Splenectomy, thrombopoietin receptor agonists and rituximab are the second-line treatments for steroid-resistant adult primary immune thrombocytopenia. The last two are becoming the most widely used treatments to avoid splenectomy adverse effects and inconveniences. However, the choice between rituximab and thrombopoietin receptor agonists is unclear. Therefore, the treatment cost may be of particular interest to prioritize the therapy option. Our aim is to determine the cost per responding-patient after 6 months of use of rituximab compared to thrombopoietin receptor agonists eltrombopag in the treatment of chronic primary immune thrombocytopenia in the Spanish National Health Service.
MethodA 26-week decision tree model was developed to assess the cost of treatment response of adult patients with chronic-refractory primary immune thrombocytopenia to eltrombopag and rituximab from the perspective of the Spanish National Health System. Effectiveness was obtained from the literature, and cost was obtained from the official rates. Costs were expressed in € (2018). Due to the short period of assessment, no discount rate was applied.
ResultsThe average cost per patient after 6 months of treatment was slightly higher for eltrombopag (€13,089.40) than for rituximab (€11,852.60). However, the greater response rate of eltrombopag decreases the bleeding costs, resulting in a 29% higher cost per responding-patient with rituximab (€18,964.15) than for eltrombopag (€14,732.65). This result is consistent with the results of the 15 sensitivity analyses carried out where eltrombopag always represents a lower cost per responding patient, except in the sensitivity analysis in which treatment with eltrombopag is performed at its maximum dose (75mg). Only in this case, the cost per responder of eltrombopag is €48 more expensive than that of rituximab. Likewise, the greatest difference in favor of eltrombopag occurs in the scenario that uses the minimum dose of this drug —25mg— (eltrombopag €7,622.14 compared to €18,964.15 for rituximab). Thus, the cost per responding patient is lower in eltrombopag even if a second cycle of retreatment with rituximab is not performed (€14,732.65 versus €15,298.61).
ConclusionsThe treatment cost of rituximab, including monitoring and bleeding costs, is higher than eltrombopag, favoring the latter over rituximab treatment.
La esplenectomía, los agonistas del receptor de trombopoyetina y el rituximab son los tratamientos de segunda línea para la trombocitopenia inmune primaria. Los dos últimos se están convirtiendo en los más utilizados para evitar los efectos adversos de la esplenectomía. Sin embargo, la elección entre ambos no está clara. El coste puede ser de interés para priorizar el tratamiento. Nuestro objetivo es determinar el coste por paciente respondedor después de 6 meses de tratamiento de la trombocitopenia inmune primaria crónica con rituximab frente al agonista del receptor de trombopoyetina eltrombopag en el Sistema Nacional de Salud español.
MétodoSe desarrolló un modelo de árbol de decisión de 26 semanas para evaluar el coste de la respuesta al tratamiento con eltrombopag y rituximab en pacientes adultos con trombocitopenia inmune primaria crónica refractaria a esteroides. Debido al corto periodo de evaluación, no se aplicó tasa de descuento.
ResultadosEl coste medio por paciente tras 6 meses de tratamiento fue ligeramente superior para eltrombopag (13.089,40 €) que para rituximab (11.852,60 €). Sin embargo, la mayor tasa de respuesta de eltrombopag disminuye los costes de hemorragia, lo que se traduce en un coste por paciente respondedor un 29% mayor con rituximab (18.964,15 €) que con eltrombopag (14.732,65 €). Este resultado concuerda con los de los 15 análisis de sensibilidad realizados, donde eltrombopag siempre representa un menor coste por paciente respondedor, excepto cuando el tratamiento con eltrombopag se realiza en su dosis máxima (75 mg). Sólo en este caso, el coste por respondedor a eltrombopag es 48 € más caro que el del rituximab. En coherencia con lo anterior, la mayor diferencia a favor de eltrombopag se da en el escenario que utiliza la dosis mínima de éste —25 mg— (eltrombopag 7622,14 € frente a 18.964,15 € de rituximab). Así, el coste por paciente respondedor es menor en eltrombopag aunque no se realice un segundo ciclo de retratamiento con rituximab (14.732,65 € frente a 15.298,61 €).
