Evaluation of the clinical and economic impact after the protocol change of basiliximab use in orthohepatic transplant.
MethodRetrospective study in which all liver transplant patients were included during the years 2013, 2014 and until February 15, 2015. The study was divided into two stages according to the protocol used: 1) administration of basiliximab only if factors of previous risk, and 2) administration of the first dose of basiliximab to all transplant patients and the second dose if it had risk factors.
Results83 patients were included, 34 according to protocol 1 and 49 according to protocol 2. No significant differences were found in the clinical variables evaluated or in the variables related to health outcomes. Considering that the percentage of patients without risk factors who received basiliximab was 43% and without differences in the stays, we could estimate an additional cost for the universal use of basiliximab in orthohepatic transplant of € 21,400.00.
ConclusionsIn our population, the protocol change making universal the first dose of basiliximab has not shown the expected benefits, but an increase in costs, so the suitability of the new protocol in consensus with the medical team must be reconsidered. The evidence regarding the use of basiliximab in orthohepatic transplant remains limited and although its benefit seems clear in patients with risk factors, especially renal failure, recommendations about its use universally remains controversial.
Evaluación del impacto clínico y económico tras el cambio de protocolo de uso de basiliximab en el trasplante ortohepático.
MétodoEstudio retrospectivo en el que se incluyó a todos los pacientes trasplantados de hígado durante los años 2013, 2014 y hasta el 15 de febrero de 2015. El estudio se dividió en dos etapas según el protocolo empleado: 1) administración de basiliximab solo si existían factores de riesgo previos, y 2) administración de la primera dosis de basiliximab a todos los pacientes trasplantados y de una segunda dosis si existían factores de riesgo.
ResultadosSe incluyeron 83 pacientes, 34 según el protocolo 1 y 49 según el protocolo 2. No se encontraron diferencias significativas en las variables clínicas evaluadas ni en las variables relacionadas con los resultados en salud. Considerando que el porcentaje de pacientes sin factores de riesgo que recibieron basiliximab fue del 43% y sin diferencias en las estancias, podríamos estimar un coste adicional por el empleo universal de basiliximab en el trasplante ortohepático de 21.400 €.
ConclusionesEn nuestra población, el cambio de protocolo haciendo universal la primera dosis de basiliximab no ha mostrado los beneficios esperados, pero sí un aumento de los costes, por lo que debe replantearse la idoneidad del nuevo protocolo en consenso con el equipo médico. La evidencia en relación con el empleo de basiliximab en el trasplante ortohepático sigue siendo limitada y aunque parece claro su beneficio en pacientes con factores de riesgo, especialmente fallo renal, las recomendaciones acerca de su uso de forma universal sigue siendo controvertido.
Liver transplant surgery is demanding, with plenty of bleeding, transfusions, and frequent use of vasoactive drugs which have a harmful effect on kidneys. Without induction, tacrolimus (TAC) must be initiated within the first 12 hours, with a patient still unstable. Additionally there is difficult dosing, which is complex during the first days, because it must be initiated with a weight-adjusted dose but with high variability between and among individuals. Acute renal damage will occur primarily because calcineurin inhibitors (CNIs) will cause vasoconstriction both in the afferent and efferent arteriole, therefore reducing the glomerular filtration rate (GFR), leading to higher morbidity with a prolonged stay at the ICU and requiring hemofiltration or dialysis. The chronic form of nephrotoxicity is characterized by the development of irreversible structural damage, which could lead to an end-stage renal disease1. For this reason, different strategies have been designed in an attempt to reduce it, such as the use of interleukin-2 antibodies (IL-2R) as immunosuppressants in the induction period, because no serum level monitoring is required, and it is considered effective and safe according to prospective or retrospective studies and randomized clinical trials, as well as having low immunogenicity2,3. In any case, it should always be used in combination with CNIs in order to prevent acute rejection4,5.
Basiliximab is a chimeric monoclonal antibody binding specifically and with high affinity to the CD25 antigen of activated T-lymphocytes that express the high-affinity IL-2R receptor. This prevents IL-2 from binding to the receptor, which is a critical signal for T-cell proliferation in the cell immune response involved in organ rejection2. This is the only option available with the mechanism of action described, although in Spain it is only indicated as prophylaxis for acute rejection in renal transplant, and its used was included in the protocol according to Royal Decree 1015/2009 on Medications in Special Situations.
