To determine the effectiveness and safety of atezolizumab, nivolumab and pembrolizumab in patients with non-small cell lung cancer.
MethodThis is a retrospective observational study including patients treated in second line and beyond. The effectiveness of treatment was assessed by means of overall survival and progression free survival measurements. Toxicity was described according to the Common Criteria for Adverse Event Terminology v5.0.
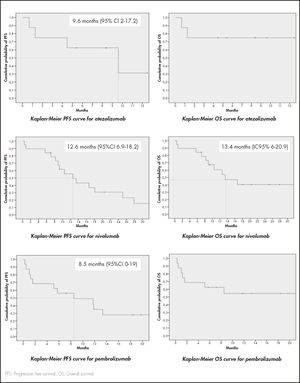
ResultsThe study included 8 patients treated with atezolizumab,19 with nivolumab, and 16 with pembrolizumab. Median progression free survival with atezolizumab was 9.6 months (95%CI 2-17.2), 12.6 months (95%CI 6.9-18.2) for nivolumab, and 8.5 months (95%CI 0-19) for pembrolizumab. Median overall survival was 13.4 months (95%CI 6-20.9) for nivolumab. Both PFS and OS were statistically higher in patients with grade 0-1 metastasis in the case of nivolumab, and in ECOG 0-1 patients for pembrolizumab. Median overall survival was not reached for atezolizumab or pembrolizumab. Around 85% of patients suffered adverse effects of some degree. Two of the patients treated with nivolumab developed vitiligo. Overall survival of both was higher than 2.5 years.
ConclusionsFor the patients included in the sample, nivolumab was less effective in those with two or more metastases; the effectiveness of pembrolizumab was lower in ECOG-2 patients. Vitiligo was related to a more durable response to treatment.
Efectividad y seguridad de atezolizumab, nivolumab y pembrolizumab en cáncer de pulmón no microcítico metastásico.
MétodoEstudio observacional retrospectivo en pacientes con cáncer de pulmón no microcítico metastásico tratados en segunda línea o posteriores. La efectividad fue evaluada mediante supervivencia global y supervivencia libre de progresión. La toxicidad mediante los Criterios Comunes de Terminología de Efectos Adversos v5.0.
ResultadosSe incluyeron 8 pacientes con atezolizumab, 19 con nivolumab y 16 con pembrolizumab. La mediana de supervivencia libre de progresión con atezolizumab fue 9,6 meses (intervalo de confianza del 95% [IC95%] 2-17,2), 12,6 meses (IC95% 6,9-18,2) para nivolumab y 8,5 meses (IC95% 0-19) para pembrolizumab. La mediana de supervivencia global con nivolumab fue 13,4 meses (IC95% 6-20,9) y no se alcanzó para atezolizumab y pembrolizumab. Ambas fueron superiores para los pacientes con 0-1 metástasis para nivolumab y en los pacientes con ECOG 0-1 para pembrolizumab. Alrededor de un 85% de los pacientes sufrieron efectos adversos. Dos pacientes tratados con nivolumab experimentaron vitíligo, con una supervivencia global mayor de 2,5 años.
ConclusionesEn la muestra analizada, la efectividad de nivolumab es menor en pacientes con dos o más metástasis, y la de pembrolizumab es menor en pacientes con ECOG 2. La aparición de vitíligo se relacionó con una respuesta duradera.
The introduction of monoclonal antibodies targeting programmed cell death protein-1 (PD-1), programmed death ligand 1 (PD-L1) and cytotoxic T lymphocyte antigen-4 (CTLA-4) has established a new paradigm in the treatment of non-small cell lung cancer (NSCLC).
A significant breakthrough was achieved when regulatory agencies approved nivolumab, pembrolizumab and atezolizumab for patients with NSCLC whose condition had progressed during or following treatment with platinum-doublet chemotherapy. The approval came following a series of clinical trials comparing the three drugs with docetaxel, the standard of care for NSCLC. In their respective clinical trials, nivolumab, pembrolizumab and atezolizumab achieved better overall survival (OS) results than docetaxel1–4.
The three drugs share a similar safety profile and are better tolerated than docetaxel1–4. However, they do present with toxic effects, particularly associated with the immune system, and have been reported to lead to an increased risk of developing immune-related adverse events (irAEs)5.
The purpose of this study was to evaluate the effectiveness and safety of atezolizumab, nivolumab and pembrolizumab in patients with locally advanced or metastatic NSCLC who had received at least one previous treatment.
MethodsThis was a retrospective observational study that included patients in a third-level hospital treated for locally advanced or metastatic NSCLC.
The inclusion period went from April 2016 to December 2018.
Inclusion criteria were as follows: age of at least 18 years; stage IIIB and IV NSCLC treated with atezolizumab, nivolumab or pembrolizumab; having received previous antineoplastic treatment for their advanced disease. Patients treated as part of a clinical trial or an expanded access program were excluded.
Patient- tumor- and treatment-related variables were analyzed in every case, as reflected in table 1.
