Venetoclax in combination with obinutuzumab has significantly improved efficacy versus immunochemotherapy (progression-free survival) in patients with chronic lymphocytic leukaemia who have not received prior treatment. The objective of this study was to evaluate its efficiency in Spain using a cost-utility analysis.
MethodUsing a partitioned-survival analysis model adapted to the Spanish context and based on three health states (progression-free survival, survival after progression, and death), a simulation of the evolution of patients who were candidates for initiating first-line treatment was conducted for a lifetime time horizon. Venetoclax in combination with obinutuzumab was compared to the most commonly used therapeutic options for these patients at the time of study design: chlorambucil in combination with obinutuzumab, ibrutinib, fludarabine in combination with cyclophosphamide and rituximab, and bendamustine in combination with rituximab. In order to estimate survival curves, efficacy data were derived from the CLL14 trial and a network meta-analysis. The analysis was conducted from the perspective of the Spanish National Healthcare System and included direct healthcare costs (i.e. pharmacological costs and their administration), and those associated with the management of the disease and adverse events. The resource use was validated by an expert group. Quality of life data were used to estimate the quality-adjusted life years obtained for each alternative. A threshold of €25,000/quality-adjusted life years was used. The robustness of the model was evaluated using deterministic and probabilistic sensitivity analyses.
ResultsVenetoclax in combination with obinutuzumab was shown to be a dominant alternative compared to the rest of the treatment alternatives, with a lower cost per patient (€-67,869 compared to chlorambucil in combination with obinutuzumab, €-375,952 compared to ibrutinib, €-61,996 compared to fludarabine in combination with cyclophosphamide and rituximab, and €-77,398 compared to bendamustine in combination with rituximab). It also had a greater gain in quality-adjusted life years (0.551 quality-adjusted life years gained compared to chlorambucil in combination with obinutuzumab and ibrutinib, 1.639 quality-adjusted life years gained compared to fludarabine in combination with cyclophosphamide and rituximab, and 1.186 quality-adjusted life years gained compared to bendamustine in combination with rituximab). Between 68% and 85% of the simulations performed in the sensitivity analysis showed that venetoclax in combination with obinutuzumab had lower costs and more quality-adjusted life years gained.
ConclusionsVenetoclax in combination with obinutuzumab is an efficient and dominant alternative for treating previously untreated patients with chronic lymphocytic leukaemia compared to the available alternatives and from the perspective of the Spanish National Health System.
Venetoclax en combinación con obinutuzumab ha mostrado frente a la inmunoquimioterapia mejoras significativas en términos de eficacia (supervivencia libre de progresión) en pacientes con leucemia linfocítica crónica que no han recibido tratamiento previo. El objetivo de este estudio fue evaluar su eficiencia en España a partir de un análisis de coste-utilidad.
MétodoA partir de un modelo de análisis de la supervivencia adaptado al contexto español y basado en tres estados de salud (supervivencia libre de progresión, supervivencia tras progresión y muerte), se llevó a cabo una simulación de la evolución de los pacientes candidatos a iniciar una primera línea de tratamiento para un horizonte temporal de toda la vida. Venetoclax en combinación con obinutuzumab se comparó frente a las opciones terapéuticas más utilizadas para estos pacientes en el momento del diseño del estudio: clorambucilo en combinación con obinutuzumab, ibrutinib, fludarabina en combinación con ciclofosfamida y rituximab, y bendamustina en combinación con rituximab. Los datos de eficacia para estimar las curvas de supervivencia fueron derivados del estudio CLL14 y de un metaanálisis en red. El análisis consideró la perspectiva del Sistema Nacional de Salud incluyendo los costes sanitarios directos, en concreto los farmacológicos y su administración, y los asociados al manejo de la enfermedad y acontecimientos adversos. El uso de recursos fue validado por un grupo de expertos. Se emplearon datos de calidad de vida para estimar los años de vida ajustados por calidad obtenidos para cada alternativa. Se consideró un umbral de 25.000 €/años de vida ajustados por calidad. La robustez del modelo se evaluó mediante análisis de sensibilidad determinísticos y probabilísticos.
ResultadosVenetoclax en combinación con obinutuzumab se mostró como una alternativa dominante frente al resto de alternativas de tratamiento, con un menor coste por paciente (-67.869 € frente a clorambucilo en combinación con obinutuzumab, -375.952 € frente a ibrutinib, -61.996 € frente a fludarabina en combinación con ciclofosfamida y rituximab, y -77.398 € frente a bendamustina en combinación con rituximab) y una mayor ganancia en años de vida ajustados por calidad (0,551 años de vida ajustados por calidad ganados frente a clorambucilo en combinación con obinutuzumab e ibrutinib, 1,639 años de vida ajustados por calidad ganados frente a fludarabina en combinación con ciclofosfamida y rituximab, y 1,186 años de vida ajustados por calidad ganados frente a bendamustina en combinación con rituximab). Entre el 68% y el 85% de las simulaciones realizadas en el análisis de sensibilidad mostraban a venetoclax en combinación con obinutuzumab con un menor coste y un mayor número de años de vida ajustados por calidad ganados.
ConclusionesVenetoclax en combinación con obinutuzumab se muestra como una alternativa eficiente y dominante como tratamiento de pacientes con leucemia linfocítica crónica no tratados previamente frente a las alternativas disponibles y desde la perspectiva del Sistema Nacional de Salud.