ConclusionesEl coste del tratamiento con rituximab, incluidos los costes de monitorización y sangrado, es más alto que el de eltrombopag, lo cual favorece a este último por encima de rituximab.
Primary immune thrombocytopenia (ITP) is an acquired autoimmune disease characterized by a platelet count less than 100 x 109 platelets/liter; this is due to platelet destruction and inadequate production1,2. Diagnosis is reached by exclusion of other diseases associated with thrombocytopenia. The annual ITP incidence rate is 3-4/100,000 in adults, increasing in older patients3. This condition is classified as newly diagnosed ITP when the evolution is shorter than 3 months from diagnosis, persistent if the duration of disease is 3-12 months and chronic when it lasts for more than 12 months1. Although 1/3 of affected persons are asymptomatic and patients with a platelet count over 50 x 109 platelets/liter do not require treatment, this long-lasting disease may threaten life due to bleeding caused by thrombocytopenia; it negatively impacts quality of life and imparts a high economic burden on the healthcare system1,2.
Classical guidelines recommended corticosteroids as first-line treatment for adult ITP followed by splenectomy as second-line treatment and the use of the anti-CD20 chimeric monoclonal antibody rituximab or a thrombopoietin receptor agonist (TPO-RA) in cases of failure or contraindication2,4.
Splenectomy achieves a 60% response after 5 years5. However, this treatment produces important adverse effects mainly derived from surgery, as well as risk of infection, thrombosis and cancer6. In contrast, rituximab and TPO-RAs cause few toxicities and spare a splenectomy. The first option permits lasting responses after a short treatment, with approximately 60% initial responses and a third of patients in remission after one year7. The second involves long-term treatments, but with high response rates (75-95%), and it has fewer side effects than rituximab and the potential of drug discontinuation7–12. Hence, those formerly considered third-line treatments have become extensively used13. In fact, the last recommendations indicate that, even if corticoids remain at the first-line treatment, in view of the lack of randomized trials directly comparing splenectomy, rituximab and TPO-RAs, all three can be used as second-line options7.
TPO-RAs, including eltrombopag and romiplostim, stimulate platelet production, increasing platelet count9,14. Unlike romiplostim subcutaneous administration, eltrombopag oral administration requires no sanitary assistance15.
The aim of this paper is to provide data for clinical decisions according to their economic implications through the per-head cost of responding patients to oral TPO-RA eltrombopag and rituximab for treating chronic ITP in the context of the Spanish Health Service.
MethodsModelWe have developed a cost-consequence model to compare the direct health costs of ITP treatment with eltrombopag and rixutimab from the perspective of Spanish public hospitals. As in a similar study comparing romiplostim and rituximab16, only direct hospital health costs of patients treated with eltrombopag and rituximab were considered. Grade 1 (petechial) bleedings, which are treated by the patients themselves or at the primary care services, were not considered.
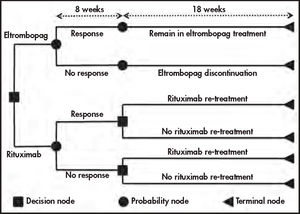
To allow the comparison with former study that evaluated the cost per response of romiplostim and rituximab16, a time horizon of 26 weeks (half a year) was set. As shown in figure 1, it was split into two periods. The first one comprised 8 weeks during which all patients were treated, and the response was evaluated. This was followed by a period of 18 weeks in which a) only patients responding to eltrombopag continued to be treated and b) patients on rituximab were treated according to previously described bases17. This structure is coherent with the previously mentioned study carried out in Spain16, so it may support decision-making.
As the time horizon is less than a year, we did not consider applying discounting to costs or effects.
In this way, over the 26 weeks, the model accumulates treatment costs (drugs plus administration), follow-up costs and the costs produced by bleedings to calculate the final cost per responding patient to both treatment alternatives.
