Nuevos roles y retos del farmacéutico de hospital. New roles and challenges of the hospital pharmacist
Más datosTo develop by consensus a dashboard model to standardise and promote the evaluation of research activity in Spanish Hospital Pharmacy Services.
MethodsThe study was carried out in 5 phases following the modified Delphi methodology: constitution of the coordinating group, elaboration of a list of scenarios, selection of participating centres, evaluation of the list of scenarios, and analysis of the results.
The coordinating group designed a questionnaire with 114 questions. General research questions and different scenarios (indicators) were included to form the dashboard. The Hospital Pharmacy Services with the highest number of publications were identified to participate in the Delphi consultation. Two rounds of consultations were conducted in which the “Need” and/or “Feasibility” of their measurement was evaluated for each of the scenarios, using a numerical scale from 1 (lowest score) to 9 (highest score).
ResultsSixteen Hospital Pharmacy Services, belonging to 8 different Autonomous Communities, participated in the Delphi consultation. A total of 100% of them responded to all the questions in the 2 rounds of consultations. It was considered that the Hospital Pharmacy Services should have a research dashboard (Need=100%) with a basic structure and a common minimum set of data for all them (Need=87.5%). The consensus was reached on distinguishing research projects led by the Hospital Pharmacy Services from those led by other groups in which the Hospital Pharmacy Services collaborate (Need=87.5%), and a definition was approved on the leadership of these projects according to whether they are single-centre or multicentre.
A consensus was reached on 40 indicators to form the dashboard, which evaluates publications (13 indicators), human resources (12 indicators), research projects (9 indicators), doctoral theses (4 indicators), and patents and intellectual property registrations (2 indicators).
ConclusionsThis is the first consensus dashboard developed to evaluate the research activity of the Hospital Pharmacy Services, which will help to analyse the productivity and impact of research systematically and continuously. In addition, it will allow comparison between them and will help to establish synergies and identify trends, patterns, and challenges.
Desarrollar mediante consenso un modelo de cuadro de mando para estandarizar y promover la evaluación de la actividad investigadora en los Servicios de Farmacia Hospitalaria españoles.
MétodoEl estudio se llevó a cabo en 5 fases siguiendo la metodología Delphi modificada: constitución del grupo coordinador, elaboración de un listado de escenarios, selección de centros participantes, evaluación del listado de escenarios y análisis de los resultados.
El grupo coordinador diseñó un cuestionario con 114 preguntas. Se incluyeron preguntas generales sobre investigación y distintos escenarios (indicadores) para formar el cuadro de mando. Se identificaron los Servicios de Farmacia Hospitalaria con mayor número de publicaciones para participar en la consulta Delphi. Se realizaron 2 rondas de consultas en las que se evaluó para cada uno de los escenarios la «Necesidad» y/o a la «Viabilidad» de su medición, utilizando una escala numérica del 1 (menor puntuación) al 9 (mayor puntuación).
ResultadosParticiparon 16 Servicios de Farmacia Hospitalaria, pertenecientes a 8 Comunidades Autónomas diferentes. El 100% respondieron a todas las preguntas en las 2 rondas. Se consideró que los Servicios de Farmacia Hospitalaria deberían tener un cuadro de mando de investigación (Necesidad = 100%) con una estructura básica y un conjunto mínimo común de datos para todos ellos (Necesidad = 87,5%). Se alcanzó consenso en distinguir los proyectos de investigación liderados por los Servicios de Farmacia Hospitalaria respecto a aquellos liderados por otros grupos en los que los Servicios de Farmacia Hospitalaria colaboran (Necesidad = 87,5%), aprobándose una definición sobre el liderazgo de estos proyectos en función de que sean unicéntricos o multicéntricos.
Se consensuaron 40 indicadores para formar el cuadro de mando, que evalúan publicaciones (13 indicadores), recursos humanos (12 indicadores), proyectos de investigación (9 indicadores), tesis doctorales (4 indicadores) y patentes y registros de propiedad intelectual (2 indicadores).
ConclusionesSe ha desarrollado el primer cuadro de mando consensuado para evaluar la actividad investigadora de los Servicios de Farmacia Hospitalaria, lo que ayudará a analizar la productividad y el impacto de la investigación de forma sistemática y continua. Además, permitirá la comparación entre los mismos y ayudará a establecer sinergias e identificar tendencias, patrones y retos.
