The present paper provides a systematic review aimed at identifying studies on pharmacological interactions in patients undergoing hematopoietic stem cell transplantation. Secondary objectives include a characterization of the prevalence of such interactions and an investigation of their specific characteristics.
MethodA search was performed of the terms “drug-drug interaction”, “drug interaction”, “stem cell transplant”, “transplantation conditioning”, and “conditioning regimen” in the PubMed database, and of the terms “drug interaction”, “stem cell transplantation”, and “transplantation conditioning” in the Embase database. Only results directly related to the objective of the review were selected. Studies in humans published between January 2000 and November 2020, written in English or Spanish, were prioritized.
ResultsThe review identified two groups of studies: epidemiological studies and studies analyzing interactions between specific drugs. The 10 epidemiological studies selected, which showed a prevalence of interactions between 60 and 100%, mainly used the Micromedex® database, focused on pharmacokinetic interactions involving azole antifungals. Results were highly heterogeneous. Of the 52 drug interaction studies reviewed, the majority were pharmacokinetic and focused primarily on the interactions of azole antifungals with calcineurin inhibitors. Some studies described the possible relationship between the interactions and specific adverse reactions or deaths from adverse events.
ConclusionsThe prevalence of drug-drug interactions in patients undergoing hematopoietic stem cell transplantation is high, with heterogeneous results both in terms of prevalence and of the profile of the interactions resulting from the use of disparate study designs and databases. The most common factor associated with drug-drug interactions was the number of drugs administered. Studies evaluating drug-drug interactions are mostly pharmacokinetic and focus mainly on azole antifungals and calcineurin inhibitors. It would be important to unify the criteria followed in epidemiological studies to obtain results that may help establish risk reduction strategies and conduct a more in-depth investigation into the pharmacodynamic mechanisms involved and into the interactions between other drugs frequently used in patients undergoing transplantation, including those recently introduced in our therapeutic arsenal.
El objetivo principal de este trabajo es identificar, mediante revisión bibliográfica sistemática, los estudios sobre interacciones farmacológicas en pacientes sometidos a trasplante de progenitores hematopoyéticos. Los objetivos secundarios son describir la prevalencia de dichas interacciones y extraer información de interacciones fármaco-fármaco concretas.
MétodoBúsquedas en PubMed con los términos “drug-drug interaction”, “drug interaction”, “stem cell transplant”, “transplantation conditioning” y “conditioning regimen” y en Embase “drug interaction”, “stem cell transplantation” y “transplantation conditioning”, seleccionando aquellos resultados relacionados directamente con el objetivo de la revisión. Se priorizaron estudios en humanos, en idiomas inglés y español, entre enero de 2000 y noviembre de 2020.
ResultadosLa revisión identificó dos grupos de estudios (epidemiológicos y de análisis de interacciones entre fármacos concretos). Los 10 estudios epidemiológicos mostraron una prevalencia de interacciones entre el 60% y el 100%, la base de datos más utilizada fue Micromedex®, el mecanismo farmacocinético y los fármacos más implicados fueron los antifúngicos azóli-cos, con resultados muy heterogéneos. Los 52 estudios de interacciones entre fármacos fueron casi todos farmacocinéticos y se centraron fundamentalmente en las interacciones de antifúngicos azólicos e inhibidores de la calcineurina. Algunos estudios describieron la posible relación entre interacciones y reacciones adversas concretas o muertes por efectos adversos.
ConclusionesLa prevalencia de interacciones en pacientes sometidos a trasplante de progenitores hematopoyéticos es elevada, siendo los resultados heterogéneos, tanto en prevalencia como en el perfil de las interacciones. En ello repercuten las diferencias en los diseños de los estudios y en las bases de datos utilizadas. Entre los factores relacionados con el riesgo de que se produzcan interacciones farmacológicas destaca el elevado número de fármacos administrados. Los estudios que evalúan las interacciones fármaco-fármaco son casi todos farmacocinéticos y se centran mayoritariamente en antifúngicos azólicos e inhibidores de la calcineurina. Sería importante unificar los criterios de los estudios epidemiológicos para obtener resultados que ayuden a establecer estrategias de reducción de riesgo, investigar en mayor profundidad las interacciones de mecanismo farma-codinámico, las interacciones entre otros fármacos de uso frecuente en el trasplante y en aquellos de introducción reciente en el arsenal terapéutico.
