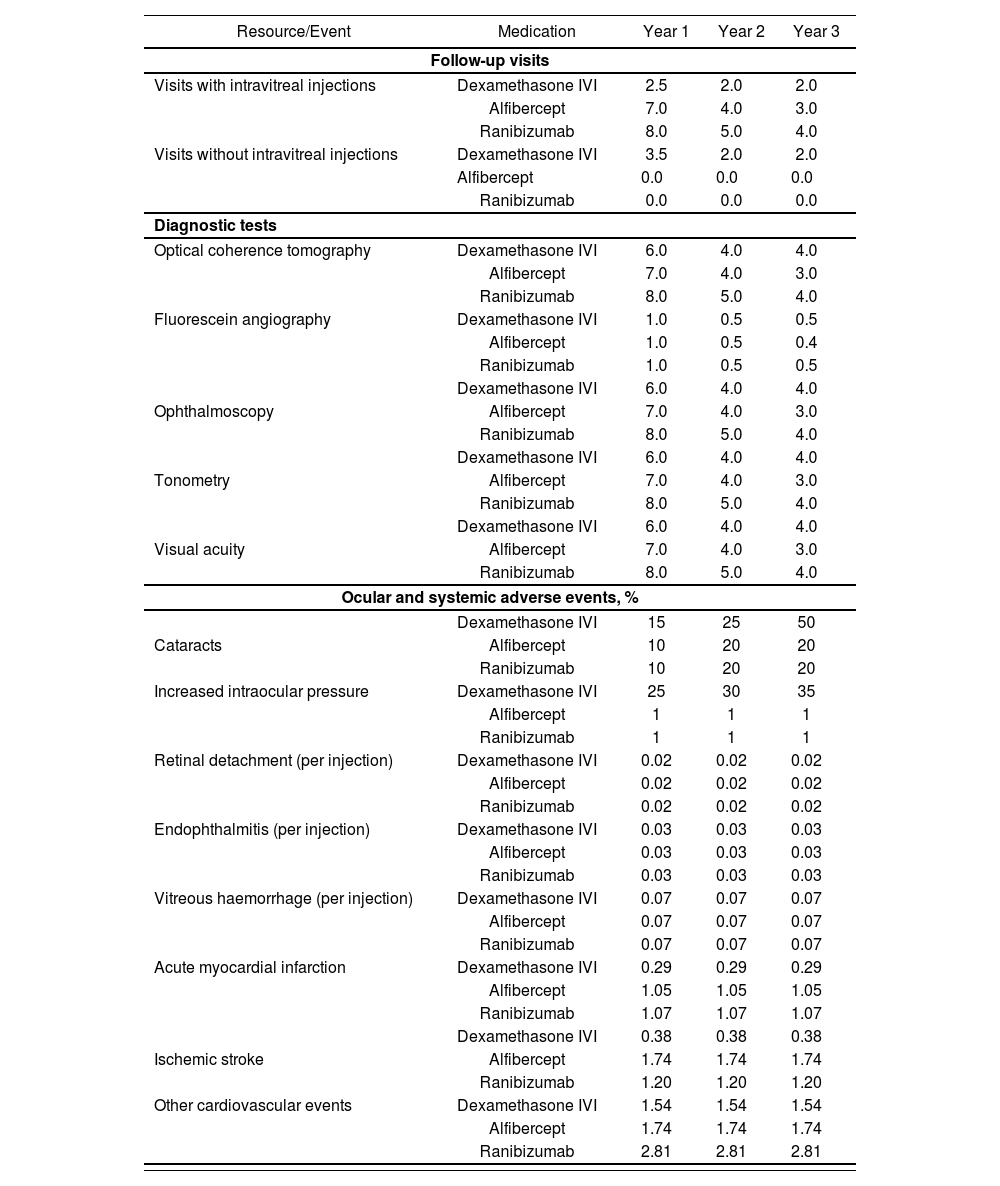
Follow-up costs were estimated according to the total number of follow-up visits required (with or without delivery of an intravitreal injection) and the diagnostic tests performed during these visits. Tests performed during the follow-up visits included optical coherence tomography, ophthalmoscopy, tonometry, visual acuity testing and, in some cases, fluorescein angiography. It was assumed that 100% of patients receiving treatment with dexamethasone for bilateral DME would require two visits to treat both eyes, whereas only 80% of patients receiving treatment with aflibercept or ranibizumab would require two visits for the treatment of bilateral DME.
