Medication reconciliation is relevant in transitional care, however, given limited resources, it is necessary to identify the patients who benefit most from this activity.
AimTo validate criteria to identify patients at high risk of medication errors undergoing major orthopedic surgery.
MethodDelphi Method in 3 phases, April–June 2023, to obtain consensus on the inclusion criteria, previously defined. Each expert rated criteria according to a 5-point Likert scale. Consensus was assumed in round 1 if the rate average was ≥4 (inclusion) or <2 (exclusion) and in rounds 2 and 3 if 50% of the responses were ≥4 (inclusion) or <2 (exclusion). It was possible to suggest the inclusion of new criteria.
Results10 experts from Faculties of Pharmacy and Medicine participated. In the first phase, consensus was reached on 18 criteria: polypharmacy, anticoagulants, oral chemotherapy (not hormone), immunosuppressants, antiretrovirals, antimyasthenics, insulin, corticoids, neuroleptics, antiarrhythmics, digoxin, carbamazepine, phenytoin, valproate, thyroid drugs, antiglaucoma, antiaggregants, and urgent surgery. Systemic antifungals and opioids were suggested. In the second phase, consensus was reached on 11 criteria: antiparkinsonics, beta-blockers, age ≥ 65 years, length of stay ≥5 days, lamotrigine, diuretics, antidepressants, angiotensin converting enzyme inhibitors, angiotensin II receptor antagonists, anxiolytics, opioids, and systemic antifungals. In the last phase, 1 criterion reached consensus (sulfonylureas) and 1 criterion did not reach consensus (calcium channel blockers).
ConclusionsWe develop and validate a list of 30 criteria to identify patients at high risk of experiencing medication errors undergoing major orthopedic surgery. These may help improve human resource management for clinical pharmacy activities by prioritizing patients who would benefit most.
La conciliación terapéutica es un proceso clínico especialmente relevante en la transición asistencial de cuidados, sin embargo, dados los recursos humanos y materiales limitados, es necesario identificar a los pacientes que más se benefician de esta actividad.
ObjetivoValidar criterios que permitan identificar pacientes sometidos a cirugía ortopédica mayor con potencial alto riesgo de sufrir un error de medicación.
MétodoMétodo Delphi en tres rondas, llevadas a cabo de abril a junio de 2023, para obtener consenso sobre los criterios de inclusión, previamente definidos por un equipo multidisciplinar. Cada experto valoró los criterios según una escala Likert de 5 puntos. Se asumió consenso en la ronda 1 si la media de las respuestas era ≥4 (inclusión) o < 2 (exclusión) y en las rondas 2 y 3 si el 50% de las respuestas eran ≥4 (inclusión) o < 2 (exclusión). Era posible sugerir la inclusión de nuevos criterios en la primera ronda.
ResultadosParticiparon 10 expertos en el área de 4 facultades de Farmacia y Medicina de Portugal. En la primera ronda, 18 criterios obtuvieron consenso: polifarmacia, anticoagulantes, quimioterapia oral (no hormonal), inmunosupresores, antirretrovirales, anti-Miastenia Gravis, insulina, corticoides, neurolépticos, antiarrítmicos, digoxina, carbamazepina, fenitoína, valproato, fármacos tiroideos y anti tiroideos, antiglaucoma, antiagregantes y cirugía urgente. Fueron sugeridos para inclusión antifúngicos sistémicos y opiáceos. En la segunda ronda, se llegó a un consenso en once criterios: anti-parkinsonianos, betabloqueantes, edad ≥65 años, duración del ingreso≥5 días, lamotrigina, diuréticos, antidepresivos, inhibidores de la Enzima Convertidora de Angiotensina, Antagonistas de los receptores de la angiotensina II, ansiolíticos, opioides y antifúngicos sistémicos. En la última ronda, un criterio alcanzó consenso (sulfonilureas) y otro no (antagonistas del calcio).
ConclusionesDesarrollamos y validamos una lista de 30 criterios para identificar a los pacientes con alto riesgo de sufrir errores de medicación sometidos a cirugía ortopédica mayor. Estos criterios pueden ayudar a mejorar la gestión de los recursos humanos para las actividades de farmacia clínica al priorizar pacientes que más se beneficiarían de la conciliación terapéutica en este ámbito.