The purpose of hematopoietic stem cell transplantation (HSCT) in the context of malignant diseases is to regenerate bone marrow function, often compromised by prior conditioning regimens, and/or to trigger a graft versus tumor effect1,2. Conditioning regimens may result in significant complications3, which tend to increase the patients’ drug intake and the potential for drug-drug interactions (DDIs). The most worrisome DDIs are those that have a negative effect on the patient. As such, these DDIs must be identified, prevented and resolved4,5.
Patients undergoing HSCT are usually treated with complex pharmacological regimens, which may lead to multiple DDIs, increasing the risk of adverse events or reducing therapeutic effectiveness. In fact, several authors have reported severe adverse events such as rhabdomyolysis6,7 and even life-threatening complications in the context of HSCT8. Other factors that may contribute to increasing the risk of DDIs include the number of drugs administered, the length of hospital stay and the type of procedure performed. Moreover, these patients present with associated comorbidities including renal and hepatic dysfunction, an impaired nutritional status and protein-binding displacement, which increases the risk of clinically significant DDIs9–11.
The main purpose of this article was to carry out a systematic literature review to identify studies on DDIs occurring in patients undergoing HSCT. Secondary goals included a description of the prevalence of such DDIs and the collection of data on specific DDIs.
MethodsA structured literature review was carried out using PubMed and Embase. The goal was to identify as many original articles as possible dealing with DDIs in patients undergoing HSCT following the PRISMA methodology. The analysis included clinical trials, observational studies, case reports or original case series, and letters to the editor. To be included, studies had to report results directly associated with the purpose of the review, the had to have been written in either English or Spanish, and their publication date had to be comprised between 1 January 2000 and 27 November 2020. Publications not related to DDIs and those related to DDIs but not to HSCT, as well as articles published only as oral papers for submission to a conference, were excluded from the analysis.
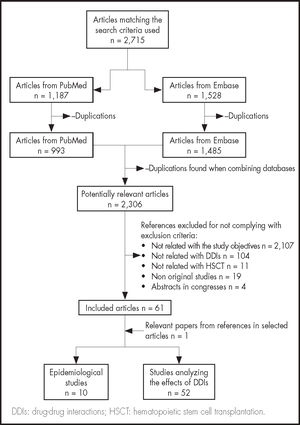
Search terms for all fields were: drug-drug interaction, drug interaction, stem cell transplant, transplantation conditioning and conditioning regimen for PubMed; and drug interaction, stem cell transplantation and transplantation conditioning for Embase, combined with Boolean operators or and and. Additional publications cited in the selected articles were also included in the search given their significance in the authors’ opinion. Figure 1 shows the process followed to select the articles included in the search.
The most relevant data in each publication was extracted by one of the authors and independently checked by another author. Any discrepancies were resolved by a third investigator. The selected publications were then run through an initial filter where the title, abstract and main text of the articles were screened to ensure that they were in line with the purpose of the present study. When the same information was repeated, the most-up-to-date, clear and comprehensive study was selected.
The epidemiological features of all the studies were duly analyzed and recorded. Such features included: study type; database used; rate and prevalence of DDIs; overall number of DDIs; number of DDIs per patient; most usual DDIs; mechanism of action; severity: risk factors; and most commonly involved drugs; as well as the DDI effect observed in drug-to-drug studies.
ResultsThe literature search resulted in 2,715 records (1,187 for PubMed and 1,528 for Embase). After removal of duplications, of articles whose title or abstract were unrelated to the purpose of this study, and of publications summarizing conference presentations, a total of 62 records were obtained that met the inclusion criteria. Ten of these were classified as epidemiological studies and 52 as studies analyzing the effects of DDIs. Tables 1 and 2 show the characteristics, objectives and main findings of the studies included.
