Heart failure is an increasingly common syndrome with a rising prevalence, which associates significant costs, mainly related to hospitalization. In fact, heart failure is the most frequent diagnosis in hospital discharges in Spain.
ObjectiveTo analyze the economic impact of new treatments for heart failure with reduced ejection fraction such as sacubitril/valsartan in out-patient and in-patient setting.
Material and methodsThe present economic evaluation study was carried out by developing a Markov model. Treatment with sacubitril/valsartan from admission or after hospital discharge was compared, with enalapril being the comparator. Total costs, years of life gained, quality-adjusted life years, and incremental cost-effectiveness ratio and incremental cost-utility ratio were analyzed. Data were obtained from the PARADIGM-HF and PIONEER-HF studies.
ResultsThe results of the base cases of the three comparisons made showed that sacubitril/valsartan produced benefits in years of life gained and quality-adjusted life years compared to enalapril showing incremental cost-utility ratio below €20,000/QALY and that this ratio was better in scenarios starting sacubitril/valsartan in the hospital setting once decompensation was resolved.
ConclusionThis study shows that starting sacubitril/valsartan from hospital admission for heart failure is cost-effective from the perspective of the National Health Service in Spain.
la insuficiencia cardiaca es un síndrome cada vez más frecuente, con una prevalencia en ascenso, que asocia importantes costes, fundamentalmente relacionados con la hospitalización. De hecho, la insuficiencia cardiaca es el diagnóstico más frecuente en las altas hospitalarias en España.
Objetivoanalizar el impacto económico de nuevos tratamientos para la insuficiencia cardiaca con fracción de eyección del ventrículo izquierdo reducida, como sacubitrilo/valsartán, instaurado tanto al alta como desde el ingreso hospitalario.
Material y métodosel presente estudio de evaluación económica se realizó mediante la elaboración de un modelo de Markov. Se comparó el tratamiento con sacubitrilo/valsartán desde el ingreso o tras el alta hospitalaria, siendo el comparador el enalapril. Se analizaron los costes totales, años de vida ganados, años de vida ajustados por calidad y ratio de coste-efectividad incremental y ratio de coste-utilidad incremental. Los datos se obtienen de los estudios PARADIGM-HF y PIONEER-HF.
Resultadoslos resultados de los casos base de las 3 comparaciones realizadas mostraron que sacubitrilo/valsartán produjo beneficios en años de vida ganados y años de vida ajustados a calidad respecto a enalapril, mostrando una ratio de coste-efectividad incremental por debajo de los 20.000 €/año de vida ajustado por calidad y que dicha ratio era mejor en los escenarios de inicio de sacubitrilo/valsartán en el ámbito hospitalario una vez resuelta la descompensación.
Conclusióneste estudio muestra que el inicio de sacubitrilo/valsartán desde el ingreso hospitalario por insuficiencia cardiaca es coste-efectivo desde el punto de vista del Servicio Nacional de Salud en España.
Heart failure (HF) is a highly prevalent syndrome that significantly affects morbidity and mortality, as well as the patients' quality of life.
Although the incidence of HF has stabilized, its prevalence continues to increase, mainly due to population aging and the emergence of treatments that improve patient survival. There are an estimated 56 million patients with HF worldwide (prevalence between 1% and 3%)1. In Spain, its prevalence is around 1.89%2 and increases with age, rising to 8% among individuals aged 65–74 years and reaching 16.1% in those aged 75 or older3.
Heart failure is considered a public health issue due to its high prevalence and its impact on survival and quality of life. The average annual cost per patient is estimated to be around €3000/year, with hospitalisations being the main cost component4.
Sacubitril/valsartan (Sac/Val) is a molecular combination of an angiotensin II receptor blocker (ARB) and a neprilysin inhibitor (ARNI). It is indicated for the treatment of HF with reduced ejection fraction (HFrEF). During its development, the PARADIGM-HF trial compared Sac/Val with enalapril, an angiotensin-converting enzyme inhibitor (ACEI), in an outpatient setting. The PIONEER-HF study compared the same medications in hospitalized patients with HFrEF who had been stabilized after an episode of acute decompensated HF. Both studies demonstrated the superiority of Sac/Val in reducing mortality, hospital admissions, and other parameters that could lead to greater resource utilization. However, since Sac/Val is an innovative drug with a higher cost than enalapril (a generic pharmaceutical product), determining its incremental cost-utility ratio (ICUR) in these settings is essential to inform prescribing decisions.
The objective of this study was to evaluate the cost-effectiveness and cost-utility of Sac/Val treatment in outpatient and inpatient settings for patients with HFrEF, from the perspective of the Spanish healthcare system, in order to determine the treatment option and setting most likely to be cost-effective, thereby supporting informed decision-making in the selection of the most appropriate treatment.
MethodologyPICO questionsFor the present study, 3 PICO questions were formulated based on the combination of the intervention (treatment with Sac/Val) and the setting in which it is initiated (during hospitalization or at discharge). The 3 questions are described below:
- •
PICO 1
- o
Population: adult patients with HFrEF admitted to the hospital due to acute decompensated HF.