The infection rate (bacterial, viral and fungal) is similar among patients receiving basiliximab or daclizumab and placebo, including infections by cytomegalovirus. No differences have been observed in the number of de novo post-transplant neoplasias at one year of treatment6,7. Its main advantage is that there will be no impact on renal function or bone marrow and, therefore, it can be used for renal impairment, as well as in anemia, leukopenia or thrombocytopenia. The negative aspects are that their influence on the relapse of Hepatitis C is unknown, because bad evolution has been described regarding Hepatitis C relapse in patients treated with daclizumab and mycophenolate mofetil (MMF), with no confirmation by subsequent studies. However, with new antivirals currently available for Hepatitis C treatment and its high cure rate, it seems that this aspect might become less relevant8,9.
There is a higher development of renal impairment immediately after liver transplant if the patient presented previous renal impairment, refractory ascites, malnutrition, an unfavorable prognostic index, and suboptimal donor; but many patients without these risk factors will also develop this complication2.
Induction with basaliximab allows to delay CNI initiation up to 4-7 days after the transplant, according to the number of basiliximab doses administered. A CNI-free period of time is allowed, so that renal function can be recovered after the aggressions received during the perioperative period; this will be usually initiated in the ward when there is clinical stability. The target therapeutic levels for TAC during the first 6 weeks without basiliximab should be within 10-15 ng/mL, while with basiliximab these could be between 8-10 ng/mL, contributing to a lower nephrotoxicity at long term and, as it has been recently observed, lower recurrence of tumors in hepatocellular carcinoma10,11.
Until 2013, the immunosuppression protocol in our center was based on the administration of basiliximab only to patients with risk factors: renal impairment previous to the transplant (GFR < 60 mL/min/1.73 m2 or serum creatinine [Cr] > 1.5 mg/dL), renal impairment immediately after the transplant (within the first 48 hours), defined as oliguria < 0.5 mL/kg/h, GFR < 60 mL/min/1.73 m2 or Cr elevation > 1.5 mg/dL, or cirrhosis with refractory ascites and severe malnutrition with renal impairment present before the transplant. In patients who presented the risk factors described, the dose to be administered was two 20 mg perfusions on the day of the transplant, and 4 days after the transplant. The second dose might not be administered if at Day 4 the renal function of the patient continued stable (GFR > 60 mL/min/1.73 m2 or Cr < 1,2 mg/dL and diuresis > 1 mL/kg/h).
According to the current immunosuppression protocol with universal administration of basiliximab, implemented since 2014, all patients would receive a first 20 mg dose on the day of the transplant and a second 20 mg dose on the fourth day after the transplant, individualized according to the basal characteristics of the patient and their clinical evolution. The introduction of the calcineurin inhibitor is indicated between Days 5 to 7 for those who have received both doses, and on the 3rd-4th day if they have only received one dose.
With this change of strategy, it was expected to achieve a reduction in the time of ICU hospitalization and a lower morbidity of patients after the transplant. Our hypothesis if that this change of protocol has not led to the results expected in terms of reduction in hospital stays and better evolution in renal function; therefore, the objective of this study is to evaluate the clinical and economic impact after the change of protocol for use of basiliximab in orthotopic liver transplantation (OLT).
MethodsA retrospective observational study comparing two protocols, including all adult patients who underwent a liver transplant in a public hospital from January, 1st, 2014 to February, 15th, 2015, with follow-up during the first 12 months after the transplant. The study has been classified by the Spanish Agency of Medicines as EPA-OD (Post-authorization study, other designs), and approved by a Research Ethics Committee.
The study was conducted with two types of patients: those who underwent a liver transplant during 2013, following the protocol in place until that date, and those who underwent a transplant in 2014-2015, according to the protocol adopted in 2014. In both cases, immunosuppressant therapy included the introduction of MMF on Day 1 after the transplant, with corticosteroids.
The data collection logbook included age, gender, year of the transplant, basiliximab doses administered, days for the initiation of treatment with TAC, days of ICU stay, days of stay for the episode, creatinine and renal function previous to transplant, at ICU discharge, at hospital discharge, and one year after transplantation, mortality within the post-operative period before discharge, at three months and one year, presence of diabetes and refractory ascites before the transplant, hypertension and diabetes as post-transplant complications, and the Model for End-stage Liver Disease (MELD) Scales12.