Chronic lymphocytic leukaemia (CLL) is a lymphoproliferative disorder characterized by the expansion of mature-appearing CD5+ B cells in the peripheral blood, secondary lymphoid tissues, and bone marrow1. CLL is the most common form of leukaemia in adults in Western countries, with an estimated incidence of 4 to 5 cases per 100,000 population-years and a particularly significant prevalence in areas in which the population is older2. More than half of these patients have age-related comorbidities, such as hypertension, diabetes, osteoarthritis, or cardiorespiratory involvement3. The treatment decision is based on the presence or absence of adverse prognostic factors (deletion [17p], deletion [11q], TP53 mutation, IGHV mutational status) and on the functional status of the patient2.
In recent years, significant advances have been made in understanding the pathophysiology of CLL, and substantial progress has been made in the clinical management of the disease due to the identification of poor prognostic genetic variables, particularly those associated with chemoresistance and progression to highly aggressive forms of CLL4,5. Currently, chemoimmunotherapy is being displaced by biologic therapies, such as B-lymphocyte receptor inhibitors and BCL-2 inhibitors6,7.
Overexpression of BCL-2 contributes to the evasion of apoptosis, making tumour cells highly dependent on BCL-2 for their survival8. High expression of BCL-2 is observed uniformly across all CLL subtypes9. Venetoclax, the first selective BCL-2 inhibitor, restores the ability of tumour cells to initiate the process of apoptosis and, administered together with obinutuzumab, has a complementary and synergistic mechanism of action that provides high efficacy rates and profound responses, with a fixed duration of 1 year of treatment5,10. In the CLL14 trial, 2-year progression-free survival (PFS) was 88.2% (95% confidence interval [CI]: 83.7%-92.6%) in patients treated with venetoclax in combination with obinutuzumab (VenO) vs 64.1% (95% CI: 83.7%-92.6%) in the control group treated with chlorambucil in combination with obinutuzumab (ClbO)5. After a median follow-up of 28.1 months, these results showed a slow-down in the risk of progression or death with VenO (hazard ratio [HR] = 0.35; 95% CI: 0.23-0.53; P < 0.001). At data cutoff, the results of overall survival (OS) were immature, with the median not having been reached in either group (less than 10% of events in each arm)5. These results were confirmed at the subsequent cutoff time, after a median follow-up of 39.6 months, where PFS was 81.9% in the VenO arm and 49.5% in the ClbO arm. These results showed a slow-down in the risk of progression or death with VenO (HR = 0.31; 95% CI: 0.22-0.44; P < 0.001)11.
The aim of this study was to conduct a cost-utility analysis to determine whether VenO, which is a therapy indicated for previously untreated patients with CLL, would be an efficient intervention within the Spanish National Health System (NHS) compared to the therapeutic alternatives available in Spain.
MethodsStudy participants and comparatorsThe characteristics of the study population were matched to those of the patients included in the CLL14 Phase 3 clinical trial5 (i.e., patients with CLL who had not received prior treatment, 33% female, and a median age of 71.1 years).
At the time of study design, VenO was also compared to the most commonly used first-line alternative treatments in CLL patients: ClbO, fludarabine in combination with cyclophosphamide and rituximab (FCR), bendamustine in combination with rituximab (BR), and ibrutinib.
Type of analysisIt was estimated the mean cost and effectiveness per patient associated with each therapeutic alternative and calculated the incremental cost-utility ratio of VenO vs the other comparators to determine the additional cost of VenO to obtain 1 quality-adjusted life year (QALY).
The analysis was conducted from the perspective of the Spanish NHS, and included direct health care costs (updated to 2020 euros using the historical year-on-year consumer price index). The time horizon was extended to the patient's lifetime. An annual discount rate of 3% was applied to costs and clinical results12. The cost-effectiveness threshold was considered to be €25,000/QALY13.
The adaptation to the Spanish context was facilitated after consultation with an expert group selected based on their experience and knowledge of clinical practice. (i.e. the co-authors of the present study). After completing individual questionnaires, a base case was proposed for validation.
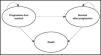
Structure of the modelA partitioned survival model14,15 was adapted to the Spanish setting. A model previously presented at the National Institute for Health and Clinical Excellence (NICE), and that simulated the evolution of patients with CLL who have not received previous treatment across different clinical situations (health states). Figure 1 shows a simplified schematic of the model, with three mutually exclusive health states: progression-free state, state after progression, and death. The proportion of patients who were alive for each cycle of the model (28-day cycles) was estimated from the area under the OS curve, and the proportion of patients who were alive free of progression was estimated from the area under the PFS curve. The proportion of patients who were alive after progression was estimated by the difference between the OS and PFS curves. The OS and PFS curves were estimated using parametric curves based on clinical evidence. To obtain the best estimate of the pharmacological cost, the model considered time-on-treatment information to measure discontinuation, which can occur due to adverse events (AEs) or disease progression. It should be noted that treatments with VenO, ClbO, FCR, and BR have a fixed duration, whereas the duration of treatment with ibrutinib is indefinite until disease progression or unacceptable toxicity. After discontinuation, patients were assumed to be able to receive subsequent treatments.
Clinical dataThe efficacy data used in the VenO and ClbO model were derived from the CLL14 trial5. Because the study follow-up period was shorter than the horizon used in the economic studies (i.e. the recommended horizon being the patient's lifetime), the PFS, OS, and time-on-treatment curves had to be extrapolated using parametric distribution functions fitted to Kaplan-Meier (KM) curves. A goodness-of-fit criteria was followed in order to decide which parametric distribution was the best fit. This criteria described how well a given distribution fits a set of observations (KM curves). Specifically, the Akaike and Bayesian information criteria15 were followed (Appendix 1). The parameterized curves were adjusted according to the overall mortality of the Spanish population16, thus preventing the survival curves estimated in the model from being above the survival of the general population.