Summary of the epidemiological studies included in the review.
| Reference | Type of study | Number of patients | Database used | Objective | Findings/main results |
|---|---|---|---|---|---|
| Valverde et al. 20189 | Retrospective observational cohort study, Brazil | 53 | Micromedex® | Prevalence of DDI between anti-infectious, antineoplastic, and immunomodulating agents during conditioning. |
|
| Hadjibabaie et al. 201312 | Cross-sectional study, Iran | 83 | Lexi-Interact On-Desktop software® | DDI frequency and profile. DDI risk factors. | Prevalence: 62.88%. 185 DDIs. Pharmacokinetic DDIs: 69.73%. Most common DDI: cotrimoxazole-fluconazole (27.27%). DDI risk factor: number of drugs. |
| Fernández de Palencia et al. 201713 | Prospective observational descriptive study, Spain | 58 | Micromedex® & Drug-Interaction Facts® | Prevalence of clinically relevant DDIs, most frequent DDIs and DDI risk factors in patients admitted to the hematology department. |
|
| Gholaminezhad et al. 201414 | Retrospective observational study, Iran | 384 | Lexi-Interact On-Desktop | Patterns and risk factors associated to potentially moderate or severe DDIs before and immediately after HSCT. | DDI prevalence: 100%. 13,600 DDIs. Median DDI/patient: 41.100% patients ≥ 1 DDI, 81.8% DDIs were moderate and 54.3% pharmacokinetic. Most common DDI: cotrimoxazole-fluconazole (95.3%). 61.5% DDIs caused by HSCT-related medication. DDI risk factors: type of HSCT and number of drugs administered. |
| Egger et al. 201015 | Retrospective observational study, Switzerland | 36 | Drug-Reax® | Prevalence and frequency of potential antifungal- related DDIs. | Prevalence: 86.11%. 57 DDIs in 31 patients. Most common DDI: voriconazole-cyclosporine (20 patients). |
| Guastaldi & Secoli. 201116 | Prospective cross-sectional study, Brazil | 70 | Drug Interactions Facts® & Drug Interactions Handbook® | Prevalence of potential antimicrobial-related DDIs (frequency and severity). Factors associated with DDIs. | Prevalence: 71.4%. 13 DDIs in 50% of patients (fluconazole 53,8%, ciprofloxacin 30.8% and cotrimoxazole 15.4%). Moderate DDIs: 92,3%, late-onset DDIs: 61.5%, and DDIs requiring treatment monitoring: 76,9%. Factors associated with DDI risk: ≥ 4 drugs, age 40-49, and male sex. |
| Jaklic et al. 201317 | Retrospective observational study, United States | 84 | University of Washington Drug Interaction Database, Stockley's Drug Interactions, Lexicomp™, Micromedex®, Drugs.com | Prevalence of mycophenolic acid- related DDIs within the first 21 days post-HSCT. | Prevalence: 87%. 135 DDIs. 87% patients ≥ 1 DDI. 5.9% of drugs ≥ 1 DDI. Median DDIs/patient 2 (0-4). 10 DDIs ↓AUC and 1 DDI ↑AUC of mycophenolic acid. Most common DDIs involved: cyclosporine (n = 58), omeprazole (n = 28) and pantoprazole (n = 20). Most DDIs were related with HSCT-specific medication. |
| Sánchez et al. 201918 | Retrospective observational study, France | 31 | Theriaque® | Prevalence and density of DDIs and evolution of renal function. |
|
| Guastaldi et al. 201119 | Cross-sectional study, Brazil | 70 | Drug-Reax® | Prevalence of potential DDIs during the preinfusion period (day -1) and description of DDIs (frequency and severity). | Prevalence: 60.0%. 128 DDIs (85.9% of moderate severity, 52.3% pharmacokinetic, 82.8% late-onset). 60% patients ≥1 DDIs, 21.4% patients ≥ 1 severe DDIs. Most common severe DDIs: fluconazole-cotrimoxazole, diazepam-fentanyl, fluconazole-levofloxacin, and fluconazole-fentanyl. |
| Trevisan et al. 201520 | Cross-sectional study, Brazil | 40 | Drug-Reax® | Prevalence of DDI on the day the hematopoietic stem cells were infused (day 0). | DDI prevalence: 82.5%. 80.9% DDI were severe and 61.9% of undetermined onset. 52.4% DDIs based on good or excellent scientific evidence. |
AUC: area under the curve; DDI: drug-drug interaction; GFR: glomerular filtration rate; HSCT: hematopoietic stem cell transplant.