Post-transplant hypertension was defined as the need for hypertension treatment or change from the treatment previous to transplant; and post-transplant diabetes was defined as the need for insulin or change of antidiabetic treatment regimen vs. previous home treatment.
Renal dysfunction was defined as GFR, calculated according to MDRD4, below 60 mL/min/1.73 m2.
Anthropometric data, use and cost of basiliximab vials, days of initiation for TAC treatment, and stay at ICU and for the episode, were obtained from the Farmatools® program at the Pharmacy Unit.
The lab test and clinical data, post-transplant complications, and MELD scale, were obtained from the Selene® Electronic Clinical Record program.
Costs per stay at ICU and hospital ward are the public prices by the Regional Health System.
The sample of this study has been limited to the number of transplants conducted during the periods of time compared. The 34 patients from the previous protocol and 49 from the new one allowed to detect relevant differences of at least 35%, and mean or median differences of at least 18 units, with an 80% power in bilateral hypothesis testing at a p ≤ 0.05 significance level.
The sample characteristics were described by summarizing the nominal variables with the relative frequency of their component categories, the scale ones that do not follow a normal distribution with median (range) and the scale ones with normal distribution with mean (SD). Normality was tested through histograms and Kolmogorov-Smirnov test. According to these characteristics of variables, the comparisons between both protocols were conducted with Pearson's chi test2 or Fisher's Exact Test, Mann-Whitney U test, or Student's t test, respectively. All these tests were bilateral, with a p ≤ 0.05 level of significance, and all relevant calculations were conducted with the SPSS statistical program by IBM Co® 21.0 in the Windows XP Professional operating system.
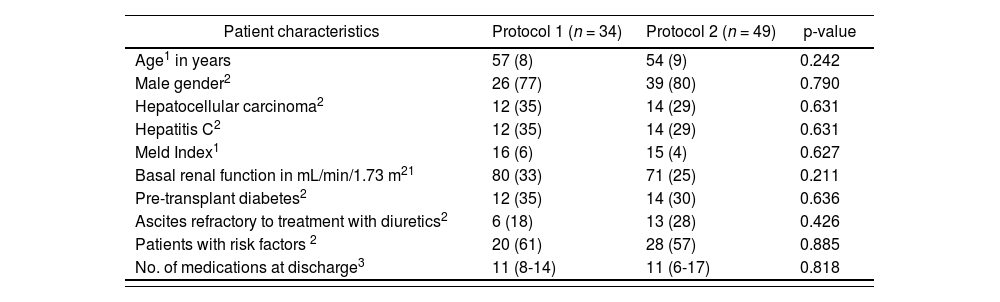
ResultsThere were 83 patients included in the study in total: 34 patients according to Protocol 1 and 49 patients according to Protocol 2. Table 1 shows the basal characteristics of both groups; no statistically significant differences were found in any of the characteristics evaluated.
Characteristics of the patient sample in the study “Clinical-economic impact of the change of protocol for use of basiliximab in liver transplant”
| Patient characteristics | Protocol 1 (n = 34) | Protocol 2 (n = 49) | p-value |
|---|---|---|---|
| Age1 in years | 57 (8) | 54 (9) | 0.242 |
| Male gender2 | 26 (77) | 39 (80) | 0.790 |
| Hepatocellular carcinoma2 | 12 (35) | 14 (29) | 0.631 |
| Hepatitis C2 | 12 (35) | 14 (29) | 0.631 |
| Meld Index1 | 16 (6) | 15 (4) | 0.627 |
| Basal renal function in mL/min/1.73 m21 | 80 (33) | 71 (25) | 0.211 |
| Pre-transplant diabetes2 | 12 (35) | 14 (30) | 0.636 |
| Ascites refractory to treatment with diuretics2 | 6 (18) | 13 (28) | 0.426 |
| Patients with risk factors 2 | 20 (61) | 28 (57) | 0.885 |
| No. of medications at discharge3 | 11 (8-14) | 11 (6-17) | 0.818 |
Protocol 1: Administration of basiliximab only to patients with risk factors. Protocol 2: Administration of first dose of basiliximab to all patients.