- o
Intervention: treatment with enalapril (up to 10 mg twice daily) during hospital stay (once decompensation is resolved), followed by initiation of Sac/Val (up to 97 mg/103 mg twice daily) in the outpatient setting after discharge.
- o
Comparator: treatment with enalapril (up to 10 mg twice daily) from hospital admission (once decompensation is resolved).
- o
Outcomes: total costs, life years gained (LYG), quality-adjusted life years (QALYs), incremental cost-effectiveness ratio (ICER), and incremental cost-utility ratio (ICUR).
- o
- •
PICO 2
- o
Population: adult patients with HFrEF admitted to the hospital due to acute decompensated HF.
- o
Intervention: treatment with Sac/Val (up to 97 mg/103 mg twice daily) during hospital stay (once decompensation is resolved), and continued in the outpatient setting.
- o
Comparator: treatment with enalapril (up to 10 mg twice daily) from hospital admission (once decompensation is resolved).
- o
Outcomes: total costs, LYG, QALYs, ICER, and ICUR.
- o
- •
PICO 3
- o
Population: adult patients with HFrEF admitted to the hospital due to acute decompensated HF.
- o
Intervention: treatment with Sac/Val (up to 97 mg/103 mg twice daily) during hospital stay (once decompensation is resolved), and continued in the outpatient setting.
- o
Comparator: treatment with enalapril (up to 10 mg twice daily) during hospital stay (once decompensation is resolved), followed by initiation of Sac/Val (up to 97 mg/103 mg twice daily) in the outpatient setting after discharge.
- o
Outcomes: total costs, LYG, QALYs, ICER, and ICUR.
- o
This economic evaluation study was conducted using a Markov model based on the structure previously published by De Gaziano et al. for an economic evaluation carried out in the United States (published in 2016 and updated in 2020)5,6. This model design enables simulation of disease progression in patients treated with the evaluated options from hospital admission—at which point they receive a diagnosis of HFrEF—until death. The model was developed using Microsoft Excel 365 and Visual Basic for Applications.
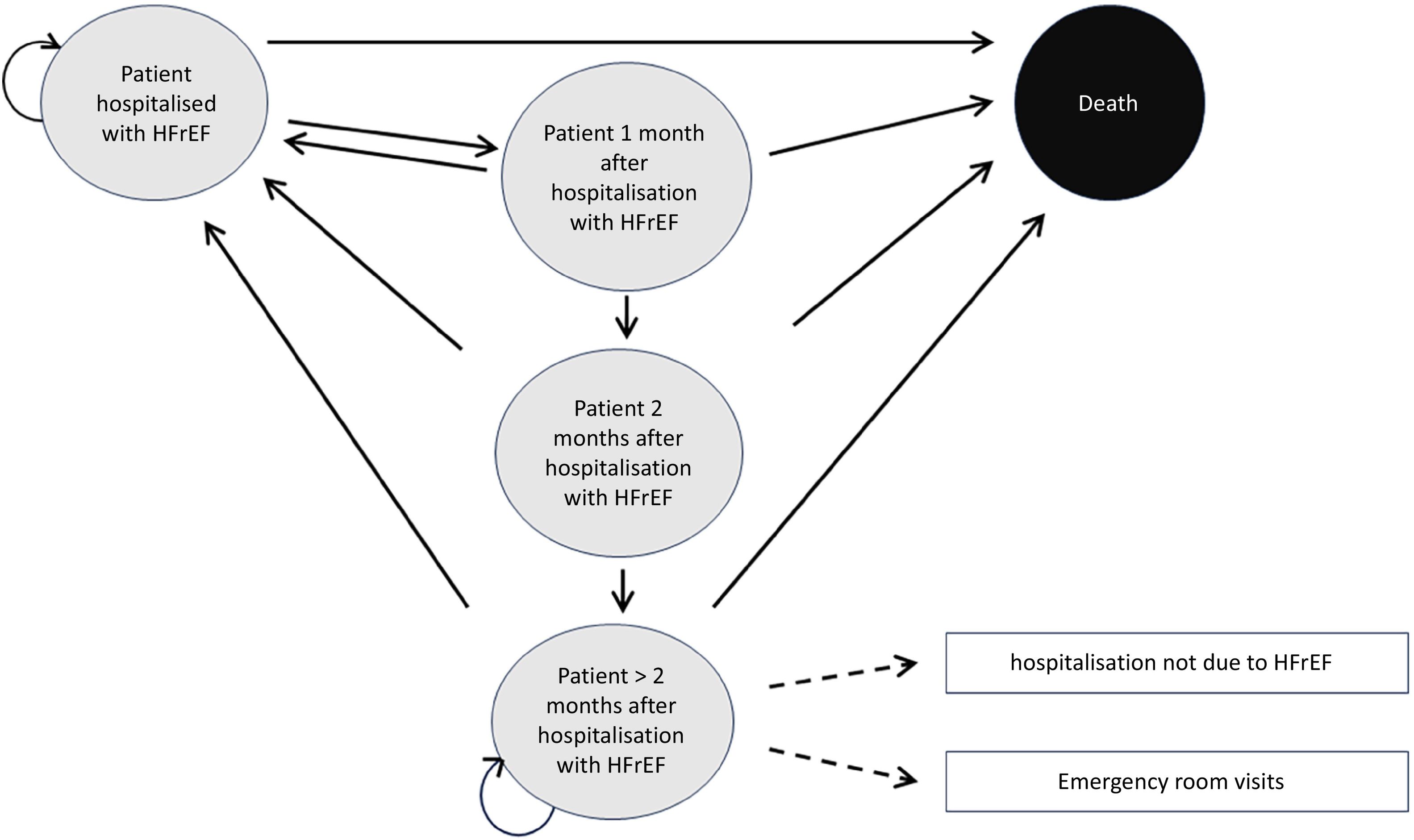
Fig. 1 shows that the model is a five-state Markov model: hospitalized patient due to HF (after stabilization following an acute decompensation episode); patient one month after hospitalization; patient two months after hospitalization; patient more than two months after hospitalization due to HF; and death (absorbing state). Patients transition from one state to another in monthly cycles, based on transition probabilities determined by the risk of all-cause mortality, hospitalization due to HF, and hospitalization for causes other than HF.