Summary of the pharmacokinetic studies included in the review
| Reference | Type of study | Number of patients | Main findings |
|---|---|---|---|
| Cool & Gulbis 20136 | Case report, United States | 1 | The patient developed rhabdomyolysis which was suspected to have been caused by a DDI between simvastatin and voriconazole. |
| Vives et al. 20087 | Letter to the editor (case report), Spain | 1 | Concomitant use of simvastatin, cyclosporine A and risperidone resulted in rhabdomyolysis and renal failure. |
| Yang et al. 20138 | Retrospective observational study, China | 104 | Itraconazole and voriconazole lead to increased concentrations of cyclosporine A. |
| Leather et al. 200621 | Prospective observational study, United States | 17 | Itraconazole leads to increased concentrations of tacrolimus and cyclosporine A. No correlation was found between the concentration of itraconazole and that of tacrolimus or cyclosporine A. 50-100% tacrolimus and cyclosporine A dose reductions are required when itraconazole is introduced in the treatment regimen. |
| Kawazoe et al. 200622 | Retrospective case series, Japan | 3 | Serum concentrations of tacrolimus increased 4.5 times after a change from fluconazole to voriconazole. This required a 20% tacrolimus dose reduction. |
| Mihara et al. 200823 | Retrospective observational study, Japan | 53 | The change from intravenous to oral fluconazole significantly increased the serum levels of cyclosporine A and tacrolimus. |
| Mori et al. 200924 | Retrospective observational study, Japan | 10 | Orally administered itraconazole leads to increased concentrations of calcineurin inhibitors. The observed increase in itraconazole serum levels was significantly correlated with an increase in calcineurin inhibitor concentrations. |
| Nara et al. 201025 | Letter to the editor (case report), Japan | 1 | Itraconazole inhibits the tacrolimus metabolism via the CYP3A4 enzyme. |
| Mori et al. 201226 | Retrospective observational study, Japan | 25 | Concentrations of tacrolimus increased significantly when administered with oral voriconazole. Serum concentrations of voriconazole were not correlated with higher concentrations of tacrolimus. |
| Kikuchi et al. 201227 | Retrospective observational study, Japan | 20 | DDIs were observed between voriconazole and cyclosporine. Voriconazole led to a significant increase in cyclosporine when both drugs were administered concomitantly. Voriconazole serum levels were not significantly correlated with an increase in cyclosporine concentrations. |
| Nara et al. 201328 | Prospective observational study, Japan | 16 |
|
| Iwamoto et al. 201529 | Prospective cohort study, Japan | 21 |
|
| El-Asmar et al. 201530 | Case report, United States | 1 | Topically applied cotrimoxazole leads to increased serum levels of sirolimus and tacrolimus. |
| Masoumi et al. 201731 | Prospective cohort study, Iran | 29 | Cyclosporine concentrations increased in a statistically significant way following the start of (both oral and intravenous) voriconazole therapy. A significant correlation was found between voriconazole concentrations and the increase in cyclosporine plasma levels. |
| Valenzuela et al. 201732 | Retrospective observational study, Chile | 7 | Voriconazole leads to increased serum concentrations of cyclosporine A. |
| Kieu et al. 201833 | Retrospective observational study, United States | 30 | Isavuconazole leads to a moderate increase in tacrolimus and sirolimus serum concentrations. |
| Collins et al. 201934 | Retrospective observational study, United States | 79 | Patients where no empirical posaconazole dose reduction was applied took longer (p < 0.05) to achieve therapeutic concentrations of tacrolimus. Their rate of subtherapeutic posaconazole levels was higher (p < 0,001) than in patients where an empirical dose reduction was applied. |
| Mimura et al. 201935 | Retrospective observational study, Japan | 52 | Voriconazole lead to greater increases in tacrolimus serum levels than fluconazole following a change from intravenous to extended-release oral tacrolimus. |
| Utano et al. 202036 | Retrospective observational study, Japan | 38 | Patients on voriconazole exhibited higher tacrolimus serum concentrations, regardless of the route of administration employed, although the increase was greater when tacrolimus was administered orally. |
| Marty et al. 200637 | Case series, United States | 11 | Voriconazole and sirolimus may be safely co-administered if a 90% empirical dose reduction of sirolimus is implemented. |