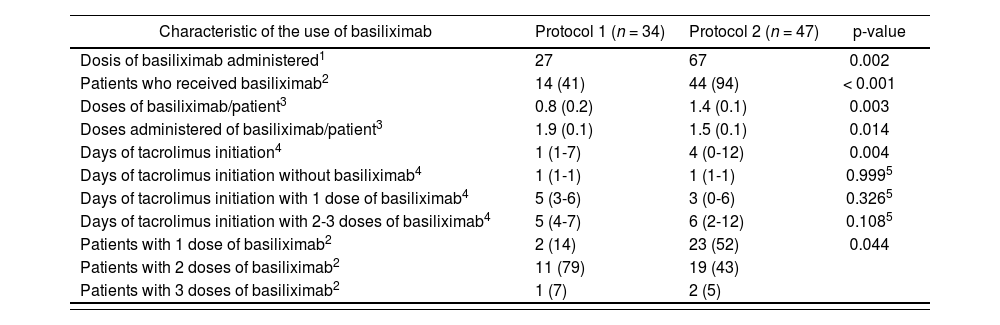
Table 2 describes the characteristics regarding basiliximab administration and TAC initiation with both protocols. In the arm of patients who received basiliximab according to Protocol 2, 2 patients were lost due to premature exitus before their second basiliximab administration. The majority of patients included in the study period 2 received basiliximab (93.6% vs. 41%). It is worth highlighting that with Protocol 1, there was a higher proportion of patients who received two doses (78.6% vs. 43.2%). The median number of days for TAC introduction was 4 for Protocol 2 vs. 1 for Protocol 1. Table 2 shows in detail the differences in the initiation of TAC administration according to the protocol and the number of basiliximab doses administered. It can be observed that when Protocol 1 was used, TAC introduction could be conducted as previously determined; when 2-3 doses of basiliximab were used, 17% of patients could initiate TAC treatment earlier than expected. However, under Protocol 2, protocol compliance when one and two doses of basiliximab were administered was of 61% and 67%, respectively. It is worth highlighting that 26% of patients received one dose of basiliximab and had TAC initiated before the established period of 3-4 days. This situation was observed at a lower extent (14%) when two doses of basiliximab were administered.
Characteristics of the use of basiliximab in the patients included in the study “Clinical-economic impact of the change of protocol for use of basiliximab in liver transplant”
| Characteristic of the use of basiliximab | Protocol 1 (n = 34) | Protocol 2 (n = 47) | p-value |
|---|---|---|---|
| Dosis of basiliximab administered1 | 27 | 67 | 0.002 |
| Patients who received basiliximab2 | 14 (41) | 44 (94) | < 0.001 |
| Doses of basiliximab/patient3 | 0.8 (0.2) | 1.4 (0.1) | 0.003 |
| Doses administered of basiliximab/patient3 | 1.9 (0.1) | 1.5 (0.1) | 0.014 |
| Days of tacrolimus initiation4 | 1 (1-7) | 4 (0-12) | 0.004 |
| Days of tacrolimus initiation without basiliximab4 | 1 (1-1) | 1 (1-1) | 0.9995 |
| Days of tacrolimus initiation with 1 dose of basiliximab4 | 5 (3-6) | 3 (0-6) | 0.3265 |
| Days of tacrolimus initiation with 2-3 doses of basiliximab4 | 5 (4-7) | 6 (2-12) | 0.1085 |
| Patients with 1 dose of basiliximab2 | 2 (14) | 23 (52) | 0.044 |
| Patients with 2 doses of basiliximab2 | 11 (79) | 19 (43) | |
| Patients with 3 doses of basiliximab2 | 1 (7) | 2 (5) |
| Doses of basiliximab administered | Tacrolimus initiation6 | |||||
|---|---|---|---|---|---|---|
| Protocol 1 (n = 34) | Protocol 2 (n = 47) | |||||
| Within range7 | Early | Delayed | Within range7 | Early | Delayed | |
| 0 | 20 (100) | − | − | 3 (100) | − | − |
| 1 | 1 (50) | − | 1 (50) | 14 (61) | 6 (26) | 3 (13) |
| 2-3 | 10 (83) | 2 (17) | − | 14 (67) | 3 (14) | 4 (19) |
| Total | 31 (91) | 2 (6) | 1 (3) | 31 (66) | 9 (19) | 7 (15) |
Protocol 1: Administration of basiliximab only to patients with risk factors. Protocol 2: Administration of first dose of basiliximab to all patients.