Thus, patients enter the model in the hospitalized state due to HF and, after one month, may remain hospitalized (hospitalized HF patient), be discharged (one month after HF hospitalization), or die (death).
After one month of discharge, patients may be readmitted (hospitalized HF patient), remain discharged (two months after HF hospitalization), or die. Two months after discharge, patients may be readmitted (hospitalized HF patient), remain discharged (>2 months after HF hospitalization), or die. Patients discharged for more than two months may remain in that state, be readmitted (hospitalized HF patient), or die. Finally, death is the absorbing state—any patient reaching this state ceases to transition through the Markov chain.
Time horizon, perspective, and discountingA lifetime horizon was considered for the analysis. As patients with HFrEF are typically older, this was assumed to correspond to a period of 30 years. The perspective adopted was that of the Spanish National Health System (SNS) (direct healthcare costs). In accordance with Spanish guidelines, a 3% annual discount rate was applied to both costs and outcomes in the base-case analysis.
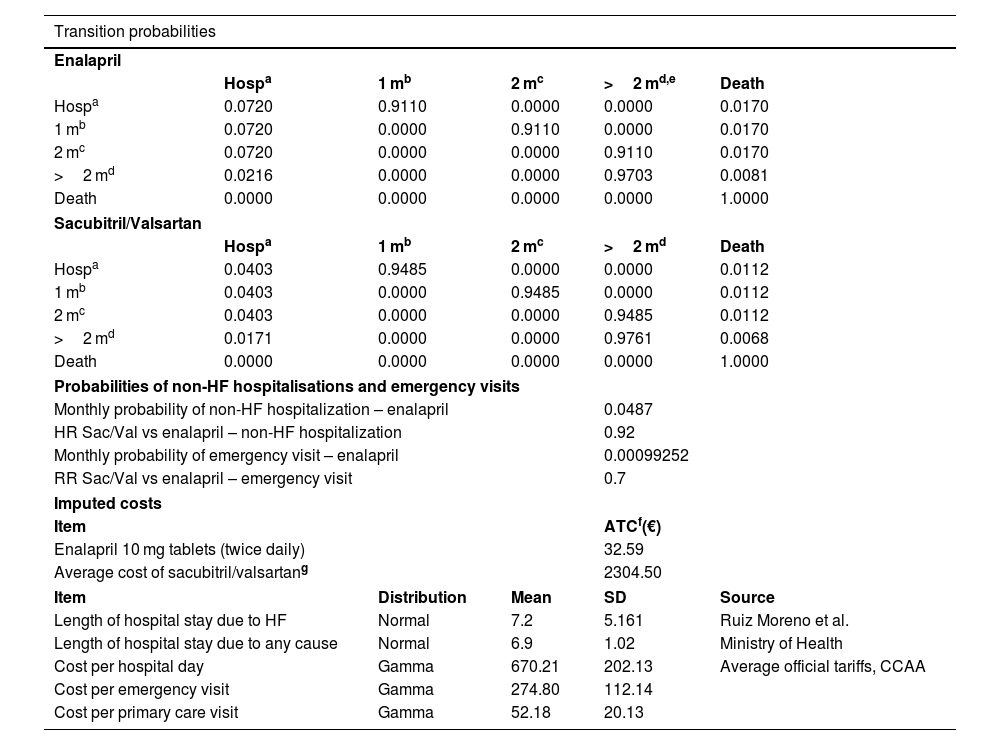
Transition probabilitiesTransition probabilities for enalapril were based on data from Gaziano et al. 2020, which were derived from the PIONEER-HF study (for transitions from hospitalization states and up to two months after discharge) and the PARADIGM-HF study (for transitions beyond two months post-discharge). Transition probabilities for the Sac/Val arms were derived from hazard ratios (HRs) from the PIONEER-HF study (during the first two months post-discharge) and the PARADIGM-HF study (beyond two months post-discharge). Table 1 shows the transition probabilities between the different health states.