The model considered the adverse ocular events (cataracts, elevated intraocular pressure, retinal detachment, endophthalmitis, and intravitreal haemorrhage) and adverse cardiovascular events (acute myocardial infarction, ischemic stroke, and other types of cardiovascular events) that may occur during treatment for DME. Endophthalmitis and increased intraocular pressure can be treated using combined medication and surgery or medication alone. Overall, 70% of patients with endophthalmitis receive pharmacologic treatment alone regardless of the treatment option for DME, whereas 100% of patients with increased intraocular pressure receive pharmacologic treatment. The model did not include surgery as a treatment for intraocular hypertension. Table 2.
Follow-up visits, diagnostic tests per year, and percentage of patients with adverse events
| Resource/Event | Medication | Year 1 | Year 2 | Year 3 |
|---|---|---|---|---|
| Follow-up visits | ||||
| Visits with intravitreal injections | Dexamethasone IVI | 2.5 | 2.0 | 2.0 |
| Alfibercept | 7.0 | 4.0 | 3.0 | |
| Ranibizumab | 8.0 | 5.0 | 4.0 | |
| Visits without intravitreal injections | Dexamethasone IVI | 3.5 | 2.0 | 2.0 |
| Alfibercept | 0.0 | 0.0 | 0.0 | |
| Ranibizumab | 0.0 | 0.0 | 0.0 | |
| Diagnostic tests | ||||
| Optical coherence tomography | Dexamethasone IVI | 6.0 | 4.0 | 4.0 |
| Alfibercept | 7.0 | 4.0 | 3.0 | |
| Ranibizumab | 8.0 | 5.0 | 4.0 | |
| Fluorescein angiography | Dexamethasone IVI | 1.0 | 0.5 | 0.5 |
| Alfibercept | 1.0 | 0.5 | 0.4 | |
| Ranibizumab | 1.0 | 0.5 | 0.5 | |
| Dexamethasone IVI | 6.0 | 4.0 | 4.0 | |
| Ophthalmoscopy | Alfibercept | 7.0 | 4.0 | 3.0 |
| Ranibizumab | 8.0 | 5.0 | 4.0 | |
| Dexamethasone IVI | 6.0 | 4.0 | 4.0 | |
| Tonometry | Alfibercept | 7.0 | 4.0 | 3.0 |
| Ranibizumab | 8.0 | 5.0 | 4.0 | |
| Dexamethasone IVI | 6.0 | 4.0 | 4.0 | |