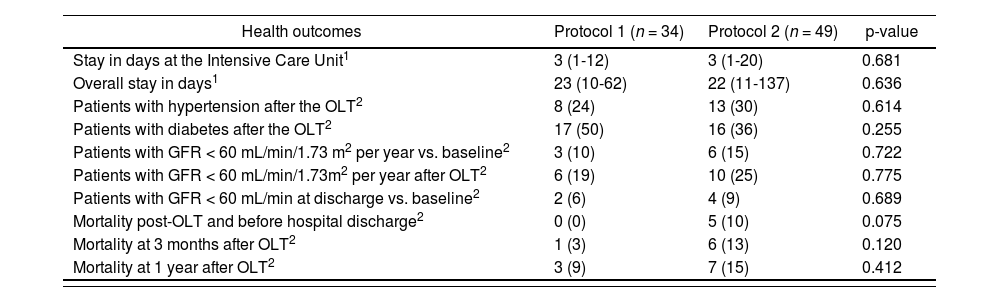
Health outcomes are shown in table 3; no statistically significant differences have been found in any of the variables evaluated: stay at ICU and for the transplant episode, hypertension and diabetes after the transplant, renal function with GFR < 60 mL/min, mortality in the immediate period after the transplant (before hospital discharge), at three months and at one year.
Variables of health outcomes and clinical in the patients of the study “Clinical-economic impact of the change of protocol for use of basiliximab in liver transplant”
| Health outcomes | Protocol 1 (n = 34) | Protocol 2 (n = 49) | p-value |
|---|---|---|---|
| Stay in days at the Intensive Care Unit1 | 3 (1-12) | 3 (1-20) | 0.681 |
| Overall stay in days1 | 23 (10-62) | 22 (11-137) | 0.636 |
| Patients with hypertension after the OLT2 | 8 (24) | 13 (30) | 0.614 |
| Patients with diabetes after the OLT2 | 17 (50) | 16 (36) | 0.255 |
| Patients with GFR < 60 mL/min/1.73 m2 per year vs. baseline2 | 3 (10) | 6 (15) | 0.722 |
| Patients with GFR < 60 mL/min/1.73m2 per year after OLT2 | 6 (19) | 10 (25) | 0.775 |
| Patients with GFR < 60 mL/min at discharge vs. baseline2 | 2 (6) | 4 (9) | 0.689 |
| Mortality post-OLT and before hospital discharge2 | 0 (0) | 5 (10) | 0.075 |
| Mortality at 3 months after OLT2 | 1 (3) | 6 (13) | 0.120 |
| Mortality at 1 year after OLT2 | 3 (9) | 7 (15) | 0.412 |
| Changes in renal function (ml/min/1.73 m2) | Protocol 1 (n = 34) | Protocol 2 (n = 49) | p-value | |
|---|---|---|---|---|
| Basal GFR > 60 ICU > 60 | 23 (70) | 27 (63) | ||
| From admission to Hospital ICU |
|
|
| 0.5151 |
| Basal GFR < 60 ICU < 60 | 4 (12) | 6 (14) | ||
| Basal GFR > 60 Discharge > 60 | 20 (61) | 26 (61) | ||
| From admission to discharge | Basal GFR > 60 Discharge < 60 | 3 (9) | 3 (7) | 0.9102 |
| Basal GFR < 60 Discharge > 60 | 5 (15) | 7 (16) | ||
| Basal GFR < 60 Discharge < 60 | 5 (15) | 7 (16) | ||
| Basal GFR > 60 Year > 60 | 19 (63) | 22 (56) | ||
| From admission to one year after transplantation | Basal GFR > 60 Year < 60 | 3 (10) | 4 (10) | 0.7351 |
| Basal GFR < 60 Year > 60 | 4 (13) | 6 (15) | ||
| Basal GFR < 60 Year < 60 | 4 (13) | 7 (18) | ||
| GFR Discharge > 60 Year > 60 | 20 (67) | 25 (63) | ||
| From discharge to one year after transplantation | GFR Discharge > 60 Year < 60 | 5 (17) | 5 (13) | 0.2831 |
| GFR Discharge < 60 Year > 60 | 3 (10) | 3 (8) | ||
| GFR Discharge < 60 Year < 60 | 2 (7) | 7 (18) | ||
Protocol 1: Administration of basiliximab only to patients with risk factors. Protocol 2: Administration of first dose of basiliximab to all patients.
OLT: orthotopic liver transplant.
Table 3 also shows the evolution of renal function considering different changes: basal value compared with discharge from ICU, basal value compared with hospital discharge; basal value compared with one year after transplantation, and the GFR presented at the time of hospital discharge compared with the GFR one year after the transplant. In all cases, no statistically significant differences were found.