Inputs. Model parameters.
| Transition probabilities | |||||
|---|---|---|---|---|---|
| Enalapril | |||||
| Hospa | 1 mb | 2 mc | >2 md,e | Death | |
| Hospa | 0.0720 | 0.9110 | 0.0000 | 0.0000 | 0.0170 |
| 1 mb | 0.0720 | 0.0000 | 0.9110 | 0.0000 | 0.0170 |
| 2 mc | 0.0720 | 0.0000 | 0.0000 | 0.9110 | 0.0170 |
| >2 md | 0.0216 | 0.0000 | 0.0000 | 0.9703 | 0.0081 |
| Death | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 1.0000 |
| Sacubitril/Valsartan | |||||
| Hospa | 1 mb | 2 mc | >2 md | Death | |
| Hospa | 0.0403 | 0.9485 | 0.0000 | 0.0000 | 0.0112 |
| 1 mb | 0.0403 | 0.0000 | 0.9485 | 0.0000 | 0.0112 |
| 2 mc | 0.0403 | 0.0000 | 0.0000 | 0.9485 | 0.0112 |
| >2 md | 0.0171 | 0.0000 | 0.0000 | 0.9761 | 0.0068 |
| Death | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 1.0000 |
| Probabilities of non-HF hospitalisations and emergency visits | |||||
| Monthly probability of non-HF hospitalization – enalapril | 0.0487 | ||||
| HR Sac/Val vs enalapril – non-HF hospitalization | 0.92 | ||||
| Monthly probability of emergency visit – enalapril | 0.00099252 | ||||
| RR Sac/Val vs enalapril – emergency visit | 0.7 | ||||
| Imputed costs | |||||
| Item | ATCf(€) | ||||
| Enalapril 10 mg tablets (twice daily) | 32.59 | ||||
| Average cost of sacubitril/valsartang | 2304.50 | ||||
| Item | Distribution | Mean | SD | Source | |
| Length of hospital stay due to HF | Normal | 7.2 | 5.161 | Ruiz Moreno et al. | |
| Length of hospital stay due to any cause | Normal | 6.9 | 1.02 | Ministry of Health | |
| Cost per hospital day | Gamma | 670.21 | 202.13 | Average official tariffs, CCAA | |
| Cost per emergency visit | Gamma | 274.80 | 112.14 | ||
| Cost per primary care visit | Gamma | 52.18 | 20.13 | ||
CCAA, Spanish autonomous communities; ATC, average annual treatment cost; Hosp, hospitalization; HR, hazard ratios; HF, heart failure; RR, relative risk.
It was assumed that non-HF hospitalisations and emergency visits occur only after two months post-discharge and do not affect patients' quality of life. The rates of non-HF hospitalisations and emergency department visits were taken from the PARADIGM-HF study. Table 1 shows the corresponding monthly probabilities derived from these data.
Resource use and costsPharmacological treatment costs were estimated using prices based on the retail price plus VAT (available at: https://www.sanidad.gob.es/profesionales/nomenclator.do, accessed 30/05/2023). The average annual treatment cost (ATC) of Sac/Val was estimated based on data from the Catalonia utilization study7.
The costs of other consumed healthcare resources were calculated by multiplying the unit cost of each resource by the number of units used. Hospitalization costs were estimated using data from the Ministry of Health and Consumer Affairs databases and the literature. Unit costs of resources were obtained from the average of official tariffs published by the Spanish autonomous communities. Regarding follow-up, 1 primary care visit was assumed after hospital discharge (assigned to the health state of 1 month after discharge), and 2 annual visits were assumed for stable patients (proportionally assigned to the health state of more than 2 months post-discharge). Table 1 shows all these costs.
Estimation of utilitiesA mixed-effects model was used to estimate age- and time-dependent utilities for the enalapril arm, based on EQ-5D-3L scores obtained in the PARADIGM-HF study (Gaziano et al.5). The same model was used to obtain utility decrements associated with patient hospitalisations and the utility increment observed in patients treated with Sac/Val.