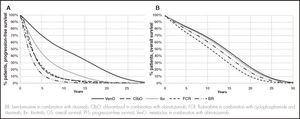
In the absence of direct evidence on ibrutinib, FCR, and BR as comparators compared to VenO, the parametric curves were estimated using HRs vs VenO (reference treatment). These were obtained from a Bayesian fixed-effect network meta-analysis17. This approach allowed an indirect comparison of VenO vs ibrutinib, FCR, or BR. To derive the PFS curve for ibrutinib, FCR and BR, it was assumed the corresponding HRs relative to VenO derived from the network meta-analysis. A log-logistic distribution for the PFS curve for VenO and ClbO was used (Figure 2A).
Regarding OS, it was assumed that there were no differences between VenO and ClbO based on the results of the CLL14 trial and corroborated by expert opinion. Thus, an exponential distribution was assumed for the purposes of extrapolation. To derive the OS curve for ibrutinib, FCR and BR, it was used the HRs relative to VenO derived from network meta-analyses (Figure 2B).
The analysis used time-on-treatment information to measure discontinuation. VenO, ClbO, FCR, and BR treatments have a fixed time duration (12 cycles for VenO and ClbO, 6 cycles for FCR and BR), whereas the duration of treatment with ibrutinib is considered to be indefinite until disease progression or unacceptable toxicity (a median of 60 months was assumed based on longer-term follow-up in the RESONATE-2 study, in which the median PFS was not reached4). After discontinuation, it was assumed that patients were able to receive subsequent treatments. For this purpose, a log-logistic distribution was assumed for VenO and ClbO, whereas the PFS curve was assumed for ibrutinib, FCR, and BR.
Quality of lifeFor the estimation of QALYs, different utility values were assumed depending on the health status of the patients. The utility values were used to represent the quality of life associated with a particular health status on a scale from 0 (death) to 1 (perfect health). Specifically, a utility of 0.80 was used for PFS health status (NICE TA174) and 0.68 for survival-after-progression status18,19. To represent the loss of utility (disutility) associated with increasing patient age, the defined utilities were adjusted by the utility according to the age of the general Spanish population derived from the Spanish National Health Survey (available from the Spanish National Institute of Statistics for 2011/12)20. The model also addressed disutility due to treatment-associated AEs. It was assumed that AEs, as well as their effects on quality of life and economic effects, occur in the first cycle of the analysis.
Resource use and costsThe analysis addressed the direct healthcare costs associated with the management of CLL (Table 1). The pharmacological cost, including treatments after progression, was calculated based on the laboratory selling price21. The use of resources and assumptions made were validated by clinical expert opinion.
Resource use and costs of analysis
| Pharmacological cost | ||||
|---|---|---|---|---|
| Treatment | Cost21 | Comment | Treatment after progression | |
| Base case | Sensitivity analysis | |||
| VenO5 | € 101,975 | Cost, 12 treatment cycles | 50% Ibr, 50% VenR | 90% Ibr, 10% VenR |
| Venetoclax | € 70,215 | 12 treatment cycles | ||
| Obinutuzumab | € 31,760 | 6 treatment cycles | ||
| ibrutinib4,22 | €400,473 | Cost until progression (assuming 60 months following RESONATE-24) | 100% Ven | 100% VenR |
| FCR23 | € 10,323 | Cost, 6 treatment cycles | 50% Ibr, 50% VenR | −– |
| BR23 | € 12,499 | Cost, 6 treatment cycles | 50% Ibr, 50% VenR | 25% Ibr, 75% VenR |
| ClbO5 | € 31,778 | Cost, 12 treatment cycles | 50% Ibr, 50% VenR | 25% Ibr, 75% VenR |
| Treatment duration after progression (mo) | Ibr: 41.022; VenR: 24.424; Ven: 16.025 | |||
| Cost by administration route26 | |||||||
|---|---|---|---|---|---|---|---|
| Intravenous administration | € 240.43 | Subcutaneous administration (first) | € 30.13 | ||||
| Cost, disutility, and percentage of patients with adverse events | |||||||
| Adverse event* | Cost26 | Disutility and duration | VenO5 | ClbO5 | Ibrutinib27 | FCR28 | BR23 |
| Weakness | € 592.61 | −0.1 1529.30 (35.3 days)29 | 2.80% | 0.50% | − | − | − |
| Diarrhoea | € 454.65 | −0.0819.31 (3.5 days)32 | 3.80% | 0.50% | 4.00% | − | 7.00% |
| Dyspnoea | € 178.52 | −0.10329.30 (12.7 days)29 | 2.40% | 0.50% | − | − | − |
| Febrile neutropenia | € 2,749.45 | −0.1530.32 (3.5 days)32 | 5.20% | 3.70% | 1.00% | − | − |
| Infusion reactions | € 892.10 | −0.232 (3.5 days)32 | 9.00% | 10.30% | − | − | − |
| Leukopenia | € 1,628.28 | −0.09** (14.0 days)29 | 2.40% | 4.70% | − | 24.00% | 48.00% |
| Neutropenia | € 1,697.72 | −0.0932.33 (3.5 days)32 | 52.80% | 47.70% | 12.00% | 34.00% | 59.00% |
| Pneumonia | € 4,460.89 | −0.19534 (18.2 days)35 | 5.70% | 4.20% | − | − | 9.00% |
| Sepsis | € 6,866.85 | −0.19534 (7.0 days)35 | 4.20% | 1.40% | − | − | 1.00% |
| Thrombocytopenia | € 942.87 | −0.10834 (23.2 days)35 | 13.70% | 15.00% | − | 7.00% | 14.00% |
| Costs associated with disease management | |||
|---|---|---|---|
| Health resources | Unit cost26 | HEALTH STATE (annual units) | |
| Without progression | After progression | ||
| Complete blood count | € 4.75 | 8 | 10 |
| Lactate dehydrogenase test | € 5.66 | 7 | 8 |
| Chest X-ray | € 38.20 | 1 | 1 |
| Bone marrow study | € 357.55 | 0 | 1 |
| Haematologist visit | € 86.76 | 8 | 10 |
| Other specialist visits | € 86.76 | 1 | 3 |
| Blood transfusion | €428.29 | 0 | 1 |
| Computed axial tomography (CAT) scan | € 162.66 | 2 | 2 |
| Biochemistry | € 1.69 | 8 | 9 |
| Liver function tests | € 17.51 | 7 | 7 |
| Blood immunoglobulin test | € 11.93 | 2 | 3 |
| Total annual cost | € 1,382 | € 2,544 | |
| Costs of terminal care at end-of-life6 | € 3,874.01 | (applied in the cycle before death) | |
BR: bendamustine in combination with rituximab; ClbO: chlorambucil in combination with obinutuzumab; FCR: fludarabine in combination with cyclophosphamide and rituximab; Ibr: ibrutinib; Ven: venetoclax; VenO: venetoclax in combination with obinutuzumab; VenR: venetoclax in combination rituximab.
