Optimization of a topical formula of N-acetylcystelne and urea for the topical treatment of ichthyosis.
MethodWe reviewed the chemical structure of the N-acetylcysteine molecule and its metabolic processes. A search was conducted of possible alternative molecules with a chemical structure similar to that of N-acetyl- cysteine that could have improved organoleptic properties. The following databases were used: PubChem®, Botplus®, the Drug Information Centre of the Spanish Agency of Medicines and Medical Devices. The molecule selection criteria were as follows: structural similarity, same therapeutic group, same mechanism of action, same authorized indication, absence of unpleasant smell, and being marketed as raw material in Spain. To complete the pharmaceutical development and validation of the compound, several tests and controls were conducted following the emulsion production procedure of the National Formulary. In order to establish the validity period, we followed the recommendations of the “Guide to Good Drug Preparation Practices in Hospital Pharmacy Services”.
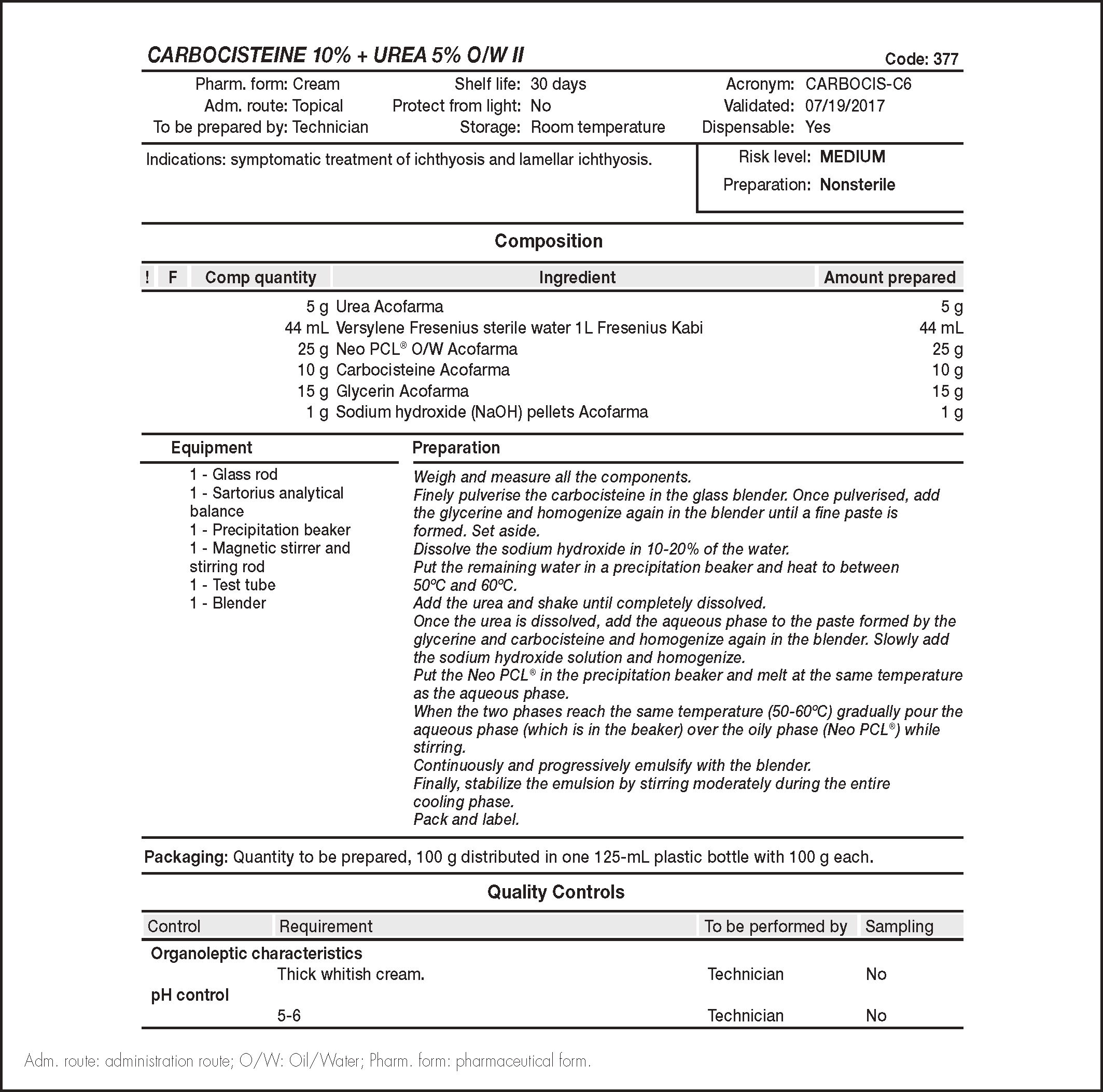
ResultsN-acetylcysteine has a free sulfhydryl group, which is responsible for its smell, and undergoes deacetylation. Its main metabolites are cystine and cysteamine. The following molecules were assessed: cystine, cyst eamine, carbocisteine, cysteine and methionine. Carbocisteine was selected because it met all the selection criteria. Carbocisteine is practically insoluble in water and soluble in mineral acids and alkaline hydroxides solutions. Unlike N-acetylcysteine, it does not have a fetid smell. It reaches its maximum stability at pH 5.5 to 7.5. The composition of the compound (100 g) was as follows: carbocisteine (10 g), urea (5 g), glycerine (15 g), water (44 mL), sodium hydroxide (1 g), and Neo PCL® Oil/Water (O/W) (25 g). It has an expiration period of 30 days. The organoleptic characteristics, emulsion type, and pH remained stable within the established expiration period. The carbocisteine compound has been incorporated into the group of topical treatments available for the treatment of patients with ichthyosis in our hospital.