Health research is an essential element for the success of any strategy aimed at improving the health of citizens. The integration of research and clinical practice ensures higher quality healthcare, better and faster translation of scientific advances into prevention, diagnosis, and treatment of disease, and more ethical and efficient patient care.1
Although research is a recognised activity for specialist pharmacists, historically, this role has been limited to supporting clinical trials or to individual efforts. It is only in recent years that pharmacists have become principal investigators in high-level research projects or heads of research groups. Thus, research is now a relevant activity in hospital pharmacy services (HPS), competing for resources and, in some cases, integrated with other hospital services with a stronger research tradition. For example, between 2014 and 2018, the number of physicians in HPSs increased by 35%.1
The Spanish Society of Hospital Pharmacists (SEFH) is fully committed to research activities. In fact, one of its 5 strategic lines is to “actively promote the research and innovation activities of hospital pharmacists as scientific healthcare professionals”.2 Currently, most Spanish HPSs have implemented the recommendations of the American College of Clinical Pharmacy regarding its advanced vision of pharmacy-directed research by 2030.3
Articles and guidelines on research in HPSs have focused more on the process itself than on systematic evaluation using objective standardised indicators, which is the approach adopted for HPS healthcare activities, where a focus on objective metrics, such as relative value units (RVUs), is more common.4
Several qualitative and quantitative indicators are available to assess research activity.5 A total of 80 different indicators were identified in a systematic review of methods for assessing the impact of healthcare research.6 However, there is currently no consensus on which indicators to use and how often to measure them. Some countries have developed initiatives to objectively and comparatively assess research quality, such as the Research Excellence Framework (REF) in the UK.7 The REF assesses the quality of research in universities and research centres, with the aim of informing the allocation of funding and promoting excellence in academic research. However, it has not been adapted to the health sector, and therefore excludes HPSs. In Spain, the Instituto de Salud Carlos III has developed a dashboard for monitoring accredited Health Research Institutes.8
The aim of this study was to develop, by consensus, a dashboard model to standardise and promote the evaluation of research activity in Spanish HPSs. Through this process, we developed a consensus model that lists the variables that need to be measured and how often this should be done.
MethodsThis study was conducted according to the modified Delphi method9 and developed in 5 phases: formation of the coordinating group; development of a list of scenarios; selection of participating centres; evaluation of the list of scenarios; and analysis of the results.
Formation of the coordinating groupA coordinating group was formed, comprising 4 pharmacists from the HPSs of 2 university hospitals with extensive research experience. This group was responsible for drawing up the list of scenarios that was presented to the expert panel.
Development of the list of scenariosThis list was developed based on the research dashboards of the HPSs of the coordinating group, as the literature searches in biomedical databases and grey literature failed to locate other dashboard models of HPS research activity.
The list of scenarios included in the expert panel consultation was divided into the following sections:
- −
General questions: 11 questions assessing the need for the proposed dashboard and its characteristics.
- −
Research infrastructure: 4 questions to assess its need.
- −
Human resources: 20 scenarios to assess their need and feasibility.
- −
Resources and results related to research projects: 5 questions to assess their need and 13 scenarios to assess their need and feasibility.
- −
Resources and results related to doctoral theses: 2 questions to assess their need and 6 scenarios to assess their need and feasibility.
- −
Results on patents, utility models, and intellectual property registrations: 3 scenarios to assess their need and feasibility.
- −
Results on publications and communications: 8 questions to assess their need and 29 scenarios to assess their need and feasibility.
- −
Individual and group results: 11 questions to assess their need and 2 scenarios to assess their need and feasibility.
Each of the questions included a space for participants to add any further comments they considered appropriate.
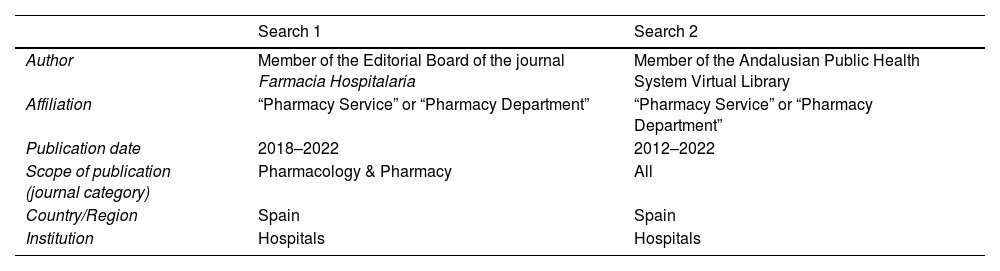
Selecting participants for the expert panelThe expert panel included 1 representative from each of the 12 HPSs with the most publications in journals indexed in the main biomedical databases. These journals were identified by 2 advanced independent Web of Science (Clarivate) searches to increase the sensitivity of the selection process (Table 1). Both searches were conducted by individuals independent of the coordinating group. The first search was limited to journals indexed in the category “Pharmacology & Pharmacy” and published in the last 5 years (2018–2022). The second search was not limited to any field and included those published in the last 10 years (2012–2022).