Several sensitivity analyses were performed to assess the uncertainty of the variables used in the model and to determine the robustness of the base case results.
Firstly, results were obtained by varying the base case discount rate (0% and 5°%).
Given the differences in resource use in patient management indicated by the clinical experts, the annual cost of patient management in both the progression-free state and after progression was varied by ±20%. Discrepancies were also noted regarding the distribution of subsequent treatments after disease progression for each alternative treatment, except in the case of FCR, where it remained the same (Table 1).
A probabilistic sensitivity analysis was also performed by simultaneously modifying the model parameters according to an established distribution (Appendix 2). Specifically, 1000 simulations were performed using the Monte-Carlo method36.
ResultsThe base case results showed that the costs per patient were lower and the gains in QALYs were greater with VenO than with other treatment alternatives (Table 2). In terms of economic assessment, VenO was the dominant alternative.
Base case results
| VenO | ClbO | Ibr | FCR | BR | |
|---|---|---|---|---|---|
| Total cost per patient | € 162,897 | € 237,587 | € 538,849 | € 232,574 | € 248,208 |
| Associated with treatment | € 95,884 | € 32,501 | € 426,031 | € 12,078 | € 16,662 |
| Associated with patient follow-up | € 17,973 | € 24,414 | € 21,759 | € 19,272 | € 23,100 |
| Associated with subsequent treatments | € 43,079 | € 175,218 | € 88,140 | € 197,313 | € 203,274 |
| Other costs* | € 5,960 | € 5,455 | € 2,919 | € 3,912 | € 5,172 |
| QALYs | 7.614 | 7.063 | 7.103 | 5.975 | 6.428 |
| Incremental cost | Reference | € −74,690 | € −375,952 | € −69,677 | € −85,311 |
| Incremental QALYs | Reference | 0.551 | 0.511 | 1.639 | 1.186 |
| ICER | € −135.554/AVAC VenO DOMINANT | € −735.718/AVAC VenO DOMINANT | € −42.512/AVAC VenO DOMINANT | € −71.932/AVAC VenO DOMINANT |
BR: bendamustine in combination with rituximab; ClbO: chlorambucil in combination with obinutuzumab; FCR: fludarabine in combination with cyclophosphamide and rituximab; Ibr: ibrutinib; ICER: incremental cost-effectiveness ratio; QALY: quality-adjusted life year; VenO: venetoclax in combination with obinutuzumab.
The lower cost of VenO vs ClbO, FCR, and BR was mainly due to a lower pharmacological cost of the subsequent treatments administered after progression. The lower cost of VenO vs ibrutinib as a comparator was mainly due to the shorter duration of time on treatment with VenO.
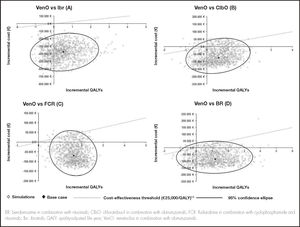
The sensitivity analyses supported these results. Table 3 shows the results of the deterministic sensitivity analyses. The probabilistic sensitivity analysis showed that 68% to 85% of the simulations performed identified VenO as the dominant alternative, with lower costs and more QALYs gained (Figure 3).