| Said et al. 200638 | Case report, United States | 1 | Itraconazole led to increased concentrations of sirolimus. This DDI could be regarded as the cause of the patient's death (disseminated adenovirus infection that resulted in terminal multi-organ failure). |
| Kubiak et al. 201239 | Case series, United States | 15 | Concomitant use of posaconazole and sirolimus is safe if a 33-50% empirical dose reduction of sirolimus is implemented. |
| Ceberio et al. 201540 | Retrospective observational study, United States | 67 | Concomitant administration of sirolimus and voriconazole is safe and well-tolerated if a 90% empirical dose reduction of sirolimus is implemented. |
| Greco et al. 201641 | Retrospective observational study, Italy | 49 | A 55-70% reduction in the daily dose of sirolimus in 19 of these patients was implemented following introduction of posaconazole. Despite the dose reduction, 1/3 of patients exhibited an increase in sirolimus serum levels during the first week of co-administration. |
| Nwaroh et al. 201842 | Case series, Canada | 3 | Discontinuation of fluconazole resulted in a marked reduction of trough plasma levels of sirolimus. Patients required a > 200% dose increase to reach therapeutic levels. |
| Marr et al. 200443 | Comparative randomized trial, United States | 105 | Patients on fluconazole were more exposed to cyclophosphamide and dechloroethyl-cyclophosphamide, while those on itraconazole were more exposed to 4-hydroxy-cyclophosphamide and 4-keto-cyclophosphamide, which are more toxic metabolites. |
| Miura et al. 201144 | Case report, Japan | 1 | S-warfarin concentrations increased 7.3 times when the drug was administered concomitantly with oral itraconazole. |
| Fakih et al. 201245 | Letter to the editor (case series), United States | 5 | Systemic concentrations of budesonide way increase in the drug is administered concomitantly with an azole. |
| Yasu et al. 201646 | Retrospective observational study, Japan | 59 | Trough levels of voriconazole were significantly higher in patients on concomitant treatment with lansoprazole as compared with rabeprazole. |
| Furrer et al. 200247 | Retrospective observational comparative study, Switzerland | 84 | Concomitant administration of amphotericin B and cyclosporine leads to a statistically significant but clinically tolerable renal function impairment. |
| Nagamura et al. 200348 | Retrospective observational study, Japan | 103 | Cyclophosphamide may reduce cyclosporine A serum concentrations for at least two weeks following conditioning. |
| Ibrahim et al. 200849 | Retrospective observational study, United States | 26 | Aprepitant led to statistically higher serum concentrations of tacrolimus when both drugs were administered concomitantly. This increase had no clinical repercussions thanks to pharmacokinetic monitoring. |
| Shayani et al. 201250 | Case series, United States | 85 | The combination of sirolimus and aprepitant leads to a dual increase in sirolimus serum levels as both agents are CYP3A4 substrates. This is not observed with the tacrolimus- aprepitant combination. |
| Fukuoka et al. 201051 | Observational study, Japan | 6 | The fact that tacrolimus concentrations remained stable with and without concomitant treatment with micafungin led to the conclusion that there was no DDI between both agents. |
| Inoue et al. 201252 | Prospective comparative study, Japan | 15 | A 150 mg daily dose of micafungin is safe and does not result in significantly interactions with cyclosporine. |
| Nishimoto et al. 201753 | Retrospective observational study, Japan | 50 | Concentrations of cyclosporine increased significantly when the drug was administered together with caspofungin. Tacrolimus concentrations, however, exhibited no differences. |
| Miceli et al. 201254 | Letter to the editor (case report), United States | 1 | Ritonavir leads to increased serum concentrations of tacrolimus when both drugs are administered concomitantly. |
| Bernard et al. 201455 | Retrospective observational study, France | 51 | Trough cyclosporine A serum levels increased significantly in patients treated with nicardipine and amlodipine, while they remained stable in patients treated with lacidipine. |
| Bleyzac et al. 201456 | Case series, France | 6 | Cyclosporine concentrations increased significantly following a 3-7 day course of imatinib. |
| Atiq et al. 201657 | Retrospective observational study, the Netherlands | 16 | Concomitant administration of cyclosporine and imatinib led to a significant increase of the former's serum concentrations in all patients. A serum concentration-based dose adjustment led to a 27% reduction in the cyclosporine dose administered. |