Searches conducted to select the expert panel.
| Search 1 | Search 2 | |
|---|---|---|
| Author | Member of the Editorial Board of the journal Farmacia Hospitalaria | Member of the Andalusian Public Health System Virtual Library |
| Affiliation | “Pharmacy Service” or “Pharmacy Department” | “Pharmacy Service” or “Pharmacy Department” |
| Publication date | 2018–2022 | 2012–2022 |
| Scope of publication (journal category) | Pharmacology & Pharmacy | All |
| Country/Region | Spain | Spain |
| Institution | Hospitals | Hospitals |
Once the HPSs had been identified, the department directors were contacted to present the project and ask for a member of their team to be included as a panellist. Prior to the evaluation, the list of scenarios (indicators) was sent to the panellists and the coordinating team held a virtual meeting with all of them in order to explain the objective of the study and its method and to resolve any questions.
Evaluation of the list of scenariosThe Delphi method is a widely used group survey technique for reaching consensus. It is conducted in several successive rounds and answered anonymously by a panel of participants with relevant experience.9 In this study, 2 rounds were conducted between June and July 2023. In each round, participants received the list of scenarios by email and were given 1 week to submit their evaluations. During each period, 2 reminders were sent to any non-responders.
The assessment of each scenario was based on the “need” (N) for the criterion to be included in the dashboard and/or the “feasibility” (F) of measuring the criterion (e.g., accessibility of data, time, and resources needed for data collection), using a numerical scale from 1 (lowest score) to 9 (highest score). Consensus was reached on each question when at least 75% of the panellists rated it as “needed” or “feasible” (score 7–9) or “not needed” or “unfeasible” (score 1–3).
In the second round, the experts were asked to re-evaluate the need for and/or the feasibility of the scenarios on which no consensus was reached in the first round. For each scenario, they were given the median and range of all the scores from the first round. This allowed the experts to re-evaluate their scores from the first round.
Analysis of the resultsWe recorded and analysed the following aspects: the composition of the expert panel; the characteristics of the panellists (i.e., gender, age, position within the HPS, and reason for being selected as a panellist); and the characteristics of HPS to which they belonged (autonomous community, reference population of the hospital, number of beds, whether the HPS is established as a research group, whether it has a research dashboard, whether there is a research coordinator, number of people involved in research, and lines of research of the service). These data are expressed as absolute frequencies and percentages, or mean and standard deviation (SD).
In evaluating the list of scenarios, we calculated the median value, the range of scores for each scenario, and the percentage of consensus obtained. Indicators for which there was consensus (≥75%; score 7, 8, or 9) on both need and feasibility were included in the final dashboard. Indicators for which there was consensus to exclude (≥75%, score 1, 2, or 3) and those for which there was no consensus after the second round were excluded from the final dashboard.
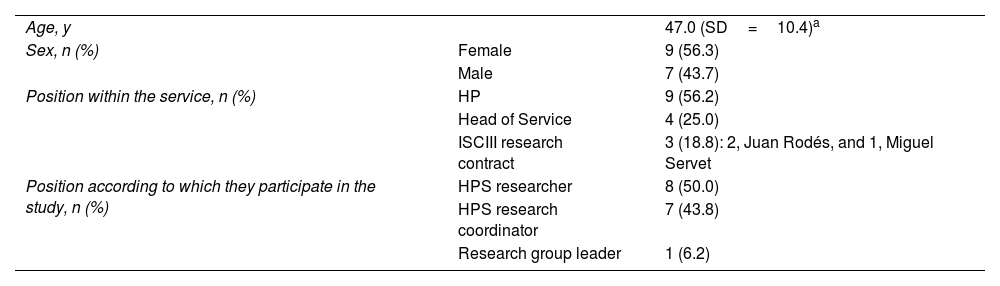
ResultsPanellistsRegarding the 2 searches to identify the 12 HPSs with the highest number of publications, 8 services were identified in both searches and 8 were identified in only 1 of the 2 searches. The coordinating group included these 16 HPSs, which were from 8 different autonomous communities (Andalusia, Aragon, Castile and Leon, Catalonia, Community of Madrid, Community of Valencia, Galicia, and the Balearic Islands). These HPSs are listed in alphabetical order as follows: Complejo Hospitalario Universitario A Coruña, Complejo Hospitalario Universitario de Santiago, Hospital Clinic de Barcelona, Hospital de la Santa Creu i Sant Pau, Hospital Universitario de Salamanca, Hospital del Mar, Hospital General Universitario Gregorio Marañón, Hospital Universitari Son Espases, Hospital Universitari Vall d'Hebron, Hospital Universitario 12 de Octubre, Hospital Universitario de Bellvitge, Hospital Universitario Miguel Servet, Hospital Universitario La Paz, Hospital Universitario Ramón y Cajal, Hospital Universitario Virgen del Rocío, and Hospital Universitario y Politécnico La Fe. Tables 2 and 3 describe the main characteristics of the panellists and the HPSs, respectively.
