The main purpose of this study is to evaluate the potential clinical impact of pharmacogenetic testing on the reduction of the toxicity in patients treated with fluoropyrimidines. This will be achieved by comparing the frequency of adverse events and the incidence of toxicity of two groups of patients that will differ from each other only in that one will receive pharmacogenetic counseling. The hypothesis is that availability of a pharmacogenetic report prior to treatment initiation has a positive effect. One of the main secondary goals is to analyze allele frequencies and the association of polymorphisms rs895819 (miR27A) and rs1801160 (DPYD*6) with toxicity by conducting an observational study to determine their clinical relevance and standardize a dose adjustment recommendation.
MethodThe study has an single-center ambispective, quasi-experimental design and is based on a multidisciplinary protocol involving implementation and standardization of DPYD*2A; DPYD*13; c.2846A>T; and HapB3 measurements. Following these measurements, pharmacogenetic counseling will be carried out and its clinical impact will be evaluated. The primary endpoint of the study is severe toxicity and/or mortality. The toxicity observed in two groups with similar epidemiological characteristics will be compared: the intervention group (candidates for treatment with fluoropyrimidines who will be subjected to the protocol) and the control group (retrospective cohort). Additionally, rs895819 (MIR27A) and rs1801160 (DPYD*6) will be determined. Testing for these variants is not part of the hospital's daily practice, nor are they included in clinical guidelines. However, according to recently published studies, the activity of dihydropyrimidine dehydrogenase might be affected by these variants, as they may be associated with toxicity. The results of the measurements of these two variants will not be incorporated to pharmacogenetics counseling until their association with toxicity is determined by means of the observational study to be conducted. The project, as well as the patient information sheet and the informed consent form, were approved by the Ethics Committee of the participating center (code 20/006).
El objetivo principal es evaluar el impacto clínico de la implementación de la farmacogenética en la reducción de toxicidad en pacientes tratados con fluoropirimidinas, comparando frecuencia y grado de toxicidad con una población de las mismas características, pero sin orientación farmacogenética, y demostrando que la disponibilidad de un informe farmacogenético previo al inicio del tratamiento tiene influencia positiva. Uno de los principales objetivos secundarios es analizar la frecuencia y la asociación del polimorfismo con toxicidad de rs895819 en MIR27A y DPYD*6, mediante un estudio observacional, para determinar su relevancia clínica y poder estandarizar una recomendación de ajuste de dosis.
MétodoEstudio con diseño ambispectivo, cuasi-experimental, unicén-trico, llevado a cabo mediante un protocolo de actuación multidisciplinar, a través del cual se implantará la determinación de DPYD*2A, DPYD*13, c.2846A>T, HapB3, se estandarizará y se realizará el consejo farmacogenético y posteriormente se evaluará su impacto clínico. La variable principal es la toxicidad severa y/o mortalidad. Se compararán dos grupos con características epidemiológicas similares, grupo intervención (pacientes candidatos a tratamiento con fluoropirimidinas y sobre los que se implantará el protocolo) y grupo control (cohorte retrospectiva). Por otra parte, se determinará rs8958l9 en MIR27A y DPYD*6, estas variantes no forman parte de la práctica diaria del hospital ni están contempladas en guías clínicas, pero según estudios publicados recientemente, pueden afectar a la actividad de la enzima y estar asociados con toxicidad. Los resultados de estas dos variantes no intervendrán en el consejo farmacogenético hasta determinar su asociación con la toxicidad, precisamente mediante el estudio observacional que se llevará a cabo. Tanto el proyecto como la hoja de información al paciente y el consentimiento informado han sido aprobados por el Comité Ético del centro participante, código: 20/006.
Approximately 30% of patients on fluoropyrimidine treatment develop severe [Common Terminology Criteria for Adverse Events (CTCAE) v5.0 grade ≥ 3] and occasionally fatal toxicities1–9.
Dihydropyrimidine dehydrogenase (DPD) is a rate-limiting enzyme in the catabolism of fluoropyrimidines. It has been reported that at least 80% of the administered 5-fluorouracil is metabolized by DPD1–5,8,10,11. Impaired DPD function results in accumulation of the active metabolite, causing severe toxicity and even death1,3,10,12–14.
DPYD is the gene that codes for DPD. Numerous variants have been studied but, according to the clinical guidelines, only four of them are capable of reducing the enzyme's activity in a clinically significant way and can therefore be implemented in clinical practice: c.190511G> A (DPYD * 2A), c.1679T> G (DPYD * 13), c.2846A> T, and c.1129–5923C> G (HapB3)1–3,5,7. Recommendations of clinical guidelines and regulatory agencies are all based on those four variants15.