Medication errors are one of the main causes of morbidity in hospitalized patients. Between 10% and 70% of medication histories contain at least an error, up to 1/3 of these errors have the potential to cause harm to the patient and more than 50% of medication errors occur in the transition between care.1 Medication reconciliation (MR) is recognized as an important tool in avoiding discrepancies. MR is defined as the process of analyzing a patient's best possible medication history (BPMH), whenever there are changes and should be carried out at vulnerable/critical points of care transition, namely at hospital admission and discharge and at unit transfers.1,2 It has previously been demonstrated that MR interventions carried out by hospital pharmacy are cost-effective.3 Healthcare institutions must promote the implementation of the MR process, namely, through the adoption of a systematic approach to MR, involving a multidisciplinary team that identifies and establishes appropriate strategies for implementing the process. Ideally, the possibility of simultaneously implementing this process at all critical points should be evaluated. If this is not possible, an operational plan must be defined, considering the first critical point as the admission that results in hospitalization.1 Pharmacist involvement at all stages of the reconciliation process for every patient may not be feasible because of reasons such as time commitment, number of pharmacists on staff, and other professional responsibilities. For this reason, Ordem dos Farmacêuticos, which is a professional public association that represents Portuguese pharmacists and regulates their activity in the national territory, published a guideline that lists some of the criteria for selecting patients. As this list is quite extensive, they recommended that the pharmaceutical intervention priorities considered most relevant in relation to the type of patients being monitored, before starting the service and in accordance to available human resources.4
Although the term “high-risk patient” is referred to in the literature as a criterion for selecting patients for whom therapeutic reconciliation should be performed, the criteria were undefined or defined in an empirical way.5 Patients undergoing major orthopedic surgeries such as hip arthroplasties/hip and knee prostheses, represent a particularly high-risk group.6,7 These patients are often elderly, with multiple comorbidities and subject to polymedication, which increases the complexity of therapeutic management and the risk of drug interactions.8 For this reason, during the development of the MR process by the hospital pharmacy team, there was a need to identify and establish a consensus over which patients are at high risk of medication errors and error-related adverse events.
This original research aims to validate the criteria that identify patients at high risk of experience medication errors and error-related adverse events undergoing major orthopedic surgery, in terms of home drug reconciliation.
MethodsDevelopment of the inicial criteria list and selection of expertsA multidisciplinary research group made of 2 hospital pharmacists and a physician (clinical pharmacologist) with expertise in MR defined, previously, and based on a literature review, clinical practice, and subsequent discussion, the criteria to be included in the form, based on the probability of risk of the adverse event to happen. For this consensus, it was defined that only patients hospitalized for major orthopedic surgery would be considered as it is a complicated surgery with a longer length of stay and with potential of pharmacist intervention.
We chose 10 experts from four Faculties of Pharmacy and Medicine nationwide that have clinical and/or academic settings (40% of the experts were clinicians and 60% academics). This selection was also based on geographic distribution and publications in impact factor journals in the field of pharmacology.
The Delphi methodAfter defining the inclusion criteria, the Delphi method was used to validate this inclusion criteria, depending on the probability and risk of an adverse event occurring. The structured Delphi method is based on the opinion of panel members and has gained acceptance in diverse fields of medicine to develop best practice guidance using collective intelligence where research is limited, ethically/logistically difficult or evidence is conflicting.9 This classic Delphi method requires 4 key features: anonymity of Delphi participants, iteration, controlled feedback, and statistical aggregation of group response. However, the technique can be effectively modified to meet the needs of the given study using modified Delphi methods.10
The research team contacted potential experts via email with a brief explanation of the project, inviting them to participate, and, if so, to appear on the published list of participants.
Between April and June 2023, 3 rounds of Delphi were conducted using Microsoft Forms® to share the questionnaire with the experts and collect their answers. Each expert rated the criteria according to a 5-point Likert scale (1-strongly disagree; 2-disagree; 3-neither agree nor disagree; 4-agree; 5-strongly agree).11
We sent an email in each round with a summary of the panel's responses and the next round form, as well as the new deadline. In the first round, it was possible to suggest the inclusion of new criteria for the second round. After receiving the responses, one research team member analyzed each result. All criteria that did not reach consensus were subject to the next round. Reminder emails were sent as necessary to encourage participation and a deadline was given for completion. Anonymity of experts was assured throughout the study.