| Visual acuity | Alfibercept | 7.0 | 4.0 | 3.0 |
| Ranibizumab | 8.0 | 5.0 | 4.0 | |
| Ocular and systemic adverse events, % | ||||
| Dexamethasone IVI | 15 | 25 | 50 | |
| Cataracts | Alfibercept | 10 | 20 | 20 |
| Ranibizumab | 10 | 20 | 20 | |
| Increased intraocular pressure | Dexamethasone IVI | 25 | 30 | 35 |
| Alfibercept | 1 | 1 | 1 | |
| Ranibizumab | 1 | 1 | 1 | |
| Retinal detachment (per injection) | Dexamethasone IVI | 0.02 | 0.02 | 0.02 |
| Alfibercept | 0.02 | 0.02 | 0.02 | |
| Ranibizumab | 0.02 | 0.02 | 0.02 | |
| Endophthalmitis (per injection) | Dexamethasone IVI | 0.03 | 0.03 | 0.03 |
| Alfibercept | 0.03 | 0.03 | 0.03 | |
| Ranibizumab | 0.03 | 0.03 | 0.03 | |
| Vitreous haemorrhage (per injection) | Dexamethasone IVI | 0.07 | 0.07 | 0.07 |
| Alfibercept | 0.07 | 0.07 | 0.07 | |
| Ranibizumab | 0.07 | 0.07 | 0.07 | |
| Acute myocardial infarction | Dexamethasone IVI | 0.29 | 0.29 | 0.29 |
| Alfibercept | 1.05 | 1.05 | 1.05 | |
| Ranibizumab | 1.07 | 1.07 | 1.07 | |
| Dexamethasone IVI | 0.38 | 0.38 | 0.38 | |
| Ischemic stroke | Alfibercept | 1.74 | 1.74 | 1.74 |
| Ranibizumab | 1.20 | 1.20 | 1.20 | |
| Other cardiovascular events | Dexamethasone IVI | 1.54 | 1.54 | 1.54 |
| Alfibercept | 1.74 | 1.74 | 1.74 | |
| Ranibizumab | 2.81 | 2.81 | 2.81 | |
IVI, intravitreal implant.
The incidence of adverse ocular events and resource utilisation in their management were determined by the expert panel according to standard clinical practice, whereas the incidence of cardiovascular events was established using data from the pivotal clinical trials of the therapeutic options5–7.
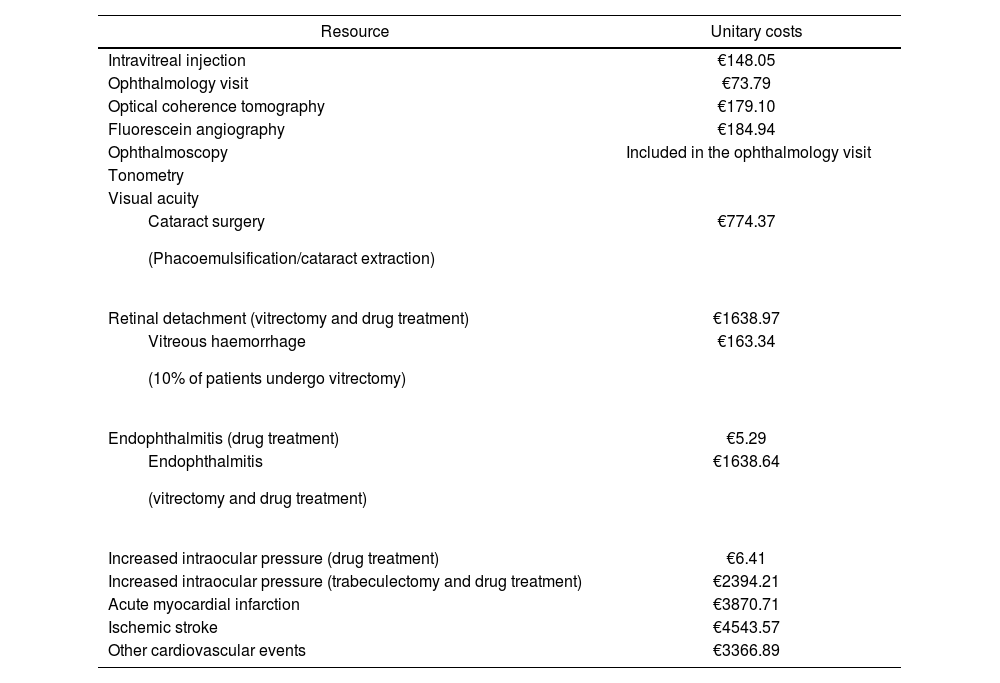
All costs are expressed in 2016 euros. Table 3 shows the unitary costs of the healthcare resources included in the analysis, which were obtained from the literature and from national cost databases19–22.