A certain variability nevertheless exists between DPYD genotype and DPD phenotype. It is therefore necessary to identify new toxicity-related variants, as such variability could be explained by other, less well-studied polymorphisms such as DPYD*67,10,14,16 or by the regulation of DPD at post-transcriptional level. The A>G rs895819 (miR-27A) polymorphism has been associated with a reduction in DPD activity716.
RationaleBefore the start of treatment, it is necessary to determine whether the patient has one the four variants of the DPYD gene and titrate the dose of the drug accordingly as this may reduce the toxicity risk which, as mentioned above, could be fatal1,11.
These determinations are a useful approach only if it can be shown that performance of a pharmacogenetic analysis prior to the initiation of treatment may have a positive impact. The clinical impact of genetic tests should be evaluated against that background.
Moreover, a certain percentage of the toxicity observed cannot be explained by those four variants10,14. For that reason, in the present study we also decided to determine the presence of the DPYD*6 and rs895819 (miR-27A) polymorphisms. Although such determinations are not part of our hospital's routine practice, nor are they recommended by any clinical guidelines, it has been shown by recently published studies that they may affect the activity of DPD and be associated with toxicity. Their inclusion could improve the predictive value of the above-mentioned tests.
Hypothesis and purpose of the studyUse of pre-treatment pharmacogenetic studies based on a consensual multidisciplinary protocol may reduce toxicity in patients treated with fluoropyrimidines.
The overarching goal of this study is to evaluate the impact of pharmacogenetic studies on the reduction of toxicity in patients treated with fluoropyrimidines, comparing the incidence and severity of adverse events with those observed in a similar population that will receive the same treatment but will not be subjected to pharmacogenetic studies. We will also seek to demonstrate that a pre-treatment pharmacogenetic report is beneficial to these patients.
The specific goals of the study will include a description of the methodology, procedures, documentation and materials required to implement pharmacogenetic tests in routine clinical practice; analyze the allele frequency of the DPYD polymorphisms of interest established in the clinical guidelines; conduct an observational analysis of polymorphisms rs895819 (miR-27A) and rs1801160 (DPYD*6); evaluate polymorphic frequencies and polymor-phism-toxicity associations to determine their clinical relevance and establish a standard dose adjustment recommendation based on the results obtained; measure the frequency of pharmacogenetic interventions and their degree of acceptance by oncologists; describe the prevalence of toxicity, classifying adverse events by severity; and evaluate the degree of satisfaction of the oncology department with the pharmacogenetic reports received and with the overall implementation of the program.
MethodsDesignThis will be a single-center ambispective quasi-experimental study intended to demonstrate the benefits of implementing a pharmacogenetic testing protocol coordinated by the pharmacy, medical oncology, and laboratory services of our hospital, supported by an external laboratory. The protocol involves the determination of genetic variants, the standardization and implementation of a genetic counseling program, and the evaluation of the clinical impact of the measures adopted.
Genetic variants to be determined under the protocolBoth testing for the four polymorphisms contemplated in the clinical guidelines (DPYD*2A, DPYD*13, c.2846A>T, and HapB3) and the genetic counseling based on the results of those tests will be incorporated to the hospital's routine practice.
A subsequent observational study will be conducted to analyze the clinical relevance of testing for two additional genetic variants [rs895819 (miR-27A) and rs1801160 (DPYD*6)]. The results of these tests will not be included in the genetic counseling protocol until the above-mentioned study determines the association of those two variants with toxicity.
ScopePatients about to start treatment with fluoropyrimidines, or who started the treatment from 1 January 2019.
VariablesThe main variable will be CTCAE grade ≥ 3 toxicity and/or mortality. Secondary variables will include patient-related sociodemographic aspects; diagnosis; functional status; treatment regimen; patient naivety; polymorphism [only for the treatment group (TG)]; genotype (TG); need for dose to be adjusted and, if so, titration percentage (TG); need to tailor the dose and, if so, percentage (TG); acceptance of recommendation (TG); toxicity, type of toxicity, cycle at which toxicity is detected and severity of toxicity; dose reduction; discontinuation of the drug; admission; death; assessment of the oncology department's satisfaction with the pharmacogenetic data received; and the implementation of the program (through a non-validated survey).
Inclusion and exclusion criteriaThe TG will include adult patients with any type of tumor about to undergo chemotherapy with fluoropyrimidines. The control group will include patients already on chemotherapy but who have not received previous courses of fluoropyrimidines.
Patients excluded from the study will be those for whom there is not enough information available on the study variables to allow proper data collection and subsequent comparisons. Patients unwilling to participate and those incapable of giving their informed consent will also be excluded.
Sample sizeTo calculate the size of the study sample it will be assumed that 30% of patients treated with fluoropyrimidines present with CTCAE grade ≥ 3 toxicity4–6,8.
Based on this hypothesis, and assuming that, following implementation of pharmacogenetic counseling, CTCAE grade ≥ 3 toxicity would decrease by 10 percentage points, i.e. to 20%, the sample must include a total of 324 subjects.