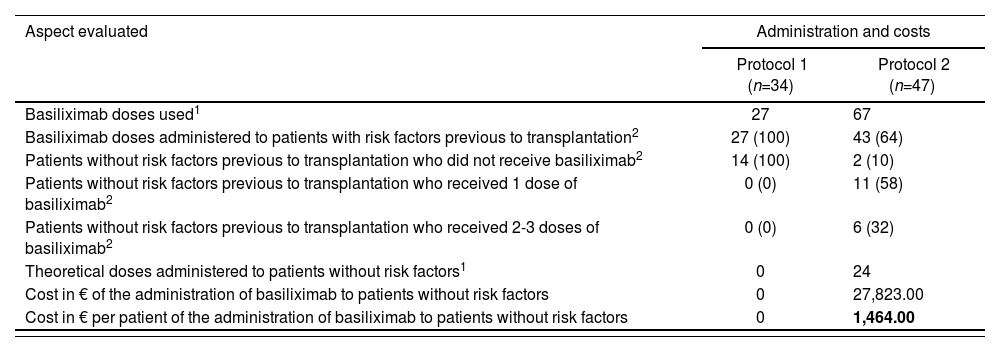
Table 4 shows economic outcomes, in terms of direct costs associated with a higher use of basiliximab at the change of regimen. It can be observed that there was a high percentage of patients without risk factors who received at least one dose of basiliximab (90% vs. 0%). The cost incurred for basiliximab administration under Protocol 2 in patients without risk factors was 1,464 € per patient. Considering that 43% of patients included in Protocol 2 did not present any risk factors before transplantation, and accepting 34 liver transplants conducted in adults in our center in one year, the incremental difference in costs was estimated in 21,400 €.
Economic outcomes from the study “Clinical-economic impact of the change of protocol for use of basiliximab in liver transplant”
| Aspect evaluated | Administration and costs | |
|---|---|---|
| Protocol 1 (n=34) | Protocol 2 (n=47) | |
| Basiliximab doses used1 | 27 | 67 |
| Basiliximab doses administered to patients with risk factors previous to transplantation2 | 27 (100) | 43 (64) |
| Patients without risk factors previous to transplantation who did not receive basiliximab2 | 14 (100) | 2 (10) |
| Patients without risk factors previous to transplantation who received 1 dose of basiliximab2 | 0 (0) | 11 (58) |
| Patients without risk factors previous to transplantation who received 2-3 doses of basiliximab2 | 0 (0) | 6 (32) |
| Theoretical doses administered to patients without risk factors1 | 0 | 24 |
| Cost in € of the administration of basiliximab to patients without risk factors | 0 | 27,823.00 |
| Cost in € per patient of the administration of basiliximab to patients without risk factors | 0 | 1,464.00 |
Protocol 1: Administration of basiliximab only to patients with risk factors. Protocol 2: Administration of first dose of basiliximab to all patients.
Risk factors: renal impairment, diabetes, or refractory ascites previous to transplantation
One of the studies showing benefit with the protocol for universal administration of basiliximab was ReSpECT3, a prospective, multicenter, randomized and open study which excluded patients with renal impairment before transplantation, and which showed a significant improvement in renal function by 10 mL/min at 52 weeks in the induction group; however, this difference in patients with GFR > 60 mL/min could lack any clinical relevance, while in our study we took into account the value at baseline and at discharge of renal function, differentiating those patients with GFR above or below 60 mL/min, so that no clinically relevant differences were found. The study by Cai J. showed that induction improves the prognosis for graft and patient in renal, liver (43,407 patients) and lung transplant, in a highly significant way, at 3 months, 1 year and 5 years13. In the case of liver transplant, survival improved by approximately 3-4%, and this was difficult to find in our sample possibly due to the sample size, as well as differences in the follow-up period. Likewise, in the retrospective study of the register of transplanted patients between 2002 and 2009, with and without hepatocellular carcinoma, where different immunosuppressant regimens were evaluated, and 14,658 patients were included in total, overall survival was analyzed at long term (3 and 5 years), though it was significant with anti-CD25 induction only in the hepatocellular carcinoma arm12. Other studies demonstrated a lower incidence of rejection confirmed by biopsy; this was not collected in our study population due to the difficult access to biopsy results13. A systematic review and meta-analysis of 18 controlled studies, of which only 13 were randomized, demonstrated a significantly lower incidence of acute rejection, including resistance to steroids, with induction; as well as a non-significant trend towards better survival, lower incidence of diabetes, and lower risk of renal dysfunction; however, no differences were found in terms of graft and patient survival, and therefore there is coincidence with our results in terms of survival data, except for the fact that we did not find any differences in complications after the transplant such as diabetes or renal function deterioration15. The study by Chih-Che Lin et al. included 45 patients split into two arms, with the objective to determine if there was an improvement in renal function in those patients who received induction therapy with basiliximab; statistically significant differences were found three months after the transplant, unlike in our study, where we conducted follow-up for 52 weeks after the transplant10.