Results of the deterministic sensitivity analysis
| Incremental cost | Incremental QALY | IC ER | ||
|---|---|---|---|---|
| VenO vs | Discount rate, 0% | |||
| ClbO | € −105,645 | 0.723 | € −146,131 | Dominant |
| FCR | € −75,703 | 2.282 | € −33,177 | Dominant |
| BR | € −86,975 | 1.613 | € −53,908 | Dominant |
| Ibr | € −436,534 | 0.706 | € −617,890 | Dominant |
| VenO vs | Discount rate, 5% | |||
| ClbO | € −59,368 | 0.468 | € −126,772 | Dominant |
| FCR | € −63,835 | 1.345 | € −47,458 | Dominant |
| BR | € −81,565 | 0.989 | € −82,474 | Dominant |
| Ibr | € −342,614 | 0.421 | € −814,349 | Dominant |
| VenO vs | Cost of patient management free of progression, (−20%) | |||
| ClbO | € −76,223 | 0.551 | € −138,259 | Dominant |
| FCR | € −71,259 | 1.639 | € −43,475 | Dominant |
| BR | € −87,151 | 1.186 | € −73,460 | Dominant |
| Ibr | € −377,024 | 0.511 | € −738,164 | Dominant |
| VenO vs | Cost of patient management free of progression, (+20%) | |||
| ClbO | € −73,158 | 0.551 | € −132,700 | Dominant |
| FCR | € −68,096 | 1.639 | € −41,545 | Dominant |
| BR | € −83,471 | 1.186 | € −70,358 | Dominant |
| Ibr | € −374,881 | 0.511 | € −733,968 | Dominant |
| VenO vs | Cost of patient management after progression, (−20%) | |||
| ClbO | € −71,870 | 0.551 | € −130,363 | Dominant |
| FCR | € −67,836 | 1.639 | € −41,387 | Dominant |
| BR | € −82,446 | 1.186 | € −69,494 | Dominant |
| Ibr | € −374,123 | 0.511 | € −732,486 | Dominant |
| VenO vs | Cost of patient management after progression, (+20%) | |||
| ClbO | € −77,511 | 0.551 | € −140,596 | Dominant |
| FCR | € −71,519 | 1.639 | € −43,634 | Dominant |
| BR | € −88,177 | 1.186 | € −74,324 | Dominant |
| Ibr | € −377,781 | 0.511 | € −739,646 | Dominant |
| VenO vs | Cost of patient management free of progression and after progression, (−20%) | |||
| ClbO | € −73,402 | 0.551 | € −133,143 | Dominant |
| FCR | € −69,418 | 1.639 | € −42,352 | Dominant |
| BR | € −84,286 | 1.186 | € −71,045 | Dominant |
| Ibr | € −375,195 | 0.511 | € −734,583 | Dominant |
| VenO vs | Cost of patient management free of progression and after progression, (+20%) | |||
| ClbO | € −75,979 | 0.551 | € −137,816 | Dominant |
| FCR | € −69,937 | 1.639 | € −42,669 | Dominant |
| BR | € −86,337 | 1.186 | € −72,773 | Dominant |
| Ibr | € −376,709 | 0.511 | € −737,549 | Dominant |
| VenO vs | Distribution of subsequent treatments after progression, (Table 1) | |||
| ClbO | € −58,233 | 0.551 | € −105,628 | Dominant |
| FCR | € −73,357 | 1.639 | € −44,755 | Dominant |
| BR | € −65,630 | 1.186 | € −55,319 | Dominant |
| Ibr | € −434,373 | 0.511 | € −850,446 | Dominant |
BR: bendamustine in combination with rituximab; ClbO: chlorambucil in combination with obinutuzumab; FCR: fludarabine in combination with cyclophosphamide and rituximab; Ibr: ibrutinib; ICER: incremental cost-effectiveness ratio; QALY: quality-adjusted life year; VenO: venetoclax in combination with obinutuzumab.
This study assessed the efficiency of VenO in the Spanish setting as a treatment for CLL in previously untreated patients against comparators that are commonly used in clinical practice and are indicated for such pathology. The results of this analysis were presented at the LXII National Congress of the Spanish Society of Haematology and Chemotherapy (SEHH) and at the XXXVI National Congress of the Spanish Society of Thrombosis and Haemostasis (SETH)37.
The modelled population was based on the CLL14 trial population, which included patients with unfavourable cytogenetic prognosis (including patients with 17p deletion and TP53 mutation). It is noteworthy that these patients were not excluded from the baseline study population, what can make the study population more representative of the real clinical practice. The pivotal ibrutinib trial, RESONATE-24, excluded patients with 17p deletion, which was thus a methodological limitation of the study design.
In the present study, VenO was considered to be the dominant alternative to all comparators because of the increased number of QALYs obtained and lower cost per patient. These results are mainly due to VenO achieving greater PFS due to its increased effectiveness and its lower pharmacological costs. The cost of using VenO was substantially lower than that of ibrutinib because VenO has a fixed treatment duration of 1 year, whereas ibrutinib has an indefinite treatment duration until disease progression or unacceptable toxicity (a median treatment duration of 60 months was assumed based on the RESONATE-24 study, in which the median PFS was not reached). This is also particularly relevant from a budgetary point of view because, in addition to being efficient, the VenO combination can generate savings in pharmaceutical expenditures (the pharmacological cost of treatment per patient is €101,975 with VenO compared to €400,473 with ibrutinib)38, while providing budgetary predictability due to the fixed duration of treatment. Compared to the other comparators, VenO has lower treatment costs after disease progression. This lower cost is due to the high rates of deep response achieved with VenO. Deep response is understood as the achievement of complete remission and undetectable minimal residual disease, which avoids or delays the initiation of a second line of treatment11. Costs related to patient follow-up are also lower with VenO than with the other alternatives because the patient remains longer in progression-free and treatment-free states.
Several cost-effectiveness studies on CLL in previously untreated patients have been conducted in other countries, but most of these studies were conducted prior to the approval of VenO and none have used this therapy as a comparator.
Soini et al. (2016) studied the cost-effectiveness of first-line treatments for CLL in patients ineligible for full-dose fludarabine39. The most cost-effective treatment was ClbO vs the other alternatives studied, such as chlorambucil in combination with ofatumumab, chlorambucil in combination with rituximab, and BR.
Furthermore, after the approval of ibrutinib, several studies conducted in other countries conducted economic analyses on its efficiency as first-line treatment for CLL40-42. Although the results of these studies are not directly applicable to Spain, all of them seem to agree that ibrutinib offers reasonable results in terms of PFS and QALYs, but it is not considered to be cost-effective.
Due to the type of analysis, the present study is limited by the need to extrapolate survival data to a longer-term horizon than the trial follow-up period. However, the extrapolation was based on the distributions with the best goodness-of-fit to the KM curves in the CLL study14. It would be relevant to perform additional analyses when longer-term CLL14 study follow-up data are obtained or to conduct real-world evidence studies.