Study groupsPatients will be distributed into two groups of similar epidemiological characteristics, a TG and a control group (CG). Each group will be assigned 162 subjects.
The TG will comprise patients about to undergo treatment with fluoropyrimidines (single-agent or combination regimen), who will also be subjected to a pharmacogenetic protocol. Patients will be sequentially recruited until the total sample size is achieved. The pharmacogenetic intervention will consist in extraction of a blood sample to find out whether subjects carried the mutation of interest or not and preparation of a pharmacogenetic report with the results and of the test and some dosing recommendations.
The CG will be formed retrospectively, following a review of the medical records of patients who have received at least one cycle of fluoropyrimidines before the pharmacogenetics protocol becomes available. All the patients started on fluoropyrimidines from 1 January 2019 will be sequentially included.
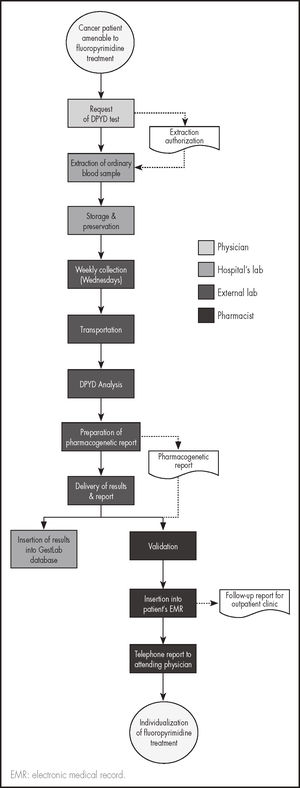
Genetic testingGenetic tests will be carried out before initiation of chemotherapy so as to be able to individualize the patients’ treatment according to the result obtained. Blood samples will be extracted on working days and will remain in the hospital's laboratory until they are analyzed. Once a week they will be sent to the external laboratory in charge of the analysis. The process is described in figure 1.
Patients will be recruited in the order in which they report to their oncology appointment. Once treatment with fluoropyrimidines has been decided, the oncologist will request the DPYD test by selecting the DPYD testing option on the hospital computer system. Given that patients often require an ordinary blood test before starting chemotherapy, that same blood extraction will be used for the genetic exam to avoid subjecting patients to unnecessary extractions.
Using the same circuit as for ordinary blood work, the sample will be sent to the hospital's laboratory where the collection tube will be stored. One morning a week, all the samples obtained during that week will be collected and transported to the external laboratory. Analysis of the samples will commence that same afternoon, the results being available the next morning. Once the results are available, the external laboratory will send them to the principal investigator (PI) of the study and to the hospital's pharmacy and laboratory services in the form of a previously defined standardized pharmacogenetic report jointly designed by the PI and the director of the external lab. The report will contain the results themselves, their interpretation as well as any relevant comments and dose titration recommendations. It will be required that less than 24 hours should elapse between collection of the samples at the hospital's lab and the delivery of results so that chemotherapy is not unduly delayed.
The hospital's pharmacy service and/or the PI will validate the report and introduce the information it contains into the patient's electronic medical record as a follow-up note under the patients’ oncology status. They will also telephone the attending oncologist to provide them with the information directly.
Analytical techniquesThe first step will be extraction and purification of genomic DNA from whole blood samples obtained by venipuncture and collected in EDTA-coated tubes. Concentration and purity will be measured by spectrophotometry. Subsequently, the 6 variants will be genotyped by real-time PCR using the TaqMan® Drug Metabolism Enzyme assay, validated in 180 individuals representing four different ethnic groups, providing a robust and reproducible signal. A StepOne thermocycler (Applied Biosystems, CA, USA) will be used with an initial denaturing step at 95° for 10 minutes followed by 40 cycles at 95° for 15 seconds and 60° for 90 seconds in 10 µl reagent volume. Finally, results will be analyzed using the StepOne v2.3 software package.
Data collectionData will be collected through a previously designed data collection sheet, which will contain a categorization of the most common toxicities and a section for other toxicities than could be identified. All the above stated variables will be studied in both groups as this will help standardize data collection thus minimizing risks and maximizing the quality and reliability of the study.
In both groups, toxicity will always be evaluated and classified by the oncologist on the basis of CTCAE v5.0 criteria. Toxicity will be monitored from the first to the sixth treatment cycle. Once included in the study, each patient will be assigned an identification number to ensure data anonymity.
Ethical considerationsThe research project itself as well as the patient information sheet and the informed consent form were approved by the hospital's Ethics Committee (code 20/006).
The hospital's Ethics Committee waived the requirement to obtain informed consent from patients in the CG given that only toxicity and treatment (dissociated and anonymized) data will be obtained from these patients as, in accordance with the International Ethical Guidelines for Biomedical Research Involving Human Subjects (CIOMS/OMS), data from these patients has to be collected retrospectively due to the fact that potentially-harmful genetic variants must be identified before treatment is initiated.