Characteristics of the panellists participating in the Delphi consultation process.
| Age, y | 47.0 (SD=10.4)a | |
| Sex, n (%) | Female | 9 (56.3) |
| Male | 7 (43.7) | |
| Position within the service, n (%) | HP | 9 (56.2) |
| Head of Service | 4 (25.0) | |
| ISCIII research contract | 3 (18.8): 2, Juan Rodés, and 1, Miguel Servet | |
| Position according to which they participate in the study, n (%) | HPS researcher | 8 (50.0) |
| HPS research coordinator | 7 (43.8) | |
| Research group leader | 1 (6.2) |
HP, hospital pharmacist; ISCIII, Instituto de Salud Carlos III; HPS, hospital pharmacy service.
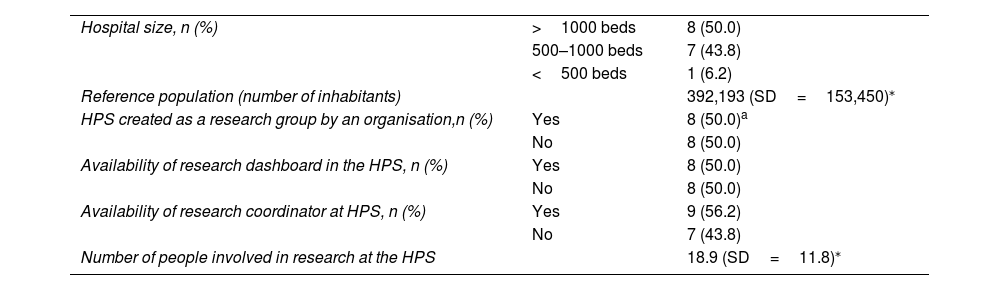
Characteristics of the pharmacy services participating in the Delphi consultation process.
| Hospital size, n (%) | >1000 beds | 8 (50.0) |
| 500–1000 beds | 7 (43.8) | |
| <500 beds | 1 (6.2) | |
| Reference population (number of inhabitants) | 392,193 (SD=153,450)⁎ | |
| HPS created as a research group by an organisation,n (%) | Yes | 8 (50.0)a |
| No | 8 (50.0) | |
| Availability of research dashboard in the HPS, n (%) | Yes | 8 (50.0) |
| No | 8 (50.0) | |
| Availability of research coordinator at HPS, n (%) | Yes | 9 (56.2) |
| No | 7 (43.8) | |
| Number of people involved in research at the HPS | 18.9 (SD=11.8)⁎ | |
HPS, hospital pharmacy service.
In total, 91.0% of the surveyed HPSs had defined lines of research (lines per service: mean 4.1; SD=1.7). Of the 14 HPSs with research lines, 9 had 4 or fewer lines and only 1 had 2 or fewer. The main research lines were as follows: oncohaematology, pharmacogenetics, pharmacokinetics, infectious diseases, nutrition, and safety in the use of medications.
Delphi consultation processIn the 2 consultation rounds, all selected panellists (100%; 16/16) answered all questions. Of the 114 questions, consensus was reached on 53 (46.5%) in the first round; therefore, 61 (53.5%) questions were resubmitted for a second round. Final consensus was reached on 71 (62.3%) questions and a dashboard with 40 indicators was constructed (Table S1; supplementary material). The results of the consultation on the list of scenarios can be found in Table S2 in the supplementary material. The results of the 2 consultation rounds are described below.
General questionsConsensus was reached on 9 of the 11 questions. The panellists agreed that all HPSs should have a research dashboard (N=100%) with a basic structure and a common minimum dataset (N=87.5%); that this information should be updated regularly (N=87.5%) on an annual basis (N=87.5%); that users should be able to explore, analyse, and extract valuable information from all the variables incorporated in the system (N=81.3%); and that this research dashboard should be promoted and maintained over time by the SEFH (N=75%).
Research infrastructureConsensus was reached on 2 of the 4 questions. The panellists also agreed that the dashboard should include outcome indicators (N=100%) and infrastructure indicators (N=81.3%). However, consensus was not reached on what should be the focus of assessment within infrastructures.