ConclusionsThe carbocisteine molecule is a good therapeutic alternative that lacks the unpleasant smell of N-acetylcysteine. The carbocisteine compound developed has been included as topical treatment for ichthyosis due to its tolerability, acceptability, and effectiveness in the treatment of patients affected by this genodermatosis.
Optimización de una fórmula magistral tópica de N-acetilcis-teína y urea para el tratamiento tópico de la ictiosis.
MétodoSe revisó la estructura química de la molécula de N-acetilcis-teína y sus procesos metabólicos. Se realizó una búsqueda de posibles moléculas alternativas con una estructura química similar a la N-acetilcis-teína que pudiesen mejorar sus propiedades organolépticas. Bases de datos: PubChem®, Botplus®, Centro de Información de Medicamentos de la Agencia Española de Medicamentos y Productos Sanitarios. Criterios de selección de la molécula: similitud estructural, mismo grupo terapéutico, mismo mecanismo de acción, misma indicación autorizada, ausencia de olor desagradable y estar comercializada como materia prima en España. Para el desarrollo galénico y validación de la fórmula se realizaron varios ensayos y controles siguiendo el procedimiento de elaboración de emulsiones del Formulario Nacional. Para establecer el periodo de validez se siguieron las recomendaciones de la “Guía de buenas prácticas de preparación de medicamentos en los servicios de farmacia hospitalaria”.
ResultadosLa N-acetilcisteína presenta grupo sulfhidrilo libre, responsable del olor, sufre desacetilación y sus principales metabolitos son cistina y cisteamina. Las moléculas evaluadas fueron: cistina, cisteamina, carbocisteína, cisteína y metionina. Se seleccionó la carbocisteína por cumplir todos los criterios de selección. La carbocisteína es prácticamente insoluble en agua y soluble en disoluciones de ácidos minerales e hidróxi- dos alcalinos. A diferencia de la N-acetilcisteína, carece de olor fétido. Presenta su máxima estabilidad a pH 5,5-7,5. La composición de la fórmula magistral (100 g): carbocisteína (10 g), urea (5 g), glicerina (15 g), agua (44 ml), hidróxido sódico (1 g) y Neo PCL® Oil/Water (O/W) (25 g). Periodo de caducidad: 30 días. Los caracteres organolépticos, signo de la emulsión y pH permanecieron estables durante el periodo de caducidad establecido. La fórmula magistral de carbocisteína elaborada se ha incorporado al arsenal de tratamientos tópicos disponibles para los pacientes con ictiosis de nuestro centro.