Study populationConsidering that eltrombopag is indicated for patients of more than one year of age with chronic ITP who are refractory to other treatments15 and that although rituximab is not officially approved for this disease, it is usually restricted to adults with ITP because of the concerns that the effects of rituximab in the childhood immune system may elicit7,18, we have limited our analysis to adults with chronic-refractory ITP.
To determine the effectiveness of these treatments, we carried out a literature review of chronic ITP treatment published in English and Spanish between 2000 and 2017. As a result, we have identified a paper focused on a group of Spanish patients treated with eltrombopag19. As no similar paper has been found for rituximab (on Spanish patients with chronic-refractory ITP), we have used the data from the Arnold DM et al. systematic review20.
To estimate rituximab dosage, we used the Dubois & Dubois formula to determine the body surface of patients21. Height and weight were determined according to microdata from the Spanish results of the European Health Survey 2014 (basal data shown in Supplementary Table 1)22.
Base case and sensitivity analysis results. Per-patient global cost of treatment with eltrombopag and rituximab
| Cost per patient (€) | |||
|---|---|---|---|
| Eltrombopag | Rituximab | ||
| BASE CASE | 13,089.40 | 11,852.60 | |
| SA 1 | Body surface | 13,089.40 | 11,454.77 |
| SA 2 | Eltrombopag dose 25 mg/day | 6,771.98 | 11,852.60 |
| SA 3 | Eltrombopag dose 50 mg/day | 11,832.08 | 11,852.60 |
| SA 4 | Eltrombopag dose 75 mg/day | 16,892.18 | 11,852.60 |
| SA 5 | No re-treatment with rituximab | 13,089.40 | 9,561.63 |
| SA 6 | Re-treatment with rituximab only for responding patients | 13,089.40 | 10,784.96 |
| SA 7 | Re-treatment with rituximab only for non-responding patients | 13,089.40 | 10,629.27 |
| SA 8 | Decrease in eltrombopag efficacy (CR patients) | 12,240.82 | 11,852.60 |
| SA 9 | Rituximab efficacy lower CI threshold | 13,089.40 | 12,057.65 |
| SA 10 | Rituximab efficacy higher CI threshold | 13,089.40 | 11,645.47 |
| SA 11 | Rituximab administration = specialist consultation cost | 13,089.40 | 10,769.87 |
| SA 12 | Monitoring decrease in rituximab | 13,089.40 | 11,537.50 |
| SA 13 | Decrease bleeding costs (−10%) | 13,039.40 | 11,771.47 |
| SA 14 | Increase bleeding costs (+10%) | 13,139.39 | 11,933.72 |
| SA 15 | Rituximab, Truxima® price | 13,089.40 | 10,572.16 |
CI: confidence interval; CR: complete response; SA: sensitivity analysts.
No study or phase III clinical trial related to rituximab response was found in Spain; therefore, the more consistent data for this treatment derive from the indicated systematic review20. Additionally, we used a retrospective French model to evaluate the need for re-treatment and its effectiveness17.
Supplementary Table 2 shows the response rates used in the model, their sources and the criteria employed to evaluate the response. The retreatment rates and their responses are shown in Supplementary Table 3.