On the other hand, in the absence of direct evidence related to the other comparators, except for ClbO5, an indirect comparison was performed by means of a network meta-analysis. This type of analysis is not free of limitations, which are mainly at the level of methodological similarity (i.e. heterogeneity between studies). Nevertheless, it is the only possible alternative in order to be able to compare treatments that have not been directly compared in clinical trials. Other limitations are the differences in methodology and the patient selection procedure between the clinical trials chosen for each treatment. To increase the external validity of this type of analysis, all pivotal clinical trials for CLL treatments should include the same type of patients (not excluding those with unfavourable prognosis) and demonstrate efficacy and safety data over the same time horizon. At the time the CLL14 study was designed, ClbO was the standard treatment for this type of patient, although currently ibrutinib is considered to be the standard treatment for previously untreated CLL patients with the 17p deletion or TP53 mutation.
In conclusion, this study shows that VenO is emerging as an efficient and dominant alternative for the treatment of CLL in previously untreated patients in Spain. According to our analysis, the introduction of VenO in the Spanish NHS could reduce treatment costs, costs associated with patient follow-up, and the costs of subsequent treatments after progression, as well as improve the life expectancy and quality of life of Spanish patients with CLL.
FundingThe study was conducted with funding from AbbVie Spain. No honoraria or authorship payments were made.
AcknowledgmentsThe authors would like to thank Ferrán Pérez Alcántara and Irene Ambatlle Jiménez of Oblikue Consulting for their support in study design, data analysis, and drafting the manuscript. These services were funded by AbbVie.
Conflicts of interestEstela Moreno Martínez has received honoraria for her participation in training activities, lectures, and expert panels from AbbVie, Amgen, Astellas, Astrazeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Fresenius Kabi, Incyte Biosciences Iberia, Ipsen, Janssen, Merck SL, Merck Sharp & Dohme, Novartis, Pierre Fabre, Roche, Sanofi and Tesaro.
Javier de la Serna Torroba has received honoraria for his participation in training activities, lectures, and expert panels from AbbVie, AstraZeneca, Beigene Gilead, Janssen and Roche.
Vicente Escudero Vilaplana has conducted training support activities or received fees for lectures or consulting services from AbbVie, Astellas, Bristol-Myers Squibb, GlaxoSmithKline, Janssen, Merck Sharp & Dohme, Novartis and Pfizer.
José Ángel Hernández Rivas has been a consultant and lecturer for Janssen, AbbVie, Gilead, Roche, BMS-Celgene, Takeda, AstraZeneca and Beigene.
Marina Sánchez Cuervo has participated in consultancies for AbbVie. Raquel Sánchez Hernández is an employee of AbbVie and may own AbbVie stock.
Presentation at CongressesLXII Congreso Nacional de la Sociedad Española de Hematología y Hemoterapia and XXXVI Congreso Nacional de la Sociedad Española de Trombosis y Hemostasia. Virtual; October 26-30, 2020.
Contribution to the scientific literature
This is the first cost-utility study to assess the potential efficiency of including venetoclax in combination with obinutuzumab in the pharmaceutical provision of the Spanish National Health System as an alternative for the treatment of chronic lymphocytic leukaemia in previously untreated patients in Spain.
Parametric distributions assessed to extrapolate the PFS and OS curves for VenO and ClbO, and the Akaike (AIC) and Bayesian (BIC) information criteria
| PLS | OS | |||||
|---|---|---|---|---|---|---|
| Distribución | AIC | BIC | AIC | BIC | ||
| VenO | ClbO | VenO | ClbO | VenO and ClbO | ||
| Exponential | 779.68 | 1,796.62 | 786.39 | 1,803.32 | 1,023.74 | 1,035.88 |
| Weibull | 780.45 | 1,766.70 | 790.52 | 1,776.74 | 1,025.50 | 1,041.68 |
| Gompertz | 778.49 | 1,773.38 | 788.56 | 1,783.42 | 1,024.99 | 1,041.17 |
| Log-logistic | 781.14 | 1,759.73 | 791.21 | 1,769.77 | 1,024.82 | 1,041.00 |
| Log-normal | 783.00 | 1,761.13 | 793.07 | 1,771.17 | 1,022.97 | 1,039.15 |
| Gamma | 780.61 | 1,763.38 | 790.68 | 1,773.43 | 1,025.41 | 1,041.59 |
| Generalized gamma | 782.08 | 1,763.03 | 795.50 | 1,776.42 | 1,024.40 | 1,044.62 |
AIC: Akaike information criteria; BIC: Bayesian information criteria; ClbO: chlorambucil in combination with obinutuzumab; OS: overall survival; PFS: progression-free survival; VenO: venetoclax in combination with obinutuzumab.