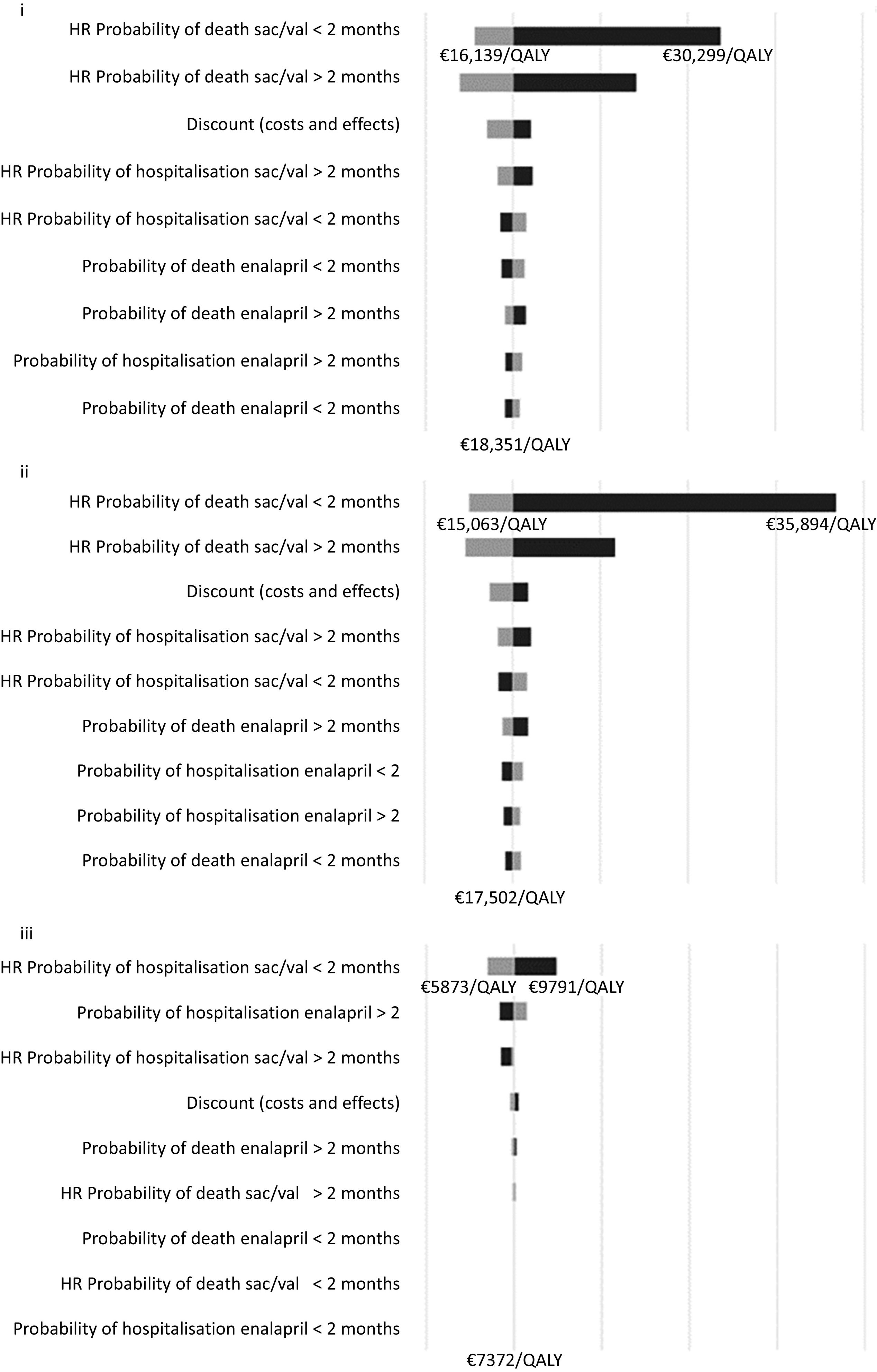
Sensitivity analysisTornado analyses were performed for the three comparisons (PICO questions) using one-way sensitivity analyses in which minimum and maximum values were applied to the following parameters:
- •
Probability of hospitalization with enalapril:
- o
During the first two months after admission
- o
More than two months after admission
- o
- •
Hazard ratio for the probability of hospitalization with Sac/Val vs enalapril:
- o
During the first two months after admission
- o
More than two months after admission
- o
- •
Probability of death while on enalapril:
- o
During the first two months after admission
- o
More than two months after admission
- o
- •
Hazard ratio for the probability of mortality with Sac/Val vs enalapril:
- o