Unitary costs (2016 euros)
| Resource | Unitary costs |
|---|---|
| Intravitreal injection | €148.05 |
| Ophthalmology visit | €73.79 |
| Optical coherence tomography | €179.10 |
| Fluorescein angiography | €184.94 |
| Ophthalmoscopy | Included in the ophthalmology visit |
| Tonometry | |
| Visual acuity | |
| €774.37 |
| Retinal detachment (vitrectomy and drug treatment) | €1638.97 |
| €163.34 |
| Endophthalmitis (drug treatment) | €5.29 |
| €1638.64 |
| Increased intraocular pressure (drug treatment) | €6.41 |
| Increased intraocular pressure (trabeculectomy and drug treatment) | €2394.21 |
| Acute myocardial infarction | €3870.71 |
| Ischemic stroke | €4543.57 |
| Other cardiovascular events | €3366.89 |
We performed several univariate and multivariate sensitivity analyses, modifying the values of different parameters in order to incorporate the uncertainty into the analysis and observe the effect of these modifications on the results. We included the following parameters in the sensitivity analysis: the percentage of patients diagnosed with DME receiving treatment, unitary costs, the cost of intravitreal injections, the number of dexamethasone IVI injections per year, the cost of managing adverse cardiovascular events, the incidence of cataracts associated with antiangiogenic drugs, and the price of therapeutic alternatives (EFP with or without the deduction stipulated in RDL 8/2010). We also modified the percentage of usage of dexamethasone IVI and the other therapeutic alternatives (Table 1). We also considered three alternative scenarios to assess different healthcare regions with bigger populations (100,000, 250,000, and 500,000 adults) than that of the initial scenario.
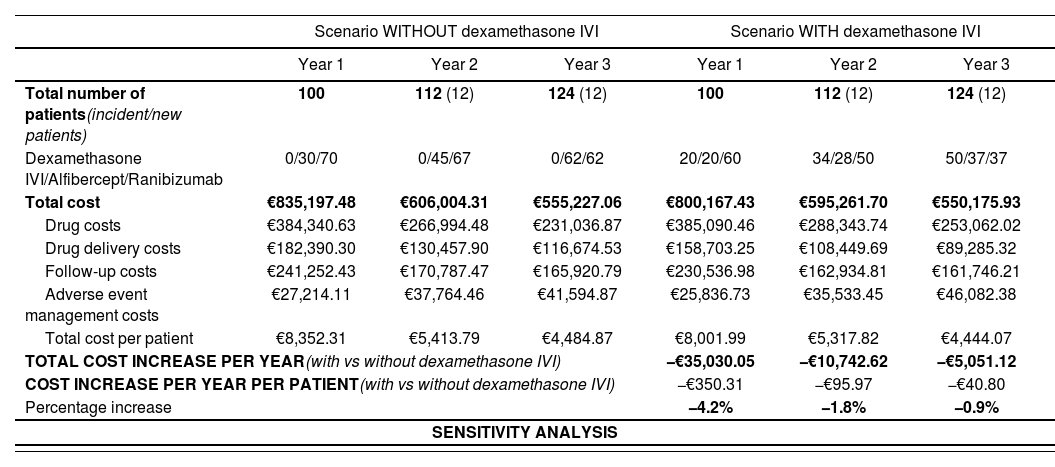
ResultsAfter applying epidemiological data for DME in Spain to a healthcare region with 25,000 adult inhabitants, we estimated that 100, 112, and 124 patients would be eligible for treatment per year over the 3-year study period.
In the scenario without dexamethasone IVI, the total annual cost of treating DME in these patients would be €835,197 (year 1), €606,004 (year 2), and €555,227 (year 3). In the scenario with dexamethasone IVI, the total annual costs would be €800,167 (year 1), €595,262 (year 2), and €550,176 (year 3). Thus, the inclusion of this treatment modality in the therapeutic arsenal for DME would produce budget savings of 4.2% (year 1), 1.8% (year 2), and 0.9% (year 3) involving reductions of €35,030, €10,743, and €5,051, respectively (Table 4). The annual cost of treatment per patient would be reduced by €350.31 (year 1), €95.97 (year 2), and €40.80 (year 3).