| Kitazawa et al. 201758 | Retrospective observational study, Japan | 6 | Fentanyl decreased tacrolimus clearance when both agents were administered together. It is proposed that a 40% tacrolimus dose reduction should be implemented when the drug is used in combination with fentanyl. |
| Guo et al. 201959 | Case series, Japan | 3 | Letermovir inhibits CYP3A4 and leads to 1-5-to-2-fold increases in the concentration of tacrolimus, regardless of the route of administration used. |
| Maples et al. 202060 | Letter to the editor, United States | 1 | Letermovir can be used safely in combination with tacrolimus or cyclosporine. Dose adjustments of calcineurin inhibitors are not recommended prior to administration of letermovir. |
| Mancini et al. 202061 | Case report, United States | 1 | Cyclosporine serum levels increased by 70% following administration of midostaurin. The cyclosporine dose was reduced by 40% to achieve therapeutic levels. |
| Nilsson et al. 200362 | Retrospective observational comparative study, Sweden | 24 | Metronidazole leads to a significant increase in busulfan serum levels. Concomitant use of both drugs must be avoided. |
| Chung et al. 201763 | Case report, South Korea | 1 | Metronidazole leads to a 57% reduction in busulfan clearance when both agents are administered together. |
| Sjoo et al. 200364 | Retrospective observational study, Switzerland | 10 | N-acetylcysteine is safe and does not alter busulfan's myeloablative effect. |
| de Castro et al. 201365 | Retrospective observational study, Brazil | 26 | Patients treated with fludarabine should receive 30% lower doses of busulfan that if they were not on fludarabine. |
| Sweiss et al. 201 966 | Case report, United States | 1 | The increase in cytokine levels observed during treatment with blinatumomab could lead to CYP3A4 suppression and an ensuing metabolization of busulfan, which may reduce its clearing ability. |
| Bubalo et al. 201267 | Randomized double-blind placebo-controlled study, United States | 1 | Aprepitant is well absorbed, does not induce its own metabolism and does not produce DDIs with cyclophosphamide or its metabolites. |
| Wasko et al. 201768 | Case report, United States | 1 | Rifampicin reduces sirolimus serum concentrations. |
| Engle & Fair 201769 | Case report, United States | 1 | Mirabegron increases serum concentrations of sirolimus. |
DDI: drug-to-drug interaction.
Eight of the 10 epidemiological studies centered exclusively on patients undergoing HSCT, while the other two included hematological patients in general12,13. The study with the largest patient cohort had 384 subjects14. Six studies analyzed the DDIs resulting from all the drugs administered, while two studies were dedicated to DDIs associated with the use of antimicrobials15,16, one of them focusing on DDIs associated with anti-infectious, antineoplastic and immunomodulating drugs9, and the other on DDIs associated to mycophenolic acid17. Sánchez et al. conducted a separate study of DDIs with the potential to affect renal function, and associated them with a slower glomerular filtration rate18.
Most studies made specific mention of the database used, the prevalence of DDI, the drugs most commonly involved in DDIs, and the drug combinations most prone to DDIs, including those usually leading to the more severe forms of DDI. The most commonly used database was Micromedex®9,13,15,17. The study resulting in the highest number of overall DDIs was Gholaminezhad et al.14 with 13,600 DDIs in 384 patients, whereas the one detecting the lowest amount of DDIs was Guastaldi & Secoli16 with 13 DDIs in 35 patients.
The per-patient prevalence of DDIs ranged between the 60% reported by Guastaldi et al.19, who used the Drug Reax database® and the 100% found by Gholaminezhad et al.14, who used Lexi-Interact®. Using a different methodology, and in a hematologic cohort including more patients than just those undergoing HSCT, Fernández de Palencia et al.13 described a DDI prevalence per drug prescribed of 56.8% using the Drug Interaction Facts database® and of 74.1% using the Micromedex database®.
The mechanism of action of DDIs was mostly pharmacokinetic12,14,19. Interactions were mostly late-onset16,19 and the damage was mostly moderate14,16,19 or severe20. However, Sánchez et al. reported a lower incidence of pharmacokinetic than of pharmacodynamic DDIs18. Azole antifungals were among the drugs most commonly involved in DDIs in six of the 10 epidemiologic studies analyzed12–16,19.
According to Guastaldi & Secoli16, factors associated with the risk of developing DDIs included the number of drugs used12,14,16, the prescription of concomitant non-antineoplastic medication13,17, advanced age, and male sex.