Probabilistic sensitivity analysis: parameters and distributions
| Variable | Deterministic value | Standard error | Distribution | Parameter 1 | Parameter 2 |
|---|---|---|---|---|---|
| Characteristics of the population | |||||
| Women (%) | 33% | 0.023 | Beta | 142.6690 | 288.3310 |
| Age | 71.08 | 0.390 | Normal | 71.0787 | 0.3897 |
| Weight | 70.00 | 0.774 | Normal | 70.0000 | 0.7736 |
| Utilities by health state | |||||
| Utility status without progression | 0.80 | 0.080 | Beta | 19.2000 | 4.8000 |
| Utility status after progression | 0.68 | 0.068 | Beta | 31.3200 | 14.7388 |
| Resource use by health state | |||||
| Without progression: Complete blood count | 0.613 | 0.061 | Gamma | 100.0000 | 0.0061 |
| Without progression: Lactate dehydrogenase test | 0.537 | 0.054 | Gamma | 100.0000 | 0.0054 |
| Progression free: Chest X-Ray | 0.077 | 0.008 | Gamma | 100.0000 | 0.0008 |
| Progression free: Haematologist visit | 0.613 | 0.061 | Gamma | 100.0000 | 0.0061 |
| Progression free: Other specialist visits | 0.077 | 0.008 | Gamma | 100.0000 | 0.0008 |
| Progression free: Computed axial tomography (CAT) scan | 0.153 | 0.015 | Gamma | 100.0000 | 0.0015 |
| Progression free: Biochemistry | 0.613 | 0.061 | Gamma | 100.0000 | 0.0061 |
| Progression free: Liver function tests | 0.537 | 0.054 | Gamma | 100.0000 | 0.0054 |
| Progression free: Blood immunoglobulin test | 0.153 | 0.015 | Gamma | 100.0000 | 0.0015 |
| After progression: Complete blood count | 0.767 | 0.077 | Gamma | 100.0000 | 0.0077 |
| After progression: Lactate dehydrogenase assay | 0.613 | 0.061 | Gamma | 100.0000 | 0.0061 |
| After progression: Chest X-ray | 0.077 | 0.008 | Gamma | 100.0000 | 0.0008 |
| After progression: Bone marrow study | 0.077 | 0.008 | Gamma | 100.0000 | 0.0008 |
| After progression: Haematologist visit | 0.767 | 0.077 | Gamma | 100.0000 | 0.0077 |
| After progression: Other specialist visits | 0.230 | 0.023 | Gamma | 100.0000 | 0.0023 |
| After progression: Blood transfusion | 0.077 | 0.008 | Gamma | 100.0000 | 0.0008 |
| After progression: Computed axial tomography (CAT) scan | 0.153 | 0.015 | Gamma | 100.0000 | 0.0015 |
| After progression: Biochemistry | 0.690 | 0.069 | Gamma | 100.0000 | 0.0069 |
| After progression: Liver function tests | 0.537 | 0.054 | Gamma | 100.0000 | 0.0054 |
| After progression: Blood immunoglobulin test | 0.230 | 0.023 | Gamma | 100.0000 | 0.0023 |
| Costs | |||||
| Complete blood count | € 4.75 | 0.475 | Gamma | 100.0000 | 0.0475 |
| Lactate dehydrogenase test | € 5.66 | 0.566 | Gamma | 100.0000 | 0.0566 |
| Chest X-ray | € 38.20 | 3.820 | Gamma | 100.0000 | 0.3820 |
| Bone marrow study | € 357.55 | 35.755 | Gamma | 100.0000 | 3.5755 |
| Haematologist visit | € 86.76 | 8.676 | Gamma | 100.0000 | 0.8676 |
| Other specialist visits | € 86.76 | 8.676 | Gamma | 100.0000 | 0.8676 |
| Blood transfusion | €428.29 | 42.829 | Gamma | 100.0000 | 4.2829 |
| Terminal care at end of life | € 3,874.01 | 387.401 | Gamma | 100.0000 | 38.7401 |
| Computed axial tomography (CAT) scan | € 162.66 | 16.266 | Gamma | 100.0000 | 1.6266 |
| Biochemistry | € 1.69 | 0.169 | Gamma | 100.0000 | 0.0169 |
| Liver function tests | € 17.51 | 1.751 | Gamma | 100.0000 | 0.1751 |
| Blood immunoglobulin test | € 11.93 | 1.193 | Gamma | 100.0000 | 0.1193 |
| Cost by administration route | |||||
| Intravenous administration | € 240.43 | 24.043 | Gamma | 100.0000 | 2.4043 |
| Subcutaneous administration | € 30.13 | 3.013 | Gamma | 100.0000 | 0.3013 |
| Treatment duration after progression | |||||
| Ibrutinib (mo) | 41.0 | 4.100 | Gamma | 100.0000 | 0.4100 |
| Venetoclax in combination con rituximab (mo) | 24.4 | 2.440 | Gamma | 100.0000 | 0.2440 |
| Venetoclax monotherapy (mo) | 16.0 | 1.600 | Gamma | 100.0000 | 0.1600 |
| Percentage of patients with adverse events | |||||
| VenO: Weakness | 2.80% | 0.011 | Gamma | 6.1070 | 0.0046 |
| VenO: Diarrhoea | 3.80% | 0.013 | Gamma | 8.3742 | 0.0045 |
| VenO: Dyspnoea | 2.40% | 0.011 | Gamma | 5.2131 | 0.0046 |
| VenO: Febrile neutropenia | 5.20% | 0.015 | Gamma | 11.6287 | 0.0045 |
| VenO: Infusion reactions | 9.00% | 0.020 | Gamma | 20.9670 | 0.0043 |