ConclusionesLa molécula de carbocisteína resultó ser una buena alternativa terapéutica que subsana el olor desagradable de la N-acetil- cisteína. La fórmula magistral de carbocisteína desarrollada fue incluida como tratamiento tópico de la ictiosis gracias a su tolerabilidad, aceptabilidad y efectividad en el tratamiento de pacientes afectos de esta genodermatosis.
Congenital ichthyoses comprise a heterogeneous group of genoder-matoses characterized by keratinization defects leading to dry skin, desquamation, hyperkeratosis, and erythema. They often also involve itching, abnormal sweating and thermoregulation, and heat intolerance1–3.
Most congenital ichthyoses are included among the rare diseases. Their prevalence has been estimated at approximately 1/100,000 to 1/1,000,000,000 individuals1–3.
The common clinical feature of hereditary ichthyosis is skin peeling, usually generalized, which is present from birth or from the first months of life. The disease has a strong impact on quality of life, due to changes in physical appearance and associated symptoms1–3.
There is currently no curative treatment for this disease, and so the therapeutic objective is symptomatic control. It is mainly managed by hydration and keratolysis of the affected areas. For this purpose, emollients and lubricants are used, such as urea, glycerol, kerosene, or propylene glycol, as well as topical keratolytics, such as lactic acid, ammonium lactate, salicylic acid, and N-acetylcysteine (NAC). In the most severe forms, oral retinoids are used1–5.
Treatment should be optimized and individualized in each case, because of great variability in skin tolerance to different topical products and in the response to each therapy. Short- and long-term side effects also guide the choice of the different therapeutic alternatives. In addition, patients with ichthyosis have reduced skin barrier function with increased transepidermal water loss. Such increased skin permeability may favour intoxication secondary to the absorption of topically applied substances6,7.
Urea is a small organic molecule that retains water in the stratum corneum, reduces epidermal proliferation, has regenerative and antimicrobial effects, and facilitates the penetration of other active ingredients. It acts as a moisturizer at concentrations of less than 5% and has keratolytic action at higher concentrations1.
N-acetylcysteine is a hypoallergenic and non-toxic amino acid derivative with an anti-proliferative effect on keratinocytes1.
Although current data are based on case series or isolated cases, formulations that combine NAC 10% and urea 5% have been shown to be effective, safe, and well tolerated in patients with ichthyosis, who show a marked response after several weeks of use1.
The magistral formula (MF) of NAC and urea has the disadvantage of an unpleasant sulphurous smell that in routine clinical practice causes a significant lack of adherence and even rejection of treatment. The NAC molecule has a free sulfhydryl (SH) group, which is responsible for this unpleasant smell. This aspect has led to the search for similar molecules and the development of new therapeutic alternatives that minimize this effect and improve treatment adherence.
Objective: Optimization of a MF of NAC and urea with improved organoleptic properties for the topical treatment of ichthyosis.
MethodsFirstly, we used PubChem to review the chemical structure of the NAC molecule and its metabolic processes8, because our aim was to search for an alternative with similar therapeutic effects. Next, we searched for possible alternative molecules with a chemical structure similar to NAC that could improve its organoleptic properties. The following databases were used: PubChem8, BotPlus9, and the Medicines Information Centre of the Spanish Agency of Medicines and Health Products (AEMPS)10.
The molecule for this indication was selected according to the following criteria: having structural similarity, belonging to the same therapeutic group, and having the same mechanism of action, the same authorized indication, and an absence of unpleasant smell. A key requirement was that any potential alternative molecule was available as raw material marketed in Spain by an AEMPS authorized laboratory. We assessed the characteristics of the different molecules using the information in the Summary of Product Characteristics of the laboratory (Acofarma, Spain) that distributes the raw material.
To design the possible formulation, we followed the same criteria that were applied regarding the MF with NAC plus urea. For the pharmaceutical development and validation of the formula, we conducted several tests and controls following the emulsion preparation procedure of the National Formulary (PN/L/002/00)11. To establish the period of validity of the MF, we followed the recommendations of the Guide to Good Drug Preparation Practices in Hospital Pharmacy Services (GBBP) for non-sterile preparations12.