Data analysisIn the first round, consensus was assumed if the rate average was ≥4 (inclusion) or <2 (exclusion).11 As it is expected that consensus would not be reached on a high number of criteria given the small sample, the cut-off method for consensus was reviewed in the following 2 rounds. So, the consensus was assumed in the second and third round if, at least 50% of the responses were ≥4 (inclusion) or <2 (exclusion).
Data analysis was carried out using Microsoft Excel® based on descriptive statistics. For each criteria the average, standard deviation and the percentage of experts who rated ≥4 were calculated.
Ethics approvalThis study was approved by Luz Saúde Ethics Committee on 2023. Reference: CES/41/2023/JAG.
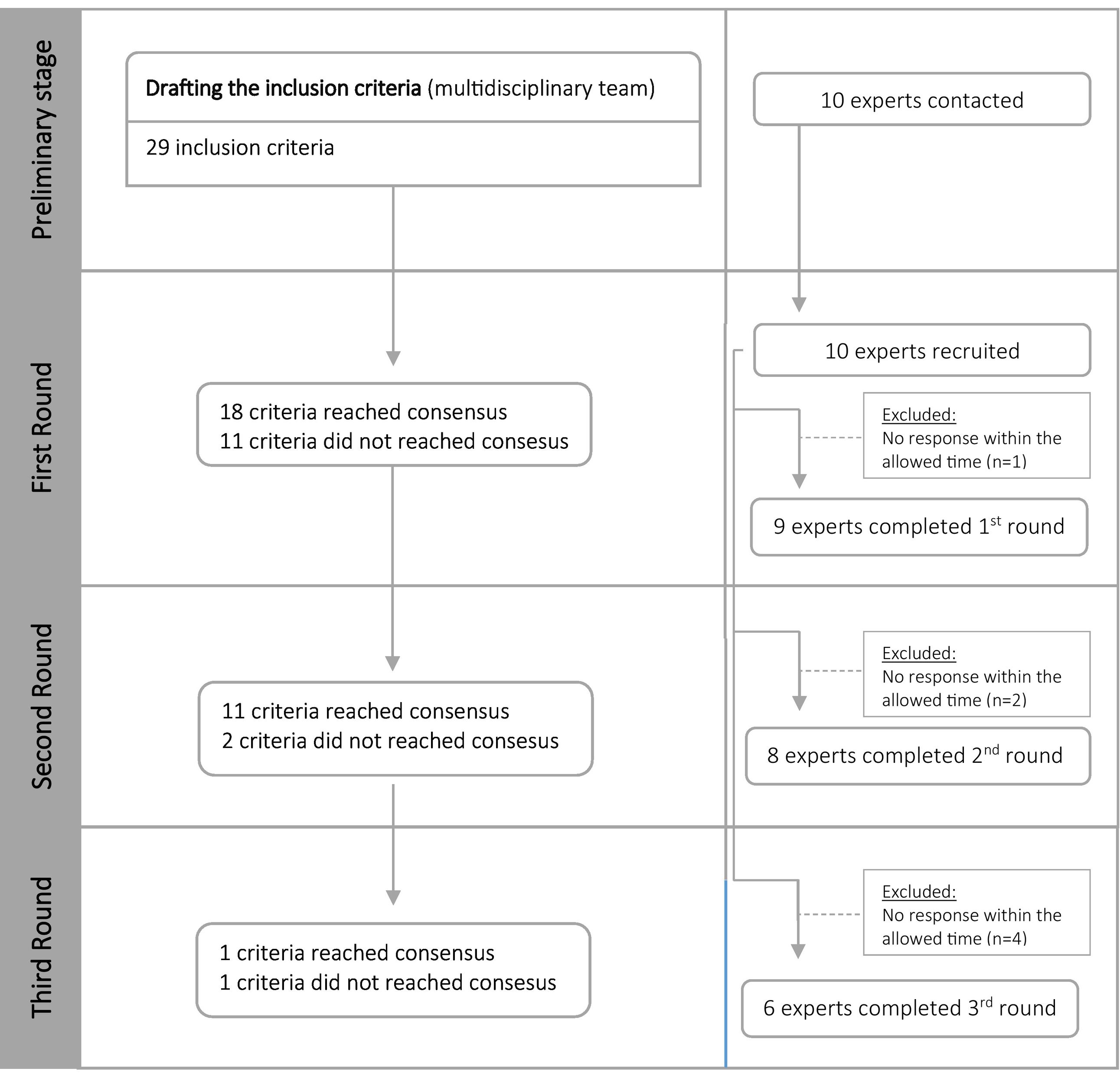
ResultsThe multidisciplinary research group drafted the form with the high-risk criteria in MR previously defined, based on the probability and risk of the adverse event to happen. This form included 29 inclusion criteria (Fig. 1) and 2 new inclusion criteria were suggested by experts: opioids and systemic antifungals. 10 experts were invited, and the invitation was accepted by all. The number of experts who responded to the form ranged from 9 in the first round to 8 in the second round and finally 6 in the third round. (Fig. 1).