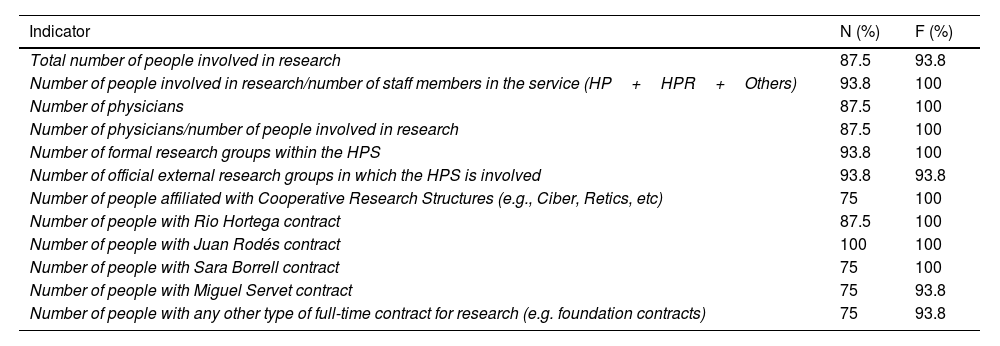
Human resourcesConsensus was reached on 12 of the 20 questions. Table 4 shows the human resources indicators for which consensus was reached regarding their inclusion in the dashboard.
Human resources indicators for which consensus was reached.
| Indicator | N (%) | F (%) |
|---|---|---|
| Total number of people involved in research | 87.5 | 93.8 |
| Number of people involved in research/number of staff members in the service (HP+HPR+Others) | 93.8 | 100 |
| Number of physicians | 87.5 | 100 |
| Number of physicians/number of people involved in research | 87.5 | 100 |
| Number of formal research groups within the HPS | 93.8 | 100 |
| Number of official external research groups in which the HPS is involved | 93.8 | 93.8 |
| Number of people affiliated with Cooperative Research Structures (e.g., Ciber, Retics, etc) | 75 | 100 |
| Number of people with Rio Hortega contract | 87.5 | 100 |
| Number of people with Juan Rodés contract | 100 | 100 |
| Number of people with Sara Borrell contract | 75 | 100 |
| Number of people with Miguel Servet contract | 75 | 93.8 |
| Number of people with any other type of full-time contract for research (e.g. foundation contracts) | 75 | 93.8 |
N, need; F, feasibility; HP, hospital pharmacist; HPR, hospital pharmacy resident; HPS, hospital pharmacy service.
Data are expressed as the percentage of consensus reached (score 7, 8, or 9).
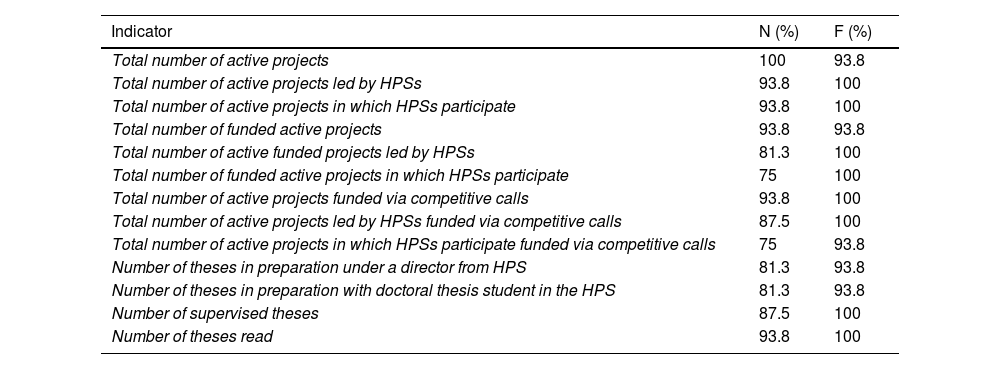
Agreement was reached on the following points: research projects and doctoral theses would be treated as research outputs rather than resources (N=81.3% and N=93.8%, respectively); HPS-led projects and projects led by other services in which HPSs participate should be treated differently (N=87.5%), using a strict definition of projects that are led (or not) by HPSs according to whether they are single-centre or multi-centre projects (Table S2; supplementary material). Consensus was reached on 13 of the 19 indicators for inclusion in the dashboard (Table 5).
Research projects and doctoral theses indicators for which consensus was reached.
| Indicator | N (%) | F (%) |
|---|---|---|
| Total number of active projects | 100 | 93.8 |
| Total number of active projects led by HPSs | 93.8 | 100 |
| Total number of active projects in which HPSs participate | 93.8 | 100 |
| Total number of funded active projects | 93.8 | 93.8 |
| Total number of active funded projects led by HPSs | 81.3 | 100 |
| Total number of funded active projects in which HPSs participate | 75 | 100 |
| Total number of active projects funded via competitive calls | 93.8 | 100 |
| Total number of active projects led by HPSs funded via competitive calls | 87.5 | 100 |
| Total number of active projects in which HPSs participate funded via competitive calls | 75 | 93.8 |
| Number of theses in preparation under a director from HPS | 81.3 | 93.8 |
| Number of theses in preparation with doctoral thesis student in the HPS | 81.3 | 93.8 |
| Number of supervised theses | 87.5 | 100 |
| Number of theses read | 93.8 | 100 |
N, need; F, feasibility; HPS, hospital pharmacy service.