Base case and sensitivity analysis results. Itemized costs of per-patient treatment with eltrombopag and rituximab
| Treatment costs (€) | Monitoring costs (€) | Bleeding costs (€) | ||||
|---|---|---|---|---|---|---|
| Eltrombopag | Rituximab | Eltrombopag | Rituximab | Eltrombopag | Rituximab | |
| BASE CASE | 11,377.51 | 10,120.27 | 1,211.91 | 921.05 | 499.97 | 811.27 |
| SA 1 | 11,377.51 | 9,722.45 | 1,211.91 | 921.05 | 499.97 | 811.27 |
| SA 2 | 10,120.20 | 10,120.27 | 1,211.91 | 921.05 | 499.97 | 811.27 |
| SA 3 | 5,060.10 | 10,120.27 | 1,211.91 | 921.05 | 499.97 | 811.27 |
| SA 4 | 15,180.29 | 10,120.27 | 1,211.91 | 921.05 | 499.97 | 811.27 |
| SA 5 | 11,377.51 | 7,829.31 | 1,211.91 | 921.05 | 499.97 | 811.27 |
| SA 6 | 11,377.51 | 9,052.64 | 1,211.91 | 921.05 | 499.97 | 811.27 |
| SA 7 | 11,377.51 | 8,896.94 | 1,211.91 | 921.05 | 499.97 | 811.27 |
| SA 8 | 10,392.60 | 10,120.27 | 1,211.91 | 921.05 | 636.31 | 811.27 |
| SA 9 | 11,377.51 | 10,208.35 | 1,211.91 | 921.05 | 499.97 | 928.25 |
| SA 10 | 11,377.51 | 10,031.30 | 1,211.91 | 921.05 | 499.97 | 693.11 |
| SA 11 | 11,377.51 | 9,037.54 | 1,211.91 | 921.05 | 499.97 | 811.27 |
| SA 12 | 11,377.51 | 10,120.27 | 1,211.91 | 605.96 | 499.97 | 811.27 |
| SA 13 | 11,377.51 | 10,120.27 | 1,211.91 | 921.05 | 449.98 | 730.14 |
| SA 14 | 11,377.51 | 10,120.27 | 1,211.91 | 921.05 | 549.97 | 892.40 |
| SA 15 | 11,377.51 | 8,839.83 | 1,211.91 | 921.05 | 499.97 | 811.27 |
SA: sensitivity analysis.
Base case and sensitivity analysis results. Per-response cost of treatment with eltrombopag and rituximab
| Cost per response (€) | |||
|---|---|---|---|
| Eltrombopag | Rituximab | ||
| BASE CASE | 14,732.65 | 18,964.15 | |
| SA 1 | Body surface | 14,732.65 | 18,327.63 |
| SA 2 | Eltrombopag dose 25 mg/day | 7,622.14 | 18,964.15 |
| SA 3 | Eltrombopag dose 50 mg/day | 13,317.49 | 18,964.15 |
| SA 4 | Eltrombopag dose 75 mg/day | 19,012.84 | 18,964.15 |
| SA 5 | No re-treatment with rituximab | 14,732.65 | 15,298.61 |
| SA 6 | Re-treatment with rituximab only for responding patients | 14,732.65 | 17,255.94 |
| SA 7 | Re-treatment with rituximab only for non-responding patients | 14,732.65 | 17,006.83 |
| SA 8 | Decrease in eltrombopag efficacy (CR patients) | 13,777.55 | 18,964.15 |
| SA 9 | Rituximab efficacy lower CI threshold | 14,732.65 | 19,292.24 |
| SA 10 | Rituximab efficacy higher CI threshold | 14,732.65 | 18,632.75 |
| SA 11 | Rituximab administration = specialist consultation cost | 14,732.65 | 17,231.79 |
| SA 12 | Monitoring decrease in rituximab | 14,732.65 | 18,460.00 |
| SA 13 | Decrease bleeding costs (10%) | 14,676.38 | 18,834.35 |
| SA 14 | Increase bleeding costs (+10%) | 14,788.93 | 19,093.96 |
| SA 15 | Rituximab, Truxima® price | 14,732.65 | 16,915.45 |
CI: confidence interval; CR: complete response; SA: sensitivity analysts.
As Supplementary Table 2 shows, there are differences in the response criteria. While the eltrombopag study used the full response rate (defined as platelet count ≥ 100 x 109/L) and the response rate (platelet count ≥ 30 and < 100), the rituximab study used a different response rate (defined as platelet count ≥50 x 109L). Despite this, a superior efficacy of eltrombopag against rituximab can be established, since its complete response rate is 77.3%, while the rituximab response rate is 62.5%.
Bleeding estimateAs petechial bleeding does not involve hospital attention —it is cared for at primary care services—the model only considers 2-, 3- and 4-grade bleeding (WHO bleeding scale23, Supplementary Table 4).