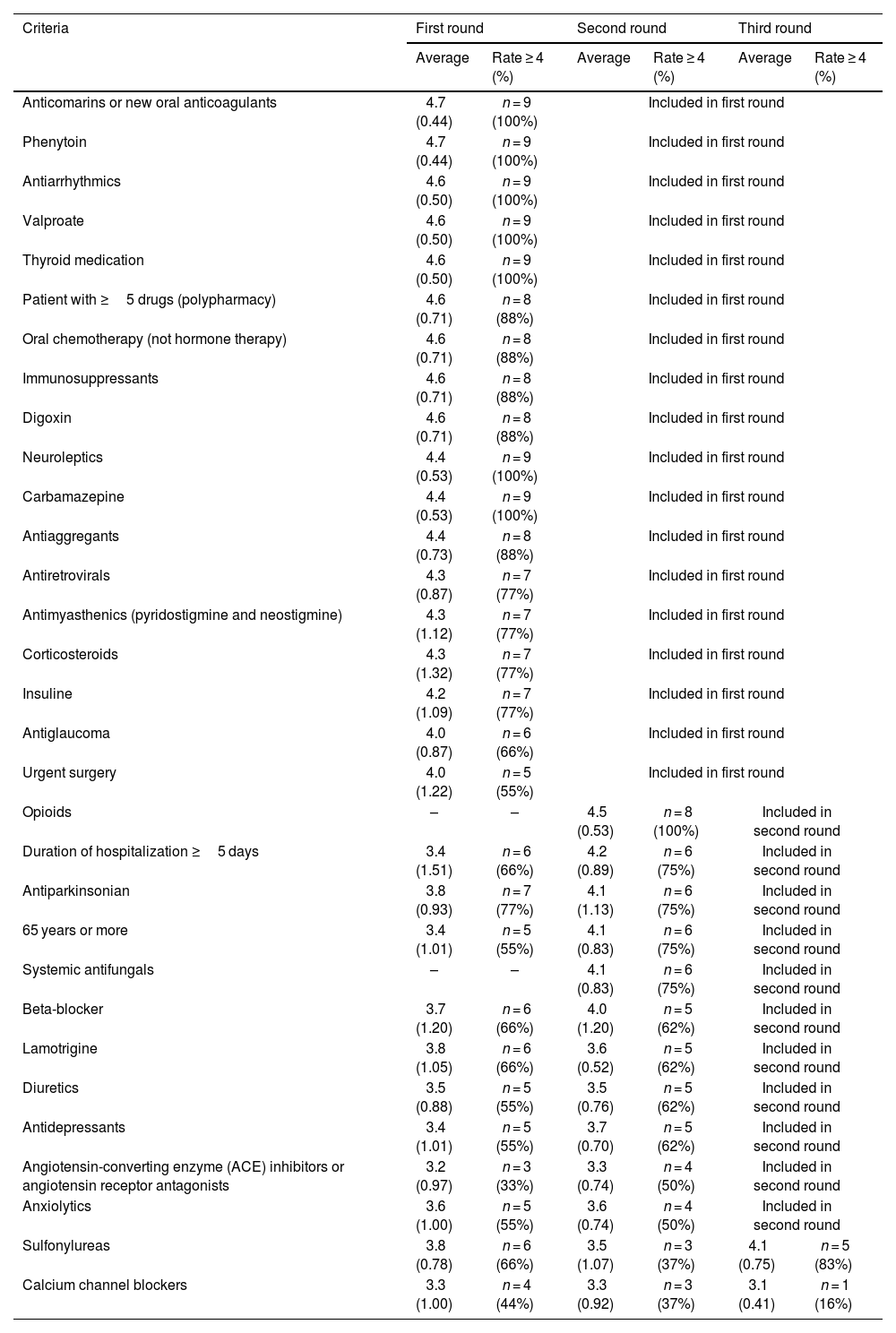
First roundIn the first phase, 9 responses were obtained in 29 inclusion criteria. Consensus for inclusion was reached on 18 criteria (Table 1). In this round, a comment was also made by an expert, asking for clarification regarding an inclusion criterion: duration of hospitalization greater than or equal to 5 days. In the next round, an explanation was provided regarding the purpose of this inclusion criterion. It was also suggested two new inclusion criteria: systemic antifungals and opioids.