In order to be able to use the new MF, authorization as an “off-label” drug was requested from the Pharmacy and Therapeutics Committee and the management of the hospital, after signed informed consent was given by patients or their legal guardians in the case of minors.
ResultsReview of the N-acetylcysteine moleculeN-acetylcysteine is a white crystalline powder that is readily soluble in water and 96% ethanol. It is a thiol derivative, which is commonly used as a mucolytic, antioxidant, nephroprotectant, and antidote for acetaminophen poisoning8.
In addition to being a keratolytic agent, NAC modulates keratinocyte proliferation and differentiation. When NAC enters cells it is rapidly hydrolysed to cysteine, a precursor of glutathione, which is a well-studied antioxidant that maintains the cellular redox state. By decreasing free radicals and increasing cysteine levels, NAC increases glutathione levels, which inhibit inflammatory factors and promotes epidermal proliferation. Applied topically, NAC prevents irritation caused by radiotherapy and protects against solar erythema2,6,13,14.
N-acetylcysteine is an amino acid derivative with multiple therapeutic uses and rare adverse effects, which include pruritus, irritation, or burning sensations. Its dermal bioavailability is less than 3%, with hepatic metabolism and renal excretion, and so adverse effects such as nausea, vomiting, urticaria, or anaphylactic reactions are more common when NAC is administered orally or intravenously6.
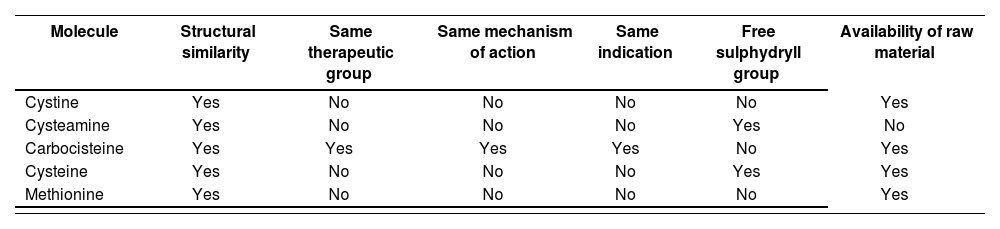
Search for possible alternatives and their characteristicsThe NAC molecule has a free SH group, which is responsible for the foul smell. This group undergoes deacetylation and its main metabolites are cystine and cysteamine. Thus, these two molecules were initially selected as possible alternatives. Carbocisteine is in the same therapeutic group (ATC classification: R05CB) and has a similar chemical structure to NAC9.
In addition, we searched PubChem8 for other different molecules with a similar structure to NAC that could be used as possible therapeutic alternatives. The molecules assessed were cystine, cysteamine, carbocisteine, cysteine, and methionine.
Cystine is a white crystalline powder that is almost insoluble in water and 96% ethanol. It dissolves in dilute solutions of alkaline hydroxides. Cystine is a sulphur-containing aliphatic amino acid biosynthesized from cysteine, and is present in many foods. It is involved in the metabolism of the skin and adnexa, and is a constituent of keratin. It is used as a dietary supplement and in the treatment of congenital homocystinuria. In addition, it is indicated in cases of diffuse alopecia, alopecia areata, acne, seborrheic eczema, nail diseases, psoriasis, and skin disorders with sulphur deficiency15.
Cysteamine is a white powder that is soluble in water and ethanol. It has an unpleasant smell due to the presence of free SH groups. Furthermore, it is not marketed as a raw material in Spain8.
Carbocisteine is a white crystalline powder that is soluble in dilute solutions of mineral acids and alkaline hydroxides, and is almost insoluble in water and 96% ethanol. It is a mucolytic agent that reduces the viscosity of bronchial secretions. Carbocisteine is used in respiratory tract disorders associated with excessive mucus such as bronchiectasis, asthmatic bronchitis, pulmonary emphysema, pneumonia, tuberculosis, cystic fibrosis, and in all conditions in which mucolytics and expectorants are required. It attains maximum stability at pH 5.5 to 7.5. It can sometimes cause nausea, headache, gastric discomfort, diarrhoea, and skin eruptions when administered orally16. Unlike NAC, the carbocisteine molecule lacks a free SH group that cannot be released during the oxidation process. This means that the carbocisteine molecule lacks a foul smell17.