Data are expressed as the percentage of consensus reached (score 7, 8, or 9).
Consensus was reached on the inclusion of the number of patents (N=87.5%, F=87.5%) and intellectual property registrations (N=75%, F=93.8%) in the dashboard, but not on utility models (N=62.5%, F=75%).
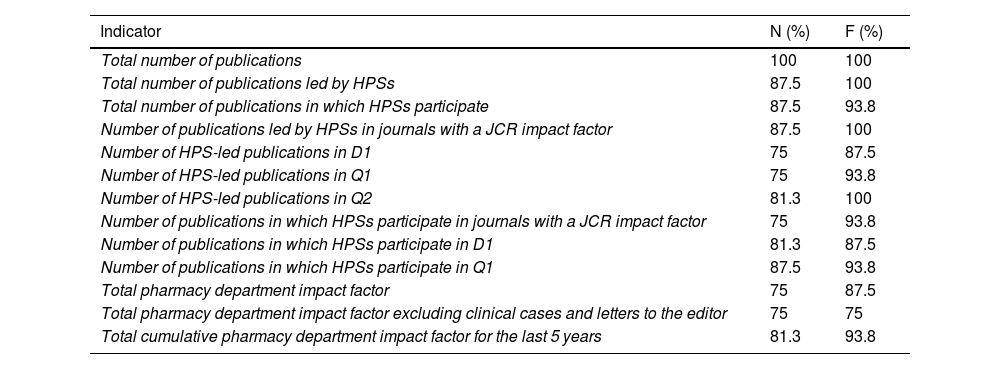
Results on publications and communicationsThe panellists agreed that the dashboard should include publications that have an assigned number and volume in the journal as well as those available online (online ahead of print) (N=75%), and HPS-led publications should be considered differently from those led by other services in which HPSs participate (N=75%). However, consensus was not reached on a definition that would distinguish between the two types of publication (supplementary material; Table S2). If the online publication year of the papers did not match the physical journal publication year, the panel used the JCR impact factor of the year corresponding to the physical journal publication (N=100%).
Consensus was reached on 13 of the 24 indicators related to publications for their inclusion in the dashboard (Table 6).
Publications indicators for which consensus was reached.
| Indicator | N (%) | F (%) |
|---|---|---|
| Total number of publications | 100 | 100 |
| Total number of publications led by HPSs | 87.5 | 100 |
| Total number of publications in which HPSs participate | 87.5 | 93.8 |
| Number of publications led by HPSs in journals with a JCR impact factor | 87.5 | 100 |
| Number of HPS-led publications in D1 | 75 | 87.5 |
| Number of HPS-led publications in Q1 | 75 | 93.8 |
| Number of HPS-led publications in Q2 | 81.3 | 100 |
| Number of publications in which HPSs participate in journals with a JCR impact factor | 75 | 93.8 |
| Number of publications in which HPSs participate in D1 | 81.3 | 87.5 |
| Number of publications in which HPSs participate in Q1 | 87.5 | 93.8 |
| Total pharmacy department impact factor | 75 | 87.5 |
| Total pharmacy department impact factor excluding clinical cases and letters to the editor | 75 | 75 |
| Total cumulative pharmacy department impact factor for the last 5 years | 81.3 | 93.8 |
N, need; F, feasibility; D1, first decile; Q1, first quartile; Q2, second quartile.
Data are expressed as the percentage of consensus reached (score 7, 8, or 9).
No consensus was reached on the need to include communications in the dashboard, nor on any of the 5 indicators proposed to assess them.
Individual and group resultsConsensus was reached (N=75%) that results stratified by research group should not be collected if there is more than one group in the HPS. No consensus was reached on the proposed indicators for assessing the results of research groups and those of individual researchers.
DiscussionThis study defined a dashboard for assessing research activity in HPSs, making it possible to define objectives, set challenges, and improve the impact of HPSs in generating knowledge. Firstly, the 16 participants in the Delphi consultation process—all from the Spanish HPSs with the highest number of publications—agreed that HPSs should have available a research dashboard with a basic structure and a common minimum dataset. A total of 40 indicators were identified to evaluate the research activity of the HPSs, and consensus (≥75%) was reached on their need and feasibility of measurement. These indicators assess publications (13 indicators), human resources (12 indicators), research projects (9 indicators), doctoral theses (4 indicators), and patents and intellectual property registrations (2 indicators). To date, this is the first published research evaluation dashboard adapted to the reality of Spanish HPSs.
It should be noted that no consensus was reached on the need to evaluate communications at conferences. Currently, they have little curricular value in the academic and professional sphere. Therefore, research groups should consider such communications as a way of making preliminary or partial results visible. The final availability of such communications to the scientific community should be in the form of scientific articles in biomedical journals. This perspective, which is quite widespread, does not yet seem to have been incorporated into the research culture of Spanish HPSs.