During the first two months after admission
- o
More than two months after admission
- o
- •
Discount rate (applied to both costs and outcomes)
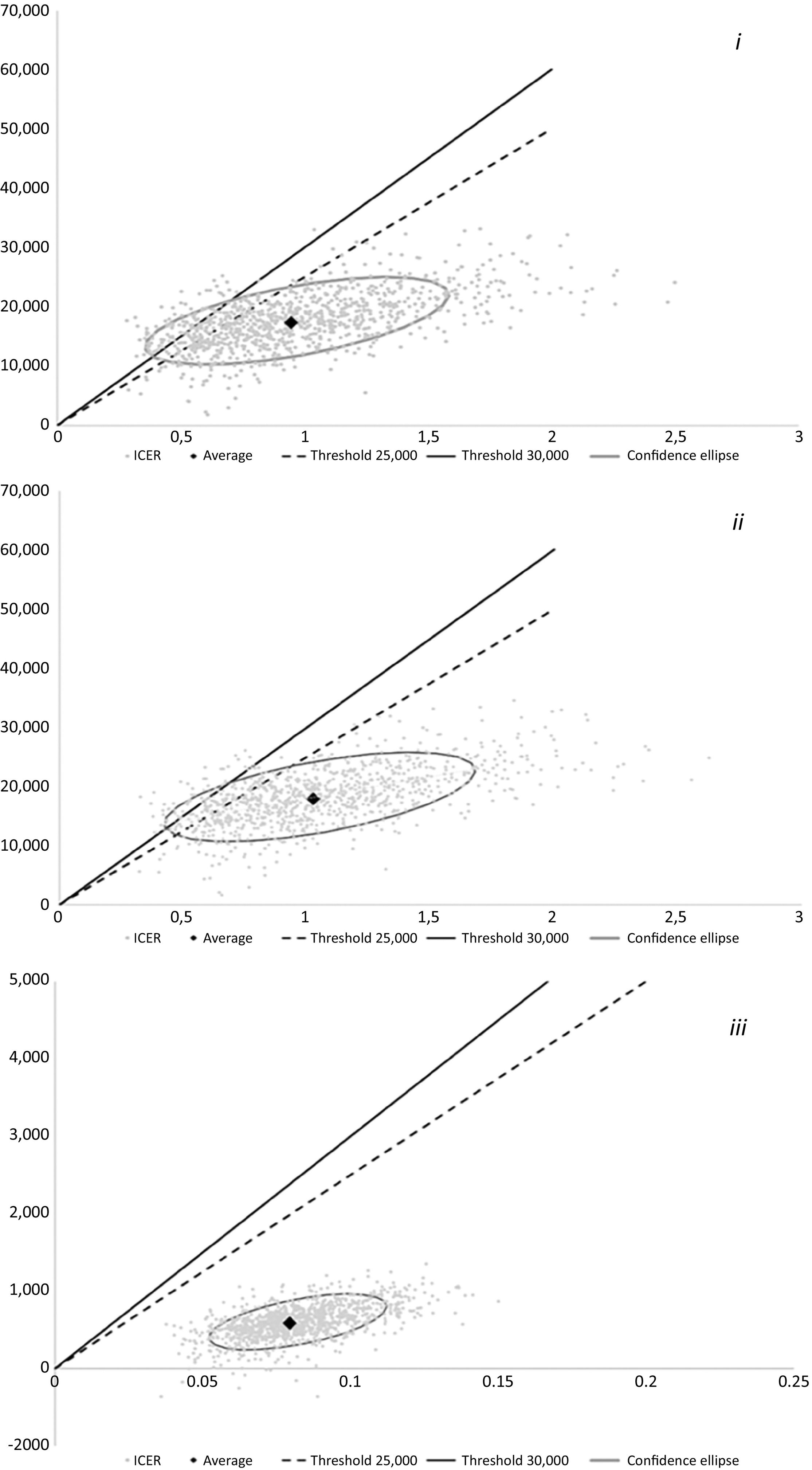
A probabilistic sensitivity analysis was also conducted for each of the three comparisons (PICO questions). A total of 1000 simulations were performed for each analysis, with model parameters randomly drawn from their respective probability distributions.
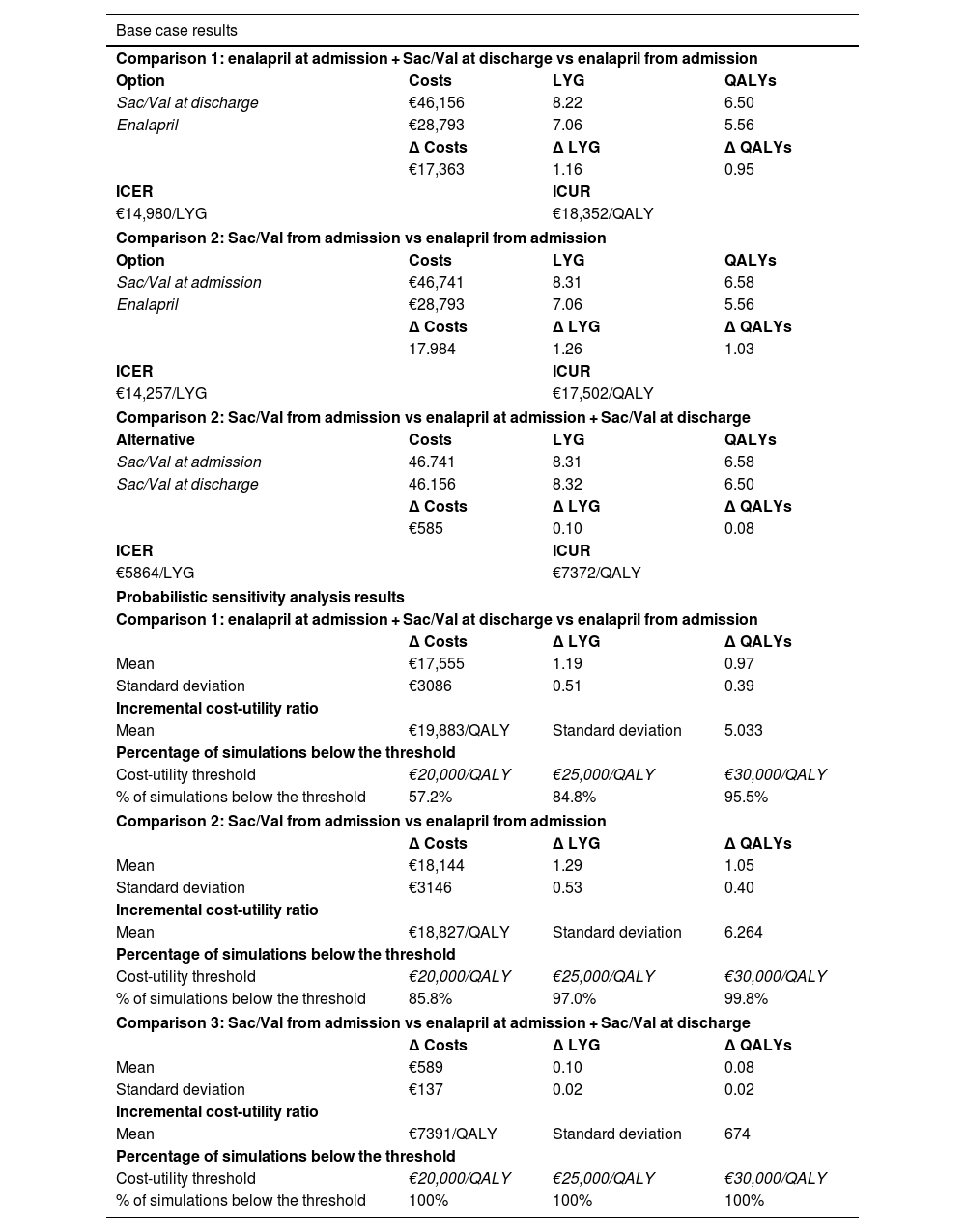
ResultsTable 2 shows the base-case results of the three comparisons. Compared to enalapril, Sac/Val yielded benefits in LYG and QALYs, with ICURs below €20,000 per QALY. The ratio was more favorable in scenarios where Sac/Val was initiated in the hospital setting once decompensation had been resolved.