Cysteine is a bright white crystalline powder. Because of its antibiotic properties, it is used in topical preparations for the treatment of various skin conditions. However, due to the presence of the free SH group, it has a certain acidic smell18.
Methionine is an almost white crystalline powder, fairly soluble in water and very slightly soluble in 96% ethanol. It dissolves in dilute acids and in dilute solutions of alkaline hydroxides. Methionine increases glutathione synthesis. It is used as an alternative to NAC in the treatment of paracetamol overdose to prevent liver damage. It also has lipotrophic action and is used as an adjuvant in the treatment of liver diseases, pancreatitis, schizophrenia, and urinary incontinence. It can be administered to treat alopecia and intervenes in keratinization processes. It can cause nausea, vomiting, drowsiness, and irritability when administered orally19.
Selection of the moleculeCysteine and cysteamine, despite being metabolites of NAC, have the free SH group in their molecule, which gives them the same unpleasant smell as NAC. Furthermore, cysteamine is not marketed as a raw material in Spain. For these reasons, they were rejected as therapeutic alternatives in the preparation of the MF (Table 1).
Molecules and selection criteria
| Molecule | Structural similarity | Same therapeutic group | Same mechanism of action | Same indication | Free sulphydryll group | Availability of raw material |
|---|---|---|---|---|---|---|
| Cystine | Yes | No | No | No | No | Yes |
| Cysteamine | Yes | No | No | No | Yes | No |
| Carbocisteine | Yes | Yes | Yes | Yes | No | Yes |
| Cysteine | Yes | No | No | No | Yes | Yes |
| Methionine | Yes | No | No | No | No | Yes |
Among the three remaining molecules, carbocisteine was selected as a possible therapeutic alternative because it met all the selection criteria: structural similarity to NAC, belonging to the same therapeutic group, having the same mechanism of action and same authorized indication as a mucolytic, lacking a free SH group, and being available as a raw material in Spain.
Both cystine and methionine were considered as a second choice in case the MF of carbocisteine could not be developed or was not effective for the indication sought (i.e. a keratolytic in the topical treatment of ichthyosis). However, although raw material is available, there is no marketed drug containing either of these molecules as a monocomponent, which implies a lack of information on their safety (Table 1).
Pharmaceutical development and formulation validationThe raw carbocisteine material was acquired from the Acofarma laboratory, which is authorized by the AEMPS for the distribution of raw materials20.
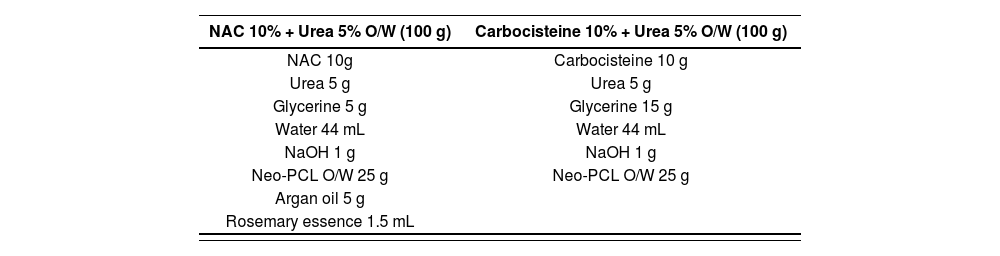
To determine the concentration of carbocisteine to be used in the MF, we took as a reference the concentration of NAC (10%) plus urea (5%). This mixture is synergetic, because urea, which is also a keratolytic agent, offers an optimal pharmacological effect and also seems to provide the most effective transcutaneous penetration of the active ingredients6,21.