Results of the budget impact analysis (base case and sensitivity analysis)
| Scenario WITHOUT dexamethasone IVI | Scenario WITH dexamethasone IVI | |||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 1 | Year 2 | Year 3 | |
| Total number of patients(incident/new patients) | 100 | 112 (12) | 124 (12) | 100 | 112 (12) | 124 (12) |
| Dexamethasone IVI/Alfibercept/Ranibizumab | 0/30/70 | 0/45/67 | 0/62/62 | 20/20/60 | 34/28/50 | 50/37/37 |
| Total cost | €835,197.48 | €606,004.31 | €555,227.06 | €800,167.43 | €595,261.70 | €550,175.93 |
| Drug costs | €384,340.63 | €266,994.48 | €231,036.87 | €385,090.46 | €288,343.74 | €253,062.02 |
| Drug delivery costs | €182,390.30 | €130,457.90 | €116,674.53 | €158,703.25 | €108,449.69 | €89,285.32 |
| Follow-up costs | €241,252.43 | €170,787.47 | €165,920.79 | €230,536.98 | €162,934.81 | €161,746.21 |
| Adverse event management costs | €27,214.11 | €37,764.46 | €41,594.87 | €25,836.73 | €35,533.45 | €46,082.38 |
| Total cost per patient | €8,352.31 | €5,413.79 | €4,484.87 | €8,001.99 | €5,317.82 | €4,444.07 |
| TOTAL COST INCREASE PER YEAR(with vs without dexamethasone IVI) | −€35,030.05 | −€10,742.62 | −€5,051.12 | |||
| COST INCREASE PER YEAR PER PATIENT(with vs without dexamethasone IVI) | −€350.31 | −€95.97 | −€40.80 | |||
| Percentage increase | −4.2% | −1.8% | −0.9% | |||
| SENSITIVITY ANALYSIS | ||||||
| Modified parameters | Value for SA | Year 1 | % Incr | Year 2 | % Incr | Year 3 | % Incr |
|---|---|---|---|---|---|---|---|
| Base case | −€35,030.05 | −4.2% | −€10,742.62 | −1.8% | −€5,051.12 | −0.9% | |
| Percentage of patients being treated (80% in the base case) | 50% | −€21,893.78 | −4.2% | −€6,714.14 | −1.8% | −€3,156.95 | −0.9% |
| Percent of patients with dexamethasone IVI | 40-70-80% (see table 1) | −€93,761.45 | −11.2% | −€56,627.75 | −9.2% | −€10,102.25 | −1.8% |
| ±10% unitary costs | −10% | −€31,455.06 | −4.0% | −€7,535.47 | −1.3% | −€2,342.45 | −0.4% |
| 10% | −€38,605.04 | −4.4% | −€13,949.77 | −2.2% | −€7,759.80 | −1.3% | |
| ± 20% cost of intravitreal injection | −20% | −€30,292.64 | −3.8% | −€6,340.98 | −1.1% | €426.72 | 0.1% |
| +20% | −€39,767.46 | −4.6% | −€15,144.26 | −2.4% | −€10,528.97 | −1.8% | |
| Number of dexamethasone IVI injections per year | 2,5-2,5-2,0 | −€35,030.05 | −4.2% | −€4,578.46 | −0.8% | €4,149.22 | 0.7% |
| Cost of managing adverse cardiovascular events | 0 € | −€32,946.17 | −4.0% | −€7,243.52 | −1.2% | €107.96 | 0.0% |
| Incidence of cataracts in aflibercept and ranibizumab treatments (−50%) | 5%-10%-10% | −€34,255.71 | −4.1% | −€8,280.90 | −1.4% | −€1,400.47 | −0.3% |
| EFP | −€34,969.25 | −4.0% | −€9,011.60 | −1.4% | −€3,265.30 | −0.6% |
| Healthcare region (number of inhabitants per area) | 100,000 | −€140,120.19 | −4.2% | −€42,970.47 | −1.8% | −€20,204.50 | −0.9% |
| 250,000 | −€350,300.47 | −4.2% | −€107,426.17 | −1.8% | −€50,511.25 | −0.9% | |
| 500,000 | −€700,600.94 | −4.2% | −€214,852.34 | −1.8% | −€101,022.49 | −0.9% |
% Incr, % increase of costs in the scenario with dexamethasone IVI vs the scenario without dexamethasone IVI; EFP, ex-factory price; IVI, intravitreal implant; RDL, Royal Decree-Law; SA, sensitivity analysis.