Agreement ratings per high-risk criteria per round. Created by the authors.
| Criteria | First round | Second round | Third round | |||
|---|---|---|---|---|---|---|
| Average | Rate ≥ 4 (%) | Average | Rate ≥ 4 (%) | Average | Rate ≥ 4 (%) | |
| Anticomarins or new oral anticoagulants | 4.7 (0.44) | n = 9 (100%) | Included in first round | |||
| Phenytoin | 4.7 (0.44) | n = 9 (100%) | Included in first round | |||
| Antiarrhythmics | 4.6 (0.50) | n = 9 (100%) | Included in first round | |||
| Valproate | 4.6 (0.50) | n = 9 (100%) | Included in first round | |||
| Thyroid medication | 4.6 (0.50) | n = 9 (100%) | Included in first round | |||
| Patient with ≥5 drugs (polypharmacy) | 4.6 (0.71) | n = 8 (88%) | Included in first round | |||
| Oral chemotherapy (not hormone therapy) | 4.6 (0.71) | n = 8 (88%) | Included in first round | |||
| Immunosuppressants | 4.6 (0.71) | n = 8 (88%) | Included in first round | |||
| Digoxin | 4.6 (0.71) | n = 8 (88%) | Included in first round | |||
| Neuroleptics | 4.4 (0.53) | n = 9 (100%) | Included in first round | |||
| Carbamazepine | 4.4 (0.53) | n = 9 (100%) | Included in first round | |||
| Antiaggregants | 4.4 (0.73) | n = 8 (88%) | Included in first round | |||
| Antiretrovirals | 4.3 (0.87) | n = 7 (77%) | Included in first round | |||
| Antimyasthenics (pyridostigmine and neostigmine) | 4.3 (1.12) | n = 7 (77%) | Included in first round | |||
| Corticosteroids | 4.3 (1.32) | n = 7 (77%) | Included in first round | |||
| Insuline | 4.2 (1.09) | n = 7 (77%) | Included in first round | |||
| Antiglaucoma | 4.0 (0.87) | n = 6 (66%) | Included in first round | |||
| Urgent surgery | 4.0 (1.22) | n = 5 (55%) | Included in first round | |||
| Opioids | – | – | 4.5 (0.53) | n = 8 (100%) | Included in second round | |
| Duration of hospitalization ≥5 days | 3.4 (1.51) | n = 6 (66%) | 4.2 (0.89) | n = 6 (75%) | Included in second round | |
| Antiparkinsonian | 3.8 (0.93) | n = 7 (77%) | 4.1 (1.13) | n = 6 (75%) | Included in second round | |
| 65 years or more | 3.4 (1.01) | n = 5 (55%) | 4.1 (0.83) | n = 6 (75%) | Included in second round | |
| Systemic antifungals | – | – | 4.1 (0.83) | n = 6 (75%) | Included in second round | |
| Beta-blocker | 3.7 (1.20) | n = 6 (66%) | 4.0 (1.20) | n = 5 (62%) | Included in second round | |
| Lamotrigine | 3.8 (1.05) | n = 6 (66%) | 3.6 (0.52) | n = 5 (62%) | Included in second round | |
| Diuretics | 3.5 (0.88) | n = 5 (55%) | 3.5 (0.76) | n = 5 (62%) | Included in second round | |
| Antidepressants | 3.4 (1.01) | n = 5 (55%) | 3.7 (0.70) | n = 5 (62%) | Included in second round | |
| Angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor antagonists | 3.2 (0.97) | n = 3 (33%) | 3.3 (0.74) | n = 4 (50%) | Included in second round | |
| Anxiolytics | 3.6 (1.00) | n = 5 (55%) | 3.6 (0.74) | n = 4 (50%) | Included in second round | |
| Sulfonylureas | 3.8 (0.78) | n = 6 (66%) | 3.5 (1.07) | n = 3 (37%) | 4.1 (0.75) | n = 5 (83%) |
| Calcium channel blockers | 3.3 (1.00) | n = 4 (44%) | 3.3 (0.92) | n = 3 (37%) | 3.1 (0.41) | n = 1 (16%) |
In the second phase, 8 experts rated 13 inclusion criteria. Consensus was reached on 11 criteria (Table 1). There was no suggestion to introduce any inclusion criteria or comments.