There is a relationship between a low platelet count and an increased risk of bleeding. In this way, patients who do not respond to treatment will have a lower count and an increased risk of bleeding than responding patients. To simulate these bleeding risks, we have used the RAISE trial data12, assuming that non-responding patients behave in the same way as the placebo arm in relation to the risk of bleeding while responding patients present a similar risk decrease to that in the treatment arm of the trial.
This assumption seems to be valid considering the effectiveness of eltrombopag and the duration of this trial, which is equivalent to that of the model (six months). The bleeding rates used in the model are shown in Supplementary Table 5. Grade 4 bleedings are potentially life threatening, with a mortality rate of 40%; 80% of patients who survive after such bleeding need rehabilitation24.
Resources and costsTo make a cost estimation, we used an average of the official lists of prices of the different Spanish regions (Supplementary Table 6). Prices are actualized to 2018 euro (€2018).
As both alternatives are hospital formulary drugs, prices to wholesalers have been used, thus avoiding the extra costs involved by distribution channels and chemist stores.
Supplementary Table 7 shows the price to wholesale (PTW) of the different drugs as they appear in BotPlusWeb Portalfarma (online drugs database of the General Council of Official Pharmaceutical Associations, https://botplusweb.portalfarma.com, accessed 1 June 2018).
To calculate the cost of the drugs, we considered the cost per mg and applied it to doses as described in the trials. Each rituximab treatment comprises 4 cycles of 375 mg for each square meter of body surface20, implying a daily dose of 25 mg for 17.13% of the patients, a dose of 50 mg for 40.89% and a dose of 75 mg for 41.98%12. In the case of rituximab, an extra administration cost must be added; as the drug is administered in the hospital, we have assumed it is equivalent to day-hospital costs.
Supplementary Table 7 also indicates the costs and their use in the model. Eltrombopag response is monitored weekly during the first 8 weeks and then once a month after week 8. For rituximab, monitoring is carried out weekly for the first 4 weeks and once a month after that. We assumed that a grade 2 bleeding cost is 0.6 times the cost of a specialist consultation plus 0.3 times the cost of an urgency consultation. For grade 3 bleedings, we assumed a diagnosis-related group (DRG) 174 (gastrointestinal bleeding) cost; for grade 4 bleedings, we assumed a cost of 0.2 times DRG 810 (medical intracranial hemorrhage) plus 0.8 times the cost of DRG 833 (surgical intracranial hemorrhage) and the cost of rehabilitation when applying. This rehabilitation process after grade 4 bleeding, when needed, was assumed to last 6 months and to include a monthly visit to the physiotherapy consultant, five physiotherapy and speech therapy sessions every week and three weekly occupational therapy sessions25.
Sensitivity analysisTo analyze the effect of the different variables on the model results, we carried out 15 sensitivity analyses, described in Supplementary Table 8.
ResultsThe average cost per patient after a 6-month treatment was €13,089.40 for eltrombopag and €11,852.60 for rituximab. Itemized costs show that the greater response rate of the first involves a decrease in bleeding costs (€811.27 with rituximab, €499.97 with eltrombopag). Due to the lower efficacy of rituximab, the average cost of response is €14,732.65 with eltrombopag and €18,964.15 with rituximab (29% higher with the latter).
Tables 1, 2 and 3 show the base case and sensitivity analysis results. The cost of eltrombopag is always lower, excluding the sensitivity analysis in which the patient received a daily dose of 75 mg of eltrombopag —a scenario where eltrombopag global cost is €5,039.58 over rituximab, but when response cost is considered, the difference is reduced to only €48 higher than that of rituximab.
DiscussionPatient refusal and hazards derived from surgery plus lifelong increased risk of infection, thromboembolic events and malignancy after splenectomy have increased the use of TPO-RAs and rituximab26,27. The last American Hematology Association guideline for ITP treatment update recommends rituximab over splenectomy and places splenectomy and TPO-RAs at the same level7. The preference between rituximab and TPO-RAs is under discussion in patients unresponsive to steroids or suffering from persistent ITP7. Hence, the cost and effectiveness of both types of treatment must be carefully evaluated to make appropriate medical decisions. We selected TPO-RA eltrombopag for this study over romiplostim due to its oral, out-of-hospital administration, in contrast to the subcutaneous administration of romiplostim, which needs sanitary assistance.