We selected the same excipients as those used in the standard NAC MF, due to the good tolerance demonstrated with their use in our hospital22. In addition, by using the same excipients, effectiveness could also be compared by making minimal variations in the MF.
Given the characteristics of the patients and the disease, in which these MFs should be applied over large areas of the body, we kept the nonionic self-emulsifying O/W absorption base. This base makes application easier, achieves better homogenization without residue or an oily sensation, and is easily absorbable and dermatologically innocuous1,5,22.
Glycerine is an osmotic dehydrating agent with hygroscopic and lubricating properties. It also has local and topical anti-inflammatory activity, and is an emollient which protects and softens the skin1,5.
Rosemary essence had already been incorporated into the initial NAC and urea MF to reduce its smell to acceptable levels5. By substituting the NAC for carbocisteine, the sulphurous smell was eliminated and it was no longer necessary to add the rosemary essence.
The first step in the development and preparation of the MF was to try to solubilize the carbocisteine with sodium hydroxide (NaOH) in the water phase. However, this was only achieved when the formula had a pH of at least 8. This pH level was not appropriate because it was outside the range of maximum stability of the active principle (AP), neither was it appropriate for chronic use over the entire body surface.
The next step was to pulverize the powder in a mortar and moisten it with glycerine. A paste was obtained which was added into the cooling phase once the emulsion was created. However, the particle size was inadequate because it was noticeable to the touch after application. To eliminate this effect, the particle size was reduced by pulverizing it using an electric grinder. The resulting powder was then moistened with glycerine and incorporated into the water phase under constant stirring.
We used NaOH to adjust the pH level to between 5 and 6, which is appropriate for application over the entire body surface and is within the maximum stability range of the AP.
Table 2 shows the composition of the original NAC MF and the new carbocisteine and urea MF.
Composition of the N-acetylcysteine magistral formula and the new carbocisteine plus urea magistral formula
| NAC 10% + Urea 5% O/W (100 g) | Carbocisteine 10% + Urea 5% O/W (100 g) |
|---|---|
| NAC 10g | Carbocisteine 10 g |
| Urea 5 g | Urea 5 g |
| Glycerine 5 g | Glycerine 15 g |
| Water 44 mL | Water 44 mL |
| NaOH 1 g | NaOH 1 g |
| Neo-PCL O/W 25 g | Neo-PCL O/W 25 g |
| Argan oil 5 g | |
| Rosemary essence 1.5 mL |
NAC: N-acetylcysteine; NaOH: sodium hydroxide; Neo PCL O/W: nonionic self- emulsifying oil/water absorption base.
We established a validity period of 30 days, which is line with the criteria described in the GBBP's risk matrix for nonsterile and nonoral watercontaining preparations12.
In accordance with the emulsion preparation procedure described in the National Formulary (PN/L/FF/002/00), we conducted the following controls11:
- •
Organoleptic characteristics: white, uniform, homogeneous, and odourless O/W cream over its established shelf life.
- •
Type of the emulsion according to the PN/L/CP/002/00 procedure: stable over its established shelf life.
- •
pH control according to PN/L/CP/001/00 procedure as a O/W emulsion: within the pH of maximum stability of the AP over its shelf life. Table 3 shows the pH values.
Figure 1 shows the standard operating procedure for the new carbocisteine plus urea MF.
Use in clinical practiceInitial authorization was obtained for the carbocisteine-urea MF developed for patients with lamellar ichthyosis who were previously receiving the NAC MF. After obtaining the relevant authorizations, the new MF was applied to small specific areas of the body to assess tolerance, the absence of adverse effects, and effectiveness17.
The carbocisteine and urea MF was incorporated into the arsenal of topical treatments available to treat patients with ichthyosis in our hospital, given that there were no side effects, tolerance was good, effectiveness was at least similar to that of the NAC MF, and there was the added benefit of greater acceptance due to the absence of a bad smell17.
DiscussionIchthyoses comprise a heterogeneous group of genetic diseases characterized by abnormal keratinization of the skin. Early symptoms are characterized by thickening of the stratum corneum, skin peeling, and dry skin. In addition, the skin's ability to perspire may be impaired leading to hyperthermia and even circulatory collapse1.