Results of the 3 comparisons.
| Base case results | |||
|---|---|---|---|
| Comparison 1: enalapril at admission + Sac/Val at discharge vs enalapril from admission | |||
| Option | Costs | LYG | QALYs |
| Sac/Val at discharge | €46,156 | 8.22 | 6.50 |
| Enalapril | €28,793 | 7.06 | 5.56 |
| Δ Costs | Δ LYG | Δ QALYs | |
| €17,363 | 1.16 | 0.95 | |
| ICER | ICUR | ||
| €14,980/LYG | €18,352/QALY | ||
| Comparison 2: Sac/Val from admission vs enalapril from admission | |||
| Option | Costs | LYG | QALYs |
| Sac/Val at admission | €46,741 | 8.31 | 6.58 |
| Enalapril | €28,793 | 7.06 | 5.56 |
| Δ Costs | Δ LYG | Δ QALYs | |
| 17.984 | 1.26 | 1.03 | |
| ICER | ICUR | ||
| €14,257/LYG | €17,502/QALY | ||
| Comparison 2: Sac/Val from admission vs enalapril at admission + Sac/Val at discharge | |||
| Alternative | Costs | LYG | QALYs |
| Sac/Val at admission | 46.741 | 8.31 | 6.58 |
| Sac/Val at discharge | 46.156 | 8.32 | 6.50 |
| Δ Costs | Δ LYG | Δ QALYs | |
| €585 | 0.10 | 0.08 | |
| ICER | ICUR | ||
| €5864/LYG | €7372/QALY | ||
| Probabilistic sensitivity analysis results | |||
| Comparison 1: enalapril at admission + Sac/Val at discharge vs enalapril from admission | |||
| Δ Costs | Δ LYG | Δ QALYs | |
| Mean | €17,555 | 1.19 | 0.97 |
| Standard deviation | €3086 | 0.51 | 0.39 |
| Incremental cost-utility ratio | |||
| Mean | €19,883/QALY | Standard deviation | 5.033 |
| Percentage of simulations below the threshold | |||
| Cost-utility threshold | €20,000/QALY | €25,000/QALY | €30,000/QALY |
| % of simulations below the threshold | 57.2% | 84.8% | 95.5% |
| Comparison 2: Sac/Val from admission vs enalapril from admission | |||
| Δ Costs | Δ LYG | Δ QALYs | |
| Mean | €18,144 | 1.29 | 1.05 |
| Standard deviation | €3146 | 0.53 | 0.40 |
| Incremental cost-utility ratio | |||
| Mean | €18,827/QALY | Standard deviation | 6.264 |
| Percentage of simulations below the threshold | |||
| Cost-utility threshold | €20,000/QALY | €25,000/QALY | €30,000/QALY |
| % of simulations below the threshold | 85.8% | 97.0% | 99.8% |
| Comparison 3: Sac/Val from admission vs enalapril at admission + Sac/Val at discharge | |||
| Δ Costs | Δ LYG | Δ QALYs | |
| Mean | €589 | 0.10 | 0.08 |
| Standard deviation | €137 | 0.02 | 0.02 |
| Incremental cost-utility ratio | |||
| Mean | €7391/QALY | Standard deviation | 674 |
| Percentage of simulations below the threshold | |||
| Cost-utility threshold | €20,000/QALY | €25,000/QALY | €30,000/QALY |
| % of simulations below the threshold | 100% | 100% | 100% |
LYG, life years gained; QALYs, quality-adjusted life years; ICER, incremental cost-effectiveness ratio; ICUR, incremental cost-utility ratio.
The tornado diagrams in Fig. 2 show that, regarding the one-way sensitivity analyses, the parameter with the greatest impact on the model is the HR for mortality in the Sac/Val arm within the first two months following admission for decompensated HF.
The base-case results are supported by those from the probabilistic sensitivity analyses shown in Table 2 and Fig. 3.