Relative to the scenario without dexamethasone IVI, the scenario with dexamethasone IVI would have led to an overall saving of 5% in drug costs over the 3-year study period (0.2%, 8%, and 9.5% per year, respectively). Drug delivery and monitoring costs would provide the greatest savings in total costs. Drug administration costs and monitoring costs would have been between 13% and 23.5% lower and between 2.5% and 4.6% lower, respectively. In the scenario with dexamethasone IVI, for the whole analysis period, drug delivery costs and monitoring costs would have been 17.01% and 3.93% lower, respectively.
Sensitivity analyses showed that an increase in the use of dexamethasone IVI would further reduce the annual costs relative to the scenario without the implant. The sensitivity analysis also showed that an increase in unitary costs of healthcare resources (excluding drug costs) would increase the total cost in the scenario without dexamethasone (drug delivery and follow-up) which is already the scenario with greater costs. Thus, this would increase the savings between scenarios due to the introduction of dexamethasone IVI. However, several factors could reduce the potential savings derived from the introduction of dexamethasone IVI. These factors include: reductions in the unitary costs of healthcare resources, not considering the cost of managing adverse systemic events, a decrease in the incidence of cataracts in antiangiogenic therapies, and increasing the number of dexamethasone IVI injections per year (Table 4).
For each of the 3-year analysis period, the total cost in a population of 100,000 adults (400 prevalent patients) would be €3.3 million, €2.4 million, and €2.2 million in the scenario without dexamethasone IVI and €3.2 million, €2.4 million, and €2.2 million in the scenario with dexamethasone IVI, respectively. In a medium-sized health area with a population of 250,000 adults (1,000 prevalent patients), for each of the 3 years the total cost of treatment for DME would be €8.4 million, €6.1 million, and €5.6 million in the scenario without dexamethasone IVI. The scenario with dexamethasone IVI would led to savings of €350,300, €107,426, and €50,511 in each year, respectively. In an area with a population of 500,000 adults, the total cost of treating DME without dexamethasone IVI would be €16.7 million, €12.1 million, and €11.1 million euros for each of the 3 years in study, respectively. The introduction of dexamethasone IVI would led to savings of €700,601, €214,852, and €101,022, respectively.
DiscussionDiabetes mellitus is a highly prevalent disease and its prevalence is expected to increase over the coming years. Poor glycaemic control and subsequent complications, such as diabetic retinopathy and DME, are a great economic burden for the Spanish National Health System due to the challenge of managing this increasing number of patients.
According to recent economic data on DME in Spain, the estimated direct annual cost per patient with DME was €6,271 (excluding drug costs)13, whereas the estimated annual cost of treatment with antiangiogenic drugs was €7,154 with follow-up costs of €47423. DME can cause partial vision loss or blindness, which would entail an additional increase in indirect costs due to disability. The mean annual cost per patient due to permanent disability is €7,051, which is especially relevant in patients with bilateral DME (€11,712 in bilateral DME vs €4,284 in unilateral DME)13. A report on blindness in Spain24 has suggested that the cost is around €5,100 per blind patient per year, entailing a total cost of around €360 million. There are additional costs that are paid by blind patients (€25,914) or those with impaired vision (€11,032)25. Besides good glycaemic control, opting for more effective treatments would reduce the economic burden on the Spanish National Health System service of treating patients with DME.