Statistical analysisA descriptive analysis of the variables will be made using frequencies for qualitative variables and minimum, maximum, and mean values and standard deviation for quantitative ones. Factors associated to the presence of toxicity will be analyzed using contingency tables, applying the chi-squared test for qualitative variables and comparisons of mean values with Student's t test for the quantitative ones.
Multivariate logistic regression models will be used to estimate the strength of associations involving toxicity. Odds ratios will also be estimated, together with their 95% confidence intervals. A stepwise variable selection process will be conducted based on the Akaike Information Criterium. Goodness of fit and predictive indicators will be used. Statistical analyses will be made using the SPSS v.26 and R v.3.6.1 software packages.
DiscussionThe study will seek to compare allele frequencies of a group of heterozygous patients carrying a “classic” variant of the DPYD gene, which is often dysfunctional in our patient population, with those reported in the literature (3-7%)1–4,12–14. Frequencies will also be compared for gene miR-27A where, according to Meulendijks et al.7, allele frequency for the rs895819 polymorphism is 33.1%. DPYD*6 will be compared with the ranges reported in the recently published studies by Iachetta et al. and Del Re et al.10,14. This will allow characterization of the studied population.
One of the main differences between the present study and those already published in the literature is related to its ambispective design. The vast majority of similar studies are retrospective and pharmacogenomic tests are performed in patients who have already developed some degree of toxicity. Prospective studies in this area are limited and often determine variants and establish polymorphism-toxicity associations without titrating the dose administered. In the present study, pharmacogenetic tests and the ensuing drug adjustment counseling will be carried out prospectively, taking advantage of the resources and clinical benefits offered by preventive genotyping.
Another important difference lies in the fact that, although numerous studies underscore the need to conduct pharmacogenetic tests, they do not evaluate the reduction in toxicity that results from implementing the genetic counseling based on the results of such tests. Following the recommendation by Henricks et al.2, this study will be aimed at determining whether performance of pharmacogenetic tests and individualization of treatment exert a positive influence on fluoropyrimidine-related toxicity and, if so, establish the degree of toxicity reduction achieved.
In the case of rs895819 (miR-27A) and rs1801160 (DPYD*6), following the work of Iachetta et al. and Del Re et al.10,14 on DPYD*6, and that of Meulendijks et al.7 on miR-27A, the association between DPYD*6 and toxicity will be analyzed in an observational study to generate further evidence and validate the results obtained by these authors, hoping that in the near future a consensus can be reached about the importance of testing for those polymorphisms and, if appropriate, adjust the dose of the patients’ medication accordingly.
Pharmacogenetic testing is not fully implemented in hospitals’ routine practice but an increasing number of them are taking steps in that direction.
LimitationsOne of the limitations of this study is its ambispective design. For ethical reasons, the data from patients in the GC will be collected retrospectively as, following the recommendations of the health authorities, the clinical guidelines and the vast evidence published, these variants must be identified before treatment is initiated, which precludes performance of prospective data gathering.
This study proposes a multidisciplinary protocol to be implemented for preventively determining DPYD variants known to be associated with a toxicity risk, tailor the treatment to each patient's genetic profile, and determine the reductions in toxicity achieved in real-life clinical practice. The protocol is meant as a useful tool in the context of personalized medicine to increase patient safety and the risk/benefit ratio of the treatment provided.
We hope to be able to contribute further evidence of the polymorphismtoxicity association by an observational study of two additional variants of the DPYD gene.
FundingThis project is being funded in part by Roche Pharmaceuticals (agreement code: SP200528001), as part of a pharmacogenetics program based on DPYD measurements. Roche has played no part in the preparation of this manuscript.
AcknowledgementsThe authors would like to thank the staff of the San Juan de Alicante Hospital for their collaboration in the development of this project. Our gratitude also goes to Ancor Genetics Laboratory for having so seamlessly adapted to the dynamics and logistics systems of our hospital.
Presentation at congressesThis project was submitted at:
- •
SEFH's 65th National Congress. Venue Barcelona (virtual); 20-22 October 2020. Submitted as an oral paper.
- •
Symposium of the Valencian Society of Hospital Pharmacists Venue; Valencia; 18-19 September 2020.
No conflict of interests.
Contribution to the scientific literature
Encouraged by the interest that this research project is attracting in many hospitals, we consider that this is the ideal time to share the protocol used with scientific community even if its results of the study are not yet available. Waiting for the results would delay publication of the protocol and deprive many healthcare providers from a model on which to base implementation of their own pharmacogenetic testing programs. This project was submitted as an oral paper at the 65th National Congress of the Spanish Society of Hospital Pharmacists.