Of the 52 studies analyzing the effect of DDIs, most of them pharmacokinetic, 18 focused on DDIs between azole antifungals and calcineurin inhibitors7,8,21–36; six on DDIs between azole antifungals and sirolimus37–42; five on DDIs between azole antifungals and other drugs (cyclophosphamide43, warfarin44, budesonide45, simvastatin6, and proton pump inhibitors46); 15 on DDIs between calcineurin inhibitors and other drugs (amphotericin B47, cyclophosphamide48, aprepitant49,50, micafungin51,52, caspofungin53, ritonavir54, calcium channel blockers55, imatinib56,57, fentanyl58, letermovir59,60, midostaurin61); five on DDIs between busulfan and other drugs (metronidazole62,63, N-acetylcysteine64, fludarabine65, blinatu-mumab66); one on DDIs between aprepitant and cyclophosphamide67; one on DDIs between rifampicin, sirolimus and voriconazole68; and one on DDIs between sirolimus and mirabegron69. The main effects of these DDIs are shown in table 2.
According to Yang et al.8, 10 patients experienced life-threatening complications potentially associated with DDIs between cyclosporine A and itraconazole or voriconazole on administration of supratherapeutic levels of cyclosporine. Six patients developed grade I to III graft-versus-host disease (GVHD) and eventually died from idiopathic pneumonia syndrome or alveolar hemorrhage. Another four patients died from neurological complications associated with cyclosporine A. Other authors have described serious adverse events such as rhabdomyolysis resulting from the interaction of azoles with statins6,7.
DiscussionThe present study was based on a compilation of studies reporting on the DDIs suffered by patients undergoing HSCT over the last 20 years. Two groups of studies can be distinguished: epidemiological studies on the one hand, and studies analyzing DDIs between two specific drugs or drug families on the other.
Epidemiological studies on the DDIs suffered by patients undergoing HSCT are scarce and tend to use dissimilar methodologies which lead to highly heterogeneous results. What can be concluded about them is that these studies show a high prevalence of DDIs, particularly potentially severe ones or between contraindicated medications. In addition, results tend to be rather heterogeneous on account, among other reasons, of the use of different databases.
Such heterogeneity can be easily seen in the literature70,71, where significant differences have been found in one same population when the data is analyzed using different databases. For example, Fernandez de Palencia et al.13 observed different prevalence rates in hematologic patients depending on whether they used the Lexi Interaction Facts® database (56.8%) or the Micromedex® database (74.1%). These differences were greater in the case of oncologic patients72 (80% using Micromedex® and 30% using Interaction Facts®) and smaller in pediatric hemato-oncologic patients73 (44.7% using Micromedex® and 51.3% using Drug Interaction Facts®). A statistical analysis of the concordances between the two databases, which compared the DDIs detected across 1,166 treatments, showed that concordance was weak in terms of the capacity to detect potential DDIs and nonexistent for the level of severity and scientific evidence attributed by each database to the same DDI71. This heterogeneity precludes the use of databases as decision-making tools in clinical practice.
Although epidemiological studies tend to focus on DDIs occurring between drugs that are commonly used in the context of HSCT and whose risk profile is well known, such as calcineurin inhibitors and azoles, other relevant yet somewhat less well-known DDIs such as those associated to CNS depressants (benzodiazepines, morphine derivatives, etc.), antiemetics, corticosteroids, proton pump inhibitors, antidepressants or antibiotics are also reported, albeit in other scenarios74–76.
Other studies, dealing mostly with DDIs between two agents, usually focus on pharmacokinetic mechanisms of action, with none of them to the best of our knowledge analyzing pharmacodynamic DDIs. All pharmacokinetic studies exhibit a similar data collection pattern.
Most published articles describe DDIs between azole antifungals and calcineurin inhibitors, which result in increased serum concentrations of the calcineurin inhibitors caused by an inhibition of the CYP3A4 metabolism. However, the intensity of DDIs tends to depend on the specific antifungal or calcineurin inhibitor used. For example, the increase in calcineurin inhibitor serum concentrations is apparently greater with voriconazole than with fluconazole22,35, whereas the intensity of DDIs involving itraconazole is significantly higher with tacrolimus than with cyclosporine28. Several studies have shown the level of azole concentration not to be associated with an increase in calcineurin inhibitor concentrations26,27. Masoumi et al., however, did find a correlation in that respect31. The route of administration also seems to play an important role. Indeed, fluconazole tends to result in more significant DDIs with intravenously administered calcineurin inhibitors when it is administered orally rather than intravenously23. In the same vein, orally administered tacrolimus is more affected by concomitant use of voriconazole36. It must be pointed out that one of the studies analyzed claims that a DDI between cyclosporine and voriconazole or itraconazole was potentially responsible for the death of 10 patients who experienced subtherapeutic levels of cyclosporine. Six of them died as a result of an idiopathic pneumonia syndrome or an alveolar hemorrhage following the occurrence of GVHD and four as a result of neurologic complications associated to cyclosporine A8.