Third roundIn the last phase, 6 experts rated 2 inclusion criteria, 1 criterion reached consensus, and 1 criterion did not reach consensus (Table 1). At the end, no criteria were excluded (rate average < 2) and 97% (n = 30) of the criteria reached consensus as high-risk criteria in MR in major orthopedic surgery (Table 1).
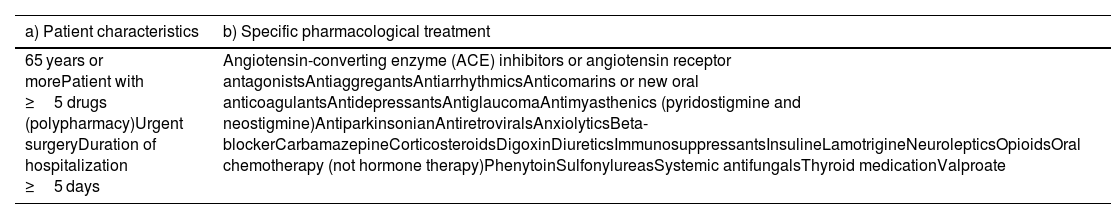
After the development of inclusion criteria, by the multidisciplinary team, and after 3 rounds, consensus was reached on 30 criteria (Table 2).
List of criteria that reached consensus. Created by the authors.
| a) Patient characteristics | b) Specific pharmacological treatment |
|---|---|
| 65 years or morePatient with ≥5 drugs (polypharmacy)Urgent surgeryDuration of hospitalization ≥5 days | Angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor antagonistsAntiaggregantsAntiarrhythmicsAnticomarins or new oral anticoagulantsAntidepressantsAntiglaucomaAntimyasthenics (pyridostigmine and neostigmine)AntiparkinsonianAntiretroviralsAnxiolyticsBeta-blockerCarbamazepineCorticosteroidsDigoxinDiureticsImmunosuppressantsInsulineLamotrigineNeurolepticsOpioidsOral chemotherapy (not hormone therapy)PhenytoinSulfonylureasSystemic antifungalsThyroid medicationValproate |
Several studies show the relevance of therapeutic reconciliation in the transition of care, as well as the pharmacist's role. This process is, for example, part of the Medication Management and Use Standard Expectation by Joint Commission International.12 This activity is time-consuming, so it is necessary to define high-risk criteria. The Ordem dos Farmacêuticos guideline mentioned before lists some of the criteria for selected patients, but this list is quite extensive, so they recommended that the pharmaceutical intervention prioritize certain ones with previously defined criteria.4 Patients undergoing major orthopedic surgeries represent a particularly high-risk group, not only due to patients characteristics (age, comorbidities, and polymedication), but also because orthopedist surgeon physicians and anesthesiologists have a greater focus on therapies directly related to the surgical procedure.13–16 For this reason, the development of a consensus regarding high-risk criteria in therapeutic reconciliation makes it possible to optimize resources and direct available resources in these patients who truly benefit from pharmaceutical care.