Other studies, coinciding with our results, did not find any statistically significant differences in the reduction of the incidence of acute rejection episodes, and in improving the graft function, though they measured survival at long term (36 weeks)16.
The recommendations from the 5th Consensus Meeting by the Spanish Society of Liver Transplant, with conclusions published by the end of 2015, coincide with our results, and only recommend induction in patients with renal impairment before transplantation or in patients at high risk of renal impairment after the transplant, without reaching a consensus about its universal use17. On the contrary, the European Guidelines for Liver Transplant published at the start of 2016 recommend, with Recommendation Level I, the use of IL-2R antibodies and TAC at lower doses and delayed initiation together with MMF and steroids, because it is safe and improves renal function significantly, though some concern is expressed about the high cost of IL-2R, an important aspect that we took into account in our study18. The American Guidelines for Clinical Practice in adults who have undergone OLT describes the use of these agents19.
The majority of the pharmacoeconomic studies published has been conducted using basiliximab as induction in renal transplant patients, in two cases using dual therapy as control arm (corticosteroids + cyclosporine), although the conclusion seems to be that using basiliximab as induction in renal transplant is cost-effective because there is a reduction in hospital stay and a lower rate of acute rejection20–22. In an editorial published in 2002, Ryutaro Hirose explained that cost represents a barrier for the routine use of IL-2R in liver transplant, and that this additional cost might not be justified, unless a significantly reduction is demonstrated in rejection rates and readmissions, aspects that have not been evaluated in our study22. A search was conducted in Pubmed using the following terms in English: “pharmacoeconomic”, “liver transplantation” and “basiliximab”; only one study was found, in a 49-patient pediatric population, comparing an arm that received TAC + basiliximab, corticosteroid-free, vs. standard therapy with corticosteroids + TAC; the conclusion was that medical costs were similar in both arms; however, neither the study population nor the immunosuppressant therapy used could be extrapolated24.
Our study presents some limitations: one could be derived of the sample size, because we have selected transplanted patients since 2013, given that data collection before this date was not possible. Other potential bias is the lack of evaluation of the development of steroid-resistant acute rejection, an aspect that could be relevant, as well as the time used to reach therapeutic levels of TAC. The cost of purchasing TAC was not included, because it was considered irrelevant, and a high variability of dosing was found. The follow-up period was of one year after OLT, but it would be interesting to assess the long-term evolution of renal function, such as has been evaluated in other series.
In our sample, the lack of improvement of renal function observed could be associated with the initiation of TAC before the time initially stated in the protocol; therefore, this was considered a critical point. These data were submitted to the liver transplant team, and the conclusion was that it was necessary to increase adherence to protocol regarding TAC initiation.
Taking into account limitations and bias, we consider that the results obtained do not confirm the objectives expected from the change of the protocol, even at the higher cost incurred. As can be deduced from our study and the new consensus guidelines, basiliximab would play an important role in patients with high creatinine levels or at risk of presenting renal impairment.
There is limited evidence published about the use of basiliximab in OLT in terms of quality, as there is high heterogeneity in the populations selected; therefore, it would be necessary to conduct randomized and blind clinical trials, preferably independent, in order to help in making clinical decisions.
FundingNo funding.
Conflict of interestsNo conflict of interests.
Contribution to scientific literature.
The relevance of our study is determined by the comparison between two different protocols of use for basiliximab in liver transplant in adult population; it aims to confirm if the use of basiliximab is cost-effective in all patients or only in those with risk factors, while other published studies compare a regimen with or without basiliximab.
- Inicio
- Todos los contenidos
- Publique su artículo
- Acerca de la revista
- Métricas