Azole antifungals are usually implicated in a different class of DDI when used together with sirolimus37–42. This combination leads to increased serum concentrations of sirolimus, requiring an empiric dose reduction before it can be combined with azoles. Antifungals may also interact with agents like cyclophosphamide, with fluconazole being safer than itraconazole as the former inhibits CYP2C9, which leads to lower levels of 4-hydroxycyclo-phosphamide, a toxic cyclophosphamide metabolite43. Azoles can also increase serum concentrations of warfarin44, budesonide45, simvastatin6, and lansoprazole46.
Calcineurin inhibitors, for their part, are involved in a significant number of DDIs with agents other than azoles. Tacrolimus and cyclosporine concentrations do not seem to be influenced by the presence of micafungin51,52, and although caspofungin does not seem to have an influence on tacrolimus concentration levels, but it does seem to increase serum concentrations of cyclosporine53. When combined with amphotericin B, cyclosporine leads to a statistically significant worsening of renal function, which may be clinically controlled if the drug is infused over a 24-hour period and if strict salt replenishment is observed47. Cyclosporine has also been found to be involved in DDIs with cyclophosphamide, leading to a decrease in the latter's serum concentrations48.
There is generalized concurrence in the literature regarding the effects of DDIs occurring between calcineurin inhibitors and other drugs such as imatinib, which leads to increased cyclosporine concentrations56,57. Nonetheless, in a study of 85 patients, Shayani et al.50 concluded that aprepitant increases sirolimus but not tacrolimus serum concentrations. Conversely, a study of 26 patients by Ibrahim et al.49 found that aprepitant did increase tacrolimus serum concentrations. Another example is provided by a study of 46 patients by Maples et al.60, who claimed that it is not necessary to adjust the dose of calcineurin inhibitors prior to administration of letermovir. However, a 3-case series published by Guo et al.59 showed that letermovir inhibits CYP3A4 and increases tacrolimus concentrations, which according to these authors warrants a dose reduction.
Some studies have shown that agents belonging to the same therapeutic group can have different DDI profiles. In this respect, Bernard et al.55 found that nicardipine and amlodipine increase serum concentrations of cyclosporine, while lacidipine had no effect of such concentrations. Finally, two studies showed that concomitant administration of metronidazole62 and fludarabine65 with busulfan increases the latter's serum concentrations.
In 2008, Vives et al. published a series of case reports that brought to light a series of severe adverse events associated with some DDIs. The authors reported that concomitant use of simvastatin, cyclosporine A and risperidone can result in rhabdomyolysis and renal failure7. Moreover, in a study describing significant DDIs resulting from recently introduced agents, Mancini et al. reported a 70% increase in the serum levels of cyclosporine A when used together with midostaurin61.
The main limitation of the present study is associated with the heterogeneity of the studies analyzed and the absence of data to quantify the impact of DDIs on patient outcomes.
In summary, all the studies analyzed describe a high prevalence of DDIs in patients undergoing HSCT, with significant disparities across the different authors in terms of the prevalence and characteristics of the DDIs identified. Such disparities are attributable to the way the studies were designed and the databases used. Factors related to the risk of DDIs include the number of drugs concomitantly administered. All studies on DDIs are of a pharmacokinetic nature and focus mainly on DDIs between azole antifungals and calcineurin inhibitors, or between these two drug families and other agents. It would be important to unify the criteria followed by epidemiological studies to produce more consistent outcomes conducive to the implementation of effective risk reduction strategies. It would also be essential to investigate pharmacodynamic DDIs and to carry out a more in-depth analysis of DDIs between other commonly used drugs in the HSCT context and between drugs that have been recently introduced in our therapeutic arsenal.
FundingNo funding.
Conflict of interestsNo conflict of interests.