Our study combined the development of inclusion criteria by the multidisciplinary group, and its validation according to the Delphi method, that is a flexible research technique well suited when there is incomplete knowledge about a phenomenon.10 It should be noted that Delphi method has some advantages when compared to a typical group interaction, namely because it allows anonymity of responses and from each other, reducing the pressure of group conformity. Another advantage is the opportunity to gather information from experts that can be geographically diverse. Disadvantages include loss of experts during rounds, weakening the consensus. Additionally, experts may be hesitant to share views that differ from other experts and may move towards consensus.17
As far as we know, this is one of the first studies to establish consensus regarding high-risk criteria for MR. Consensus was reached on 30 criteria: polypharmacy (defined as 5 or more medications daily),18 anticoagulants, oral chemotherapy (not hormone therapy), immunosuppressants, antiretrovirals, antimyasthenics (pyridostigmine and neostigmine), insulin, corticoids, neuroleptics, antiarrhythmics, digoxin, carbamazepine, phenytoin, valproate, thyroid drugs, antiglaucoma therapy, antiaggregants, urgent surgery, systemic antifungals, opioids, antiparkinsonics, beta-blockers, age ≥ 65 years, length of stay ≥5 days, lamotrigine, diuretics, antidepressants, ACE inhibitors, sulfonylureas, and anxiolytics. At the end, 1 criterion did not reach consensus: calcium channel blockers. Regarding calcium channel blockers, there is lack of controlled evidence, so no recommendations can be made either for or against the use of these drugs. Therapy should be continued or restarted based on the same criteria that would apply in the non-operative setting.19
Some included criteria deserve further discussion, like anxiolytics, namely benzodiazepines since they are the most commonly prescribed drugs in this class, we found in the published literature either indication to maintain or withdrawal in the perioperative period, due to the increased risk of serious withdrawal symptoms versus the risk of adverse effects.20
With the criteria that did not reach consensus, we proposed to hold a focus group to determine the reasons for not having consensus and decide whether to include it as a high-risk criterion. However, using modified Delphi method, it is important to know that this active participation of the steering group can cause bias through opinion of members.9 It should also be noted that the criterion of length of stay ≥5 days required clarification in the second round, at the request of an expert, as he did not understand the objective of including the length of stay in the definition of criteria, and it was explained that is related to an increased risk of developing complications.
With this work, in addition to having established a consensus regarding the high-risk criteria in therapeutic reconciliation, it was possible to develop an informatic alert system, based on these criteria, which will help us in the management of patients who require more attentive therapeutic reconciliation.
It is also important that, despite being an innovative study, there are methodological limitations, namely the small sample of experts who answered the questionnaire and, consequently, the relatively large attrition rate throughout the rounds, which may impact the final results. This limitation was acknowledged from the outset, and justifies the use of a flexible methodology for consensus, as explained in the methods.
In conclusion, we developed and validated a list of 30 criteria to identify patients at high risk of experience medication-related adverse events undergoing major orthopedic surgery. These criteria may help improve human resource management for clinical pharmacy activities by prioritizing patients who would benefit most from MR. This methodology could be replicated in other clinical areas.
Contributions to scientific literatureMedication reconciliation is relevant in the transitional care. As hospital pharmacists, we would like to go further in our ability to optimize the quality of the pharmacotherapy our patients receive, but, given the limited resources, it is necessary to identify those who could benefit most from this activity. This publication is of special interest because it is the first, as far as we know, to provide a list of criteria to prioritize patients at high risk of experience medication-related adverse events.
These criteria may help to improve human resource management for clinical pharmacy activities by prioritizing patients who would benefit most from medication reconciliation.
FundingNo funding.
CRediT authorship contribution statementMafalda Cavalheiro: Writing – review & editing, Writing – original draft, Visualization, Validation, Formal analysis. Jesús Cotrina-Luque: Validation, Supervision, Methodology, Investigation, Conceptualization. Gonçalo Duarte: Methodology, Investigation, Conceptualization. Patricia Silva: Writing – review & editing, Writing – original draft. Cátia Pereira: Supervision. Miriam Capoulas: Validation. Cláudia Santos: Validation.
This word has been presented previously at International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Congress in 2023.
We thank Professor Dr. Helder Mota Filipe (Faculty of Pharmacy of Universidade de Lisboa), Professor Dr. Fátima Falcão (Faculty of Pharmacy of Universidade de Lisboa), Professor Dr. Daniel Caldeira (Faculty of Medicine of Universidade de Lisboa), Professor Dr. João Costa (Faculty of Medicine of Universidade de Lisboa), Professor Dr. Ricardo Pereira (Faculty of Medicine of Universidade de Lisboa), Professor Dr. Isabel Victoria Pereira (Faculty of Pharmacy of Universidade de Coimbra), Professor Dr. Marta Lavrador (Faculty of Pharmacy of Universidade de Coimbra), Professor Dr. Maria Margarida Castel-Branco (Faculty of Pharmacy of Universidade de Coimbra), Professor Dr. Paula Fresco (Faculty of Pharmacy of Universidade do Porto), Professor Dr. Fernando Fernandez-Llimos (Faculty of Pharmacy of Universidade do Porto), for accepting the invitation to participate as experts in the Delphi Panel.