| VenO: Leukopenia | 2.40% | 0.011 | Gamma | 5.2131 | 0.0046 |
| VenO: Neutropenia | 52.80% | 0.034 | Gamma | 237.1525 | 0.0022 |
| VenO: Pneumonia | 5.70% | 0.016 | Gamma | 12.8144 | 0.0044 |
| VenO: Sepsis | 4.20% | 0.014 | Gamma | 9.2944 | 0.0045 |
| VenO: Thrombocytopenia | 13.70% | 0.024 | Gamma | 33.6547 | 0.0041 |
| ClbO: Weakness | 0.50% | 0.005 | Gamma | 1.0754 | 0.0046 |
| ClbO: Diarrhoea | 0.50% | 0.005 | Gamma | 1.0754 | 0.0046 |
| ClbO: Dyspnoea | 0.50% | 0.005 | Gamma | 1.0754 | 0.0046 |
| ClbO: Febrile neutropenia | 3.70% | 0.013 | Gamma | 8.2222 | 0.0045 |
| ClbO: Infusion reactions | 10.30% | 0.021 | Gamma | 24.5730 | 0.0042 |
| ClbO: Leukopenia | 4.70% | 0.014 | Gamma | 10.5540 | 0.0045 |
| ClbO: Neutropenia | 47.70% | 0.034 | Gamma | 195.1778 | 0.0024 |
| ClbO: Pneumonia | 4.20% | 0.014 | Gamma | 9.3820 | 0.0045 |
| ClbO: Sepsis | 1.40% | 0.008 | Gamma | 3.0385 | 0.0046 |
| ClbO: Thrombocytopenia | 15.00% | 0.024 | Gamma | 37.7647 | 0.0040 |
| FCR: Leukopenia | 24.00% | 0.021 | Gamma | 127.5789 | 0.0019 |
| FCR: Neutropenia | 34.00% | 0.024 | Gamma | 208.1212 | 0.0016 |
| FCR: Thrombocytopenia | 7.00% | 0.013 | Gamma | 30.4086 | 0.0023 |
| BR: Diarrhoea | 7.00% | 0.015 | Gamma | 21.0000 | 0.0033 |
| BR: Leukopenia | 48.00% | 0.030 | Gamma | 257.5385 | 0.0019 |
| BR: Neutropenia | 59.00% | 0.029 | Gamma | 401.4878 | 0.0015 |
| BR: Pneumonia | 9.00% | 0.017 | Gamma | 27.5934 | 0.0033 |
| BR: Sepsis | 1.00% | 0.006 | Gamma | 2.8182 | 0.0035 |
| BR: Thrombocytopenia | 14.00% | 0.021 | Gamma | 45.4186 | 0.0031 |
| Ibrutinib: Diarrhoea | 4.00% | 0.017 | Gamma | 5.6667 | 0.0071 |
| Ibrutinib: Febrile neutropenia | 1.00% | 0.009 | Gamma | 1.3737 | 0.0073 |
| Ibrutinib: Neutropenia | 12.00% | 0.028 | Gamma | 18.5455 | 0.0065 |
| Disutilities and duration by adverse event | |||||
| Weakness | 0.115 | 0.012 | Gamma | 100.0000 | 0.0012 |
| Diarrhoea | 0.080 | 0.005 | Gamma | 256.0000 | 0.0003 |
| Dyspnoea | 0.103 | 0.010 | Gamma | 100.0000 | 0.0010 |
| Febrile neutropenia | 0.150 | 0.015 | Gamma | 100.0000 | 0.0015 |
| Infusion reactions | 0.200 | 0.020 | Gamma | 100.0000 | 0.0020 |
| Leukopenia | 0.090 | 0.009 | Gamma | 100.0000 | 0.0009 |
| Neutropenia | 0.090 | 0.002 | Gamma | 3164.0625 | 0.0000 |
| Pneumonia | 0.195 | 0.004 | Gamma | 2500.0000 | 0.0001 |
| Sepsis | 0.195 | 0.004 | Gamma | 2500.0000 | 0.0001 |
| Thrombocytopenia | 0.108 | 0.012 | Gamma | 81.0000 | 0.0013 |
| Weakness, duration (d) | 35.33 | 3.533 | Gamma | 100.0000 | 0.3533 |
| Diarrheal, duration (d) | 3.50 | 0.350 | Gamma | 100.0000 | 0.0350 |
| Dyspnoea, duration (d) | 12.70 | 1.270 | Gamma | 100.0000 | 0.1270 |
| Febrile neutropenia, duration (d) | 3.50 | 0.350 | Gamma | 100.0000 | 0.0350 |
| Infusion reactions, duration (d) | 3.50 | 0.350 | Gamma | 100.0000 | 0.0350 |
| Leukopenia, duration (d) | 14.00 | 1.400 | Gamma | 100.0000 | 0.1400 |
| Neutropenia, duration (d) | 3.50 | 0.350 | Gamma | 100.0000 | 0.0350 |
| Pneumonia, duration (d) | 18.21 | 1.821 | Gamma | 100.0000 | 0.1821 |
| Sepsis, duration (d) | 7.00 | 0.700 | Gamma | 100.0000 | 0.0700 |
| Thrombocytopenia, duration (d) | 23.20 | 2.320 | Gamma | 100.0000 | 0.2320 |
| Cost of managing adverse events | |||||
| Weakness | € 592.61 | 59.261 | Gamma | 100.0000 | 5.9261 |
| Diarrhoea | € 454.65 | 45.465 | Gamma | 100.0000 | 4.5465 |
| Dyspnoea | € 178.52 | 17.852 | Gamma | 100.0000 | 1.7852 |
| Febrile neutropenia | € 2,749.45 | 274.945 | Gamma | 100.0000 | 27.4945 |
| Infusion reactions | € 892.10 | 89.210 | Gamma | 100.0000 | 8.9210 |
| Leukopenia | € 1,628.28 | 162.828 | Gamma | 100.0000 | 16.2828 |
| Neutropenia | € 1,697.72 | 169.772 | Gamma | 100.0000 | 16.9772 |
| Pneumonia | € 4,460.89 | 446.089 | Gamma | 100.0000 | 44.6089 |
| Sepsis | € 6,866.85 | 686.685 | Gamma | 100.0000 | 68.6685 |
| Thrombocytopenia | € 942.87 | 94.287 | Gamma | 100.0000 | 9.4287 |
BR: bendamustine in combination with rituximab; ClbO: chlorambucil in combination with obinutuzumab; FCR: fludarabine in combination with cyclophosphamide and rituximab; VenO: venetoclax in combination with obinutuzumab.
Early Access date (04/19/2022).