DiscussionThe results of this study show that treatment options with Sac/Val are cost-effective compared to ACEIs, and more efficient when Sac/Val is initiated in hospital once the patient has been stabilized, rather than delaying initiation until after hospital discharge in the outpatient setting. These findings were consistent across both deterministic and probabilistic analyses.
When reviewing the results of the probabilistic analyses to determine the scenarios in which Sac/Val exceeded the efficiency threshold (defined as €25,000/QALY), it was observed that the variables influencing this outcome were similar to those identified in the probabilistic sensitivity analyses: mortality-related variables in Sac/Val vs enalapril comparisons, and hospitalization-related variables in the comparisons between initiating ARNI during hospitalization or after discharge. This finding was expected in the case of the comparisons with enalapril, as an HR value of 1 indicates equal effectiveness between the drugs being compared. This means that the most efficient option is also the least costly. The same applies to comparisons between Sac/Val initiation timing strategies, where, with mortality rates similar in both arms, the differentiating factor is the number of hospital admissions, which is reduced by early initiation of ARNI. This finding is in line with those reported in the systematic review by Proudfoot et al8.
Regarding efficiency (cost-utility), the results are consistent with Proudfoot's findings8, and are similar to those of the models developed by Gaziano5,6, which were adapted to the Spanish setting in this study. They are also in line with other models published in Europe, such as those in Switzerland9, Germany10, the Netherlands11, and, closer to home, Portugal12. All of these results are consistent with the assessment conducted by the UK's NICE13. A similar result in favor of Sac/Val efficiency was also found in studies using a societal perspective14, although it should be assessed whether the findings of this US study can be extrapolated to the Spanish healthcare system.
Cost-effectiveness (cost-utility) studies are a key source of information to inform funding decisions. This study identifies the most efficient option for the healthcare system (i.e., the option that offers the greatest benefit for each monetary unit). These studies complement budget impact analyses, which only consider treatment costs and exclude those arising from other aspects of healthcare provision.
As with all analyses of this nature, the main limitation of pharmacoeconomic models is the data used to populate them. In this case, the efficacy data for the drugs were obtained from clinical trials, which may differ from real-world clinical practice due to inclusion and exclusion criteria or the limited duration of the studies. Therefore, modeling requires extrapolating efficacy data to the broader population eligible for funding and over a lifetime horizon.
To mitigate the uncertainty introduced by this extrapolation, a probabilistic sensitivity analysis was conducted, generating different efficacy scenarios based on probability distributions. The results of this analysis show that Sac/Val is the cost-effective option in most scenarios. Moreover, it can be assumed that the impact of temporal extrapolation is minimal in this instance, given that HFrEF typically affects older patients with relatively short life expectancies.
Additionally, a conservative approach was adopted by not including other potential savings attributable to Sac/Val. These include those associated with avoiding implantable cardioverter-defibrillators (ICDs), which have a unit cost exceeding €20,000 (average of official tariffs across Spain's autonomous communities). In this regard, an Italian cost-effectiveness study showed that treatment with Sac/Val was the dominant strategy compared to ICD implantation15.
It is also worth noting that the benefits of Sac/Val vs enalapril are likely due to the fact that, while ACEIs act solely on the angiotensin II pathway, Sac/Val also targets the neprilysin pathway. The pathophysiology of HFrEF involves five distinct pathways: the angiotensin II and neprilysin pathways, as previously mentioned, plus the norepinephrine, aldosterone, and sodium-glucose cotransporter type 2 (SGLT-2) pathways. Accordingly, the latest international guidelines recommend early intervention across all five pathways using quadruple therapy, which includes an ARNI targeting two pathways. In this context, and to adapt the US cost-effectiveness study on this topic16 to the Spanish setting, a cost-effectiveness analysis should be conducted to compare the full five-pathway quadruple therapy—ARNI, beta-blocker (BB), mineralocorticoid receptor antagonist (MRA), and SGLT-2 inhibitor—with the current treatment recommended by Spanish funding criteria. The latter involves a stepwise approach targeting only three pathways (ACEI, BB, and MRA). Clinical trials show that patients treated with Sac/Val from hospital admission experience a very early reduction in clinical events, which would explain the favorable cost-effectiveness outcomes observed.
ConclusionsThe results of this study show that, under the assumptions used in the model, initiating treatment with Sac/Val from hospital admission onwards in patients with HFrEF is cost-effective, is the most efficient option for the SNS, and is superior to treatment with Sac/Val at discharge or treatment with enalapril.
Contribution to the scientific literatureThis study demonstrates the efficiency of sacubitril/valsartan—where earlier initiation yields greater benefit—for treating patients with heart failure with reduced ejection fraction within the Spanish National Health System. Although sacubitril/valsartan has been available for some time, this is the first study to be conducted and published from the perspective of the Spanish National Health System. The results of this type of study are useful in supporting the decision-making process for drug funding.
CRediT authorship contribution statementAntonio García-Quintana: Writing – review & editing, Visualization, Validation, Supervision, Methodology, Formal analysis, Conceptualization. Héctor Alonso Ramos: Writing – review & editing, Visualization, Validation, Methodology, Formal analysis, Conceptualization. Javier Parrondo: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
FundingThe authors declare that this study was funded by Novartis Farmacéutica SA.
None declared.