According to the model, the inclusion of dexamethasone IVI in the therapeutic arsenal for DME would save €35,030, €10,743, and €5,051 over each of the 3 years of analysis, respectively. These savings would mainly be due to the lower rate of treatments with dexamethasone IVI, which would in turn reduce drug delivery and follow-up costs. Annual savings per patient would be between €41 and €350. These estimations are corroborated by the alternative scenario in which there is increased use of dexamethasone IVI, suggesting that there would be even greater savings over the study period.
To the best of our knowledge, this study is the first to assess the impact on the healthcare budget of currently available treatments for DME in Spain, including dexamethasone IVI. A review of the literature found three recent economic assessment studies conducted in different countries that analysed the efficiency of several anti-VEGF therapies26–28. However, we found only two studies that included dexamethasone IVI25,29. One of these was a cost-effectiveness analysis conducted in Spain, which suggested that dexamethasone IVI was an efficient option with a cost of €2,050 per line of visual acuity gained25.
The present study has some limitations. The main limitation is related to the estimation of the target population. The robustness of the data is affected by the reliability of the epidemiological data available for DME, because the studies from which the data were obtained were conducted with relatively small reference populations17,18. The percentages of usage of the different therapeutic alternatives represent possible future trends in the usage of these drugs. The results obtained could vary if the estimated values of medication use do not closely reflect changes in the market.
The lack of robust information on the real-world management of patients with DME meant that some parameters had to be provided by the expert panel. Therefore, the following estimates may not accurately represent daily clinical practice in all Spanish healthcare centres: the number of intravitreal injections, the number of annual visits needed to treat patients with the therapeutic alternatives, resource utilisation caused by adverse events and patient follow-up.
Complete economic assessments, such as cost-effectiveness or cost-utility analyses, allow us to determine the comparative efficiency of the available alternatives for the treatment of a given disease. Budget impact analyses, such as that conducted in this study, involve partial economic assessments which analyse the economic impact of different therapies without assessing their effectiveness or efficacy, thus making them complementary to other assessments. This type of assessment should be seen as a useful tool for decision-making in healthcare, particularly in settings in which healthcare budget control is a priority. However, future studies should help to complement the information presented in this study and therefore assist in this decision-making process.
In conclusion, the use of dexamethasone IVI for the treatment of DME would entail savings to healthcare budgets. These savings would mainly be due to the reduced costs of drug delivery and patient follow-up.
FundingThe project was conducted with unconditional funding from Allergan, SAU.
Conflicts of interestsFernando de Andrés and Itziar Oyagüez work at PORIB, a consultancy specializing in economic assessments of health technologies that received funds from Allergan SAU to conduct this project. Enrique Cervera, Luis Arias and Félix Armadá received honoraria from Allergan SAU for this project as consultants to Allergan SAU. Concha Martínez works at Pricing and Market Access, Allergan SAU. The authors declare that this financial support does not represent a conflict of interest in relation to the development of this article.
This article was partially presented as a brief report at the 19th Annual European Congress of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR), held in Vienna (Austria), from October 29, 2016 to November 2, 2016.
The authors declare that this work has not been published nor is it in the process of being considered for publication in any other journal.
Contribution to the literature
This study assessed the budget impact on the Spanish National Health System of the inclusion of dexamethasone intravitreal implant as a treatment for diabetic macular oedema. The field of ophthalmology has become of increasing relevance in budgetary terms because of the recent incorporation of new intravitreal therapies. The results of this study should be of aid in decision-making in the setting of ophthalmology.