Here, we show that the cost of a 6-month treatment is similar using rituximab and eltrombopag, €11,852.60 and €13,089.40, respectively. Both treatments accomplish responses and have low side effects, but lower beneficial effects have been observed with rituximab13. Therefore, as the response to treatment with eltrombopag is greater, the cost per-responding patient is smaller, even though the treatment cost itself is higher, turning the budget to €14,732.65 for eltrombopag and €18,964.15 for rituximab in that period of time. These results are indirectly consistent with those from other economic evaluations in Spain showing that eltrombopag was cost-effective against romiplostim and romiplostim was cost-effective against rituximab16,25. Additionally, a recent meta-analysis indirectly comparing rituximab and TPO-RAs eltrombopag and romiplostim treatment for persistent or chronic ITP suggests that the second type of treatment is superior to the former when considering response (platelet ≥ 50 × 109/L), clinically significant and severe bleeding28. Additionally, although treatment with eltrombopag is considered chronic, there is evidence that suggests that it is possible to discontinue the treatment19.
Another issue to consider is that the rituximab administration route is intravenous or subcutaneous after the first dose, and it has to be monitored at the hospital for undesired side effects versus oral administration at home in the case of eltrombopag. Therefore, treatment with eltrombopag lessens the workload at day hospitals, allowing resources to be focused on other patients who need day-hospital facilities for the administration of their treatments (such as chemotherapy). A limitation of this study is that the model does not take into account the adverse effects caused by treatments, which may potentially be more severe in the first perfusions of the monoclonal antibody than in the case of the thrombopoietin receptor agonist.
As data for rituximab do not inform of splenectomized patients, our model considers the Spanish average of 22% splenectomized patients, but it cannot itemize the splenectomized group of patients. Clinical studies have shown that eltrombopag is more effective in non-splenectomized patients12,29,30, so an increase in the number of splenectomized patients could mean a decrease in the response rate.
A final limitation is related to the use of rituximab at a lower dose (100 mg). In the absence of efficacy data at this dose, this option has not been considered for the present analysis (it should be noted that the use of data that are not sufficiently comprehensible would in turn imply another limitation). Additionally, using a standard dose of rituximab of 375 mg is consistent with a similar article in which rituximab was evaluated against romiplostim16, and may allow comparison between both.
In conclusion, the treatment budget of rituximab, considering monitoring and bleeding costs, is higher than that of eltrombopag. This, together with long response rates and the reduced undesirable effects, supports the recommendation of the latter treatment over rituximab. This type of analysis should be required to guide healthcare policies and treatment decision-making.
FundingNo funding.
Conflict of interestsNo conflicts of interest.
Financial activities outside the submitted work: José Ramón González-Porras has received fees for consulting services by Amgen, Novartis, SOBI, Grifols and CSL Behring and speaking honoraria from Novonordisk, Shire, SOBI, Roche, Daiichi Sankyo, Pfizer, Rovi, Amgen, and Novartis. Francisco Javier Parrondo-García has received consultancy fees from Takeda Farmacéutica España, Tesaro España, Pierre Fabre España, Servier España, Bristol-Myers Squibb S.L., Lilly S.A., Biogen Spain S.L., ViiV Healthcare Spain and Pfizer SLU. Eduardo Anguita has received speaking honoraria and support for attending conferences from Amgen and Novartis.
Presentation at congressThis work was an oral presentation at the LXI National Conference of SEHH and XXXV Congress of SETH, Valencia, October 24-26, 2019.
Contribution to the scientific literatureThe cost for responding primary immune thrombocytopenia patient in the Spanish Health Service.
Our data favor eltrombopag over rituximab for primary immune thrombocytopenia treatment in Spain.