As these are rare diseases, few high-quality studies have assessed the available treatments. Most of the published literature is based on expert opinion and small case series. The development of new treatment options is hampered by the low number of patients, which makes it difficult to conduct controlled studies and clinical trials23.
The search for molecules with a similar chemical structure is one of the strategies used to develop new drugs or treatments for a given disease that have better efficacy or fewer adverse effects than existing drugs. In our study, this was the fundamental criterion followed in the search for an alternative to NAC with improved organoleptic properties and analogous efficacy.
From the pharmaceutical point of view, a determining factor is the availability of raw material to prepare the formula. It is also relevant that the raw material is marketed as a monocomponent and is authorized for human use — which means that safety data are available— although the administration route may differ. In any case, since the drug is used in the topical treatment of ichthyosis, it would be reasonable to assume that any potential adverse effects would be milder than those derived from its systemic administration.
It has been shown that the NAC 10% plus urea 5% MF is an effective and safe therapeutic alternative in the treatment of ichthyosis within a few weeks of use6. Despite the advantages initially presented by topical treatment with NAC, the foul smell of the emulsion due to the presence of free SH groups makes it difficult for many patients to continue treatment17.
Among all the alternatives studied, carbocisteine has a similar chemical structure to that of NAC, belongs to the same therapeutic group, has the same indications and mechanism of action, is available as raw material in Spain, and does not carry the free SH radical. These aspects led to its selection as an alternative AP to NAC.
The same concentration of carbocisteine was selected as that used in the NAC (10%) MF and it was combined with urea 5%. Several authors have reported that this association not only has a synergistic effect, because urea is another keratolytic agent, but also seems to improve the transcutaneous absorption of NAC6,21. We hypothesised that this effect obtained with NAC would also be obtained with carbocisteine.
The development of the carbocisteine plus urea MF was hampered by its low solubility and high concentration (10%), which necessitated a reduction in particle size to make it easier to spread. Particle size was reduced by pulverising it in an electric grinder.
The key advantage of the new carbocisteine and urea MF was that, unlike NAC, it did not have a foul smell. The replacement of NAC by carbocisteine improved the organoleptic characteristics of the MF, making its use possible as a therapeutic alternative to NAC treatment.
Batalla et al. conducted a study in our hospital of 4 patients diagnosed with ichthyosis who received treatment with a carbocisteine plus urea MF. All but one patient reported that the effectiveness of the carbocisteine and urea MF was at least similar to that of the initial NAC MF. Acceptability of the MF was higher because of the absence of a foul smell. All patients reported good tolerance and an absence of adverse effects17.
However, it was harder to spread the MF. This obstacle was overcome by reducing particle size and moistening with glycerine during the manufacturing process17.
Treatment should be individualized according to the patients’ degree of tolerance to different topical products6,7. If needed, the NAC and urea concentrations can be modified. This possibility is maintained with the new carbocisteine plus urea MF.
The carbocisteine molecule proved to be a good therapeutic alternative that eliminates the foul smell of NAC. The carbocisteine and urea MF developed has been included as a topical treatment for ichthyosis due to its tolerability, acceptability, and effectiveness in the treatment of patients with this genodermatosis.
FundingNo funding.
Conflict of interestsNo conflict of interests.
Contribution to the scientific literature
Congenital ichthyoses are a group of genodermatoses included among the rare diseases. Currently, there is no curative treatment, but the therapeutic objective is to palliate the symptoms. Lubricants, emollients, and keratolytics are used for this purpose. Moreover, because these are rare diseases, few studies have assessed the available treatments. Several case series have shown that N-acetylcysteine and urea formulations are effective; however, their unpleasant smell makes continuity of treatment difficult.
This study presents the development of a new magistral formula for the topical treatment of congenital ichthyosis using an alternative molecule to N-acetylcysteine that lacks an unpleasant smell, meets the requirements established by the Guide to Good Drug Preparation Practices in Hospital Pharmacy Services and the National Formulary, and, in addition to being effective, is accepted and tolerated by patients diagnosed with ichthyosis.
Early Access date (02/15/2022).