One of the main achievements of this study is to have reached consensus on distinguishing HPS-led research projects from those led by other groups in which HPSs participate and to have approved a definition of this criterion according to whether the projects are single-centre or multicentre. From now on, it will be possible to compare both types of project conducted in Spanish HPSs using a common framework. Unfortunately, consensus was not reached on the issue of publications. The panel were inconsistent in that they agreed that HPS-led publications should be distinguished from those led by other groups in which HPSs participate, but failed to reach consensus on a definition of these aspects. Future work will be needed to standardise the quantitative figures that may eventually be collected in Spain on the number of articles led by the 2 types of group.
A consensus was reached on excluding from the dashboard results stratified by individual researcher or by research group, if there is more than one group in the HPS. This aspect is reasonable in reference to publicly shared dashboards or if results are integrated into the dashboard system of a scientific society. However, in reference to the HPS internal dashboards, we suggest that it could be useful to replicate all the approved indicators for each research group in the HPSs, so that their performance can be assessed comparatively. Similarly, individual indicators, such as the h-index, have a strategic function in the internal dashboards of HPSs to measure researcher performance and growth. This information can be used to customise recognition and incentive programs to acknowledge and motivate researchers at the organisational level.
No consensus was reached on how to measure research infrastructure, as there are no accepted standards and it is difficult to assess in hospital departments or units.
A dashboard enhances efficiency in managing research, enables effective monitoring of the results, and supports strategic reflection on the role of each HPS in research activity. Previous studies have focussed on the definition or analysis of the best indicators to be included in such dashboards. Research Centers in Minority Insitutions funded by the National Institute of Health (NIH) worked on defining a global dashboard for all centres included.10 Indicators were established based on 4 main targets: increasing scientific productivity, fostering scientific collaborations, promoting professional growth, and expanding research resources. On the other hand, a systematic review found that the most common research performance indicators in 50 studies were as follows: number of publications (n=38), number of citations (n=27), impact factor (n=15), research funding (n=10), degree of co-authorship (n=9), and h-index (n=5).5 Overall, this study found that there was limited research on feasibility, validity, reliability, and acceptability. In 2017, another systematic review on assessing the impact of health research identified 5 broad categories: (1) direct research-related impact; (2) influence on policy-making; (3) health and health systems impact; (4) health-realted and societal impact; and (5) broader economic impact.6 The first category was the most common scenario and addresses the assessment of new knowledge generation and dissemination, capacity building, training, and leadership, and the development of research networks. Most of the indicators included in our dashboard are from the first category (i.e., direct research-related impact), such as the number of publications, impact factor, research projects, and so on. This result may be due to the fact that these indicators are well established, objective, relatively easy to obtain, and are in line with the scales used by most research funding agencies and institutions. The availability of such a dashboard is the first step in further exploring research results in a more holistic way, thereby facilitating data-driven and evidence-based decision-making.
However, as the scientific landscape evolves, new forms of evaluation are emerging. Any such dashboard should therefore be dynamic and be able to adapt to new evidence and trends in research assessment. There is ongoing debate on identifying indicators that better reflect how research results can change or improve clinical practice and their real impact on society, as traditional metrics are geared toward measuring the dissemination of knowledge in the scientific world rather than its real-world impact.11 It is important to understand the advantages and disadvantages of these indicators and to consider other qualitative and contextual factors when evaluating scientific research.12 The Leiden Manifesto and the San Francisco Declaration are 2 initiatives that seek to improve the assessment of the quality and impact of scientific results.12–14 The Leiden Manifesto emphasises the need for a more balanced and accountable evaluation that goes beyond traditional metrics. It aims to avoid an over-reliance on quantitative indicators and to encourage a more comprehensive and contextualised evaluation of research.12 The San Francisco Declaration, also known as the Declaration on Research Assessment (DORA), challenges the overuse of the impact factor and promotes a more holistic assessment that emphasises the quality, originality, and real impact of research, rather than relying solely on bibliometric indicators.13,14 Large investments in research have sparked debate on the importance of incentivising researchers and organisations to conduct responsible research15 that has a tangible impact on society, provides a return on investment, and saves costs.16
The development of dashboards to assess responsible research is essential to promote change at the institutional level. The European Commission's report Next-Generation Metrics: Responsible Metrics and Evaluation for Open Science provides a detailed analysis of alternative metrics for research evaluation and advocates for a responsible and equitable approach to the development and implementation of next-generation metrics.17 The proposed framework highlights plurality of metrics, contextualisation, transparency, qualitative assessment, and equity as key principles for improving the evaluation of open science. The report stresses that the European Commission should encourage academic publishers across Europe to reduce the importance of journal impact factors as a promotional tool and to use them only in the context of a variety of metrics that provide a more complete picture. However, methodological and temporal difficulties in assessing the real impact of research mean that traditional indicators are still the most widely used metrics for assessing research performance and securing funding.10 In the field of hospital pharmacy, the Granada Declaration on improving the quality of publications and advancing research paradigms in clinical and social pharmacy practice recommends that decision-makers should consider broader bases and not just journal-based metrics for assessing quality and achievements in the disciplines.18
On the other hand, the collation of the main lines of research in HPSs shows a notable degree of homogeneity in the fields where research is being conducted, which is expected to facilitate the creation of synergies between different services. Pharmacists involved in networks and/or collaborative research groups have a greater chance of producing more impactful research than those working individually. In 2020 and 2021, the 3 most common areas addressed in the articles published in Farmacia Hospitalaria were as follows: management and organisation of pharmacy services (n=28), pharmacogenetics and pharmacogenomics (n=18), and oncology (n=18).19 Nevertheless, it should be noted that during this period, there were 2 special issues on COVID-19 crisis management and on personalised medicine.
The main limitation of this study is that the creation of the dashboard was based on expert opinion, as no previously published evidence on the composition of HPS dashboards was found. In addition, a repository designed for archiving research activity might be misconstrued as a system fostering undue competition. There is also a risk of that the ultimate goal would be to achieve high scores on the dashboard metrics, rather than to pursue research with a real impact on the health system and society. Finally, the dashboard needs to be dynamic and able to adapt to new forms of evaluation, especially by incorporating a qualitative assessment of research activity.
In conclusion, this study has developed by consensus the first dashboard model for assessing HPS research activity. This dashboard will facilitate the systematic and continuous analysis of productivity and research impact in this setting. It can also be used to compare different HPSs in Spain, to create synergies, and to identify trends, patterns, and challenges. A further step would be to create a system for collecting and sharing these data among hospitals under the coordination of the SEFH.
FundingNone declared.
Statement of authorshipBernardo Santos and Ana Herranz proposed the study and coordinated its implementation. Bernardo Santos designed the study method. All authors participated in drafting the proposed dashboard scenarios, selecting the experts, and in project presentation meetings. Ana Belén Guisado and Vicente Escudero analysed the data obtained from the Delphi consultation process. Vicente Escudero wrote the first draft of the manuscript which was reviewed and approved by all authors.
Responsibility and transfer of rightsAll the authors accept the responsibilites defined by the International Committee of Medical Journal Editors (available at: http://www.icmje.org/).
In the event of publication, the authors grant exclusive rights of reproduction, distribution, translation, and public communication (by any means or sound, audiovisual or electronic support) of their work to Farmacia Hospitalaria and, by extension, to the SEFH. To this end, a letter of assignment of rights will be signed at the time of sending the paper through the online manuscript management system.
CRediT authorship contribution statementVicente Escudero-Vilaplana: Data curation, Formal analysis, Investigation, Project administration, Writing – original draft, Writing – review & editing. Ana Belén Guisado-Gil: Data curation, Formal analysis, Investigation, Project administration, Writing – review & editing. Bernardo Santos-Ramos: Conceptualization, Investigation, Methodology, Project administration, Writing – review & editing. Ana Herranz: Conceptualization, Investigation, Methodology, Project administration, Writing – review & editing.
We would like to acknowledge the participation of all the panellists who made this project possible: Laila Abdel-kader Martín (Hospital Universitario Virgen del Rocío), Purificación Cid Silva (Complejo Hospitalario Universitario A Coruña), Eva Delgado Silveira (Hospital Universitario Ramón y Cajal), Fernando Do Pazo (Hospital Universitari Son Espases), Daniel Echeverría Esnal (Hospital del Mar), Anxo Fernández Ferreiro (Complejo Hospitalario Universitario de Santiago), Carmen García Muñoz (Hospital Universitario 12 de Octubre), Vicente Gimeno Ballester (Hospital Universitario Miguel Servet), Luis López Fernández (Hospital General Universitario Gregorio Marañón), Juan Eduardo Megías Vericat (Hospital Universitario y Politécnico La Fe), María José Otero (Hospital Universitario de Salamanca), Nuria Padullés (Hospital Universitario de Bellvitge), María Queralt Gorcas (Hospital Universitari Vall d'Hebron), Pau Riera (Hospital de la Santa Creu i Sant Pau), Dolors Soy (Hospital Clinic de Barcelona), Elena Villamaña Bueno (Hospital Universitario La Paz). We would also like to thank Luis Margusino Framiñán, member of the Editorial Committee of the journal Farmacia Hospitalaria and the Biblioteca Virtual del Sistema Sanitario Público de Andalucía for his help in identifying the pharmacy services with the most publications. Finally, we would like to thank Yolanda Alonso for her help in distributing, collecting, and transcribing the questionnaires.