Baseline characteristics of patients treated with immune therapy
| Atezolizumab (n = 8) | Nivolumab (n = 19) | Pembrolizumab (n = 16) | |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Sex | |||
| Males | 6 (75) | 13 (68.4) | 10 (62.5) |
| Females | 2 (25) | 6 (31.6) | 6 (37.5) |
| Age | |||
| < 70 years | 6 (75) | 11 (57.9) | 14 (87.5) |
| > 70 years | 2 (25) | 8 (42.1) | 2 (12.5) |
| Smoking | |||
| Never/former smoker | 4 (50) | 10 (52.6) | 7 (43.75) |
| Active smoker | 4 (50) | 9 (47.4) | 9 (56.25) |
| ECOG | |||
| 0 | 2 (25) | 1 (5.3) | 4 (25) |
| 1 | 6 (75) | 15 (78.9) | 9 (56.25) |
| 2 | − | 3 (15.8) | 2 (12.5) |
| 3 | − | − | 1 (6.25) |
| Histology | |||
| Squamous | 3 (37.5) | 13 (68.4) | 4 (25) |
| Adenocarcinoma | 4 (50) | 6 (31.6) | 11 (68.75) |
| NOS | 1 (12.5) | − | 1 (6.25) |
| Stage | |||
| III | 1 (12.5) | 4 (21.1) | 2 (12.5) |
| IV | 7 (87.5) | 15 (78.9) | 14 (87.5) |
| Nr of metastatic sites | |||
| 1 | 2 (25) | 11 (57.9) | 7 (43.75) |
| 2 | 4 (50) | 1 (5.3) | 4 (25) |
| 3 | 1 (12.5) | 3 (15.8) | 2 (12.5) |
| > 3 | − | − | 1 (6.25) |
| Types of metastatic sites | |||
| Pulmonary | 5 (62.5) | 10 (52.6) | 7 (43.75) |
| Lymph nodes | 3 (37.5) | 2 (10.5) | 3 (18.75) |
| Adrenal | 2 (25) | 2 (10.5) | 2 (12.5) |
| Cerebral | 1 (12.5) | 2 (10.5) | 4 (25) |
| Bone | 1 (12.5) | 4 (21.1) | 4 (25) |
| Hepatic | 1 (12.5) | − | − |
| Splenic | − | 1 (5.3) | 1 (6.25) |
| Pleural | − | − | 2 (12.5) |
| Mediastinum | − | − | 1 (6.25) |
| Mutations (adenocarcinoma) | |||
| Negative | 4 (100) | 5 (83.3) | 10 (62.5) |
| EGFR +, T790+ | − | 1 (16.7) | 1 (6.25) |
| PDL-1 expression | |||
| Negative (<1%) | 7 (87.5) | 5 (26.3) | 1 (6.25) |
| Low (1-49%) | − | 6 (31.6) | 7 (43.75) |
| High (>50%) | − | − | 8 (50) |
| Undetermined | 1 (12.5) | − | − |
| No data | − | 8 (42.1) | − |
| Nr previous treatments | |||
| 1 | 5 (62.5) | 17 (89.5) | 12 (75) |
| 2 | − | 1 (5.3) | 4 (25) |
| 3 | 2 (25) | − | − |
| 4 | 1 (12.5) | 1 (5.3) | − |
| Time elapsed from previous treatment | |||
| < 6 months | 1 (12.5) | 2 (10.5) | 6 (37.5) |
| > 6 months | 7 (87.5) | 17 (89.5) | 10 (62.5) |
| Cycles administered | |||
| Median (interquartile range) | 7.5 (4.75-13) | 10 (4-18.5) | 4.5 (2-11) |
| Radiotherapy | |||
| Previous | 3 (37.5) | 7 (36.8) | 5 (31.25) |
| Concomitant | 1 (12.5) | 1 (5.3) | 2 (12.5) |
| Baseline steroid therapy | |||
| Yes | 3 (37.5) | 4 (21.1) | 7 (43.75) |
| No | 5 (62.5) | 15 (78.9) | 9 (56.25) |
| Antibiotic therapy | |||
| During treatment | 4 (50) | 9 (47.4) | 5 (31.25) |
NOS: not otherwise specified.
The effectiveness of the treatment was analyzed by means of OS and progression-free survival (PFS) measurements.
Safety was evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE) v5.0 of the National Cancer Institute of the United States.
Data were obtained from the patients’ electronic medical records (Orion-Clinic v11.0) and from the pharmacotherapeutic management application for the onco-hematological patients (Farmis-Oncofarm v3.0)
Data analysisCategorical variables were described by means of frequencies while quantitative ones were expressed as central tendency and dispersion. Survival was assessed using Kaplan-Meier curves. A univariate analysis of factors associated with OS and PFS was performed using the Kaplan-Meier method; the Long-rank test was used to statistically compare the curves. Cox's proportional hazards model was employed to calculate the hazard ratio and 95% confidence intervals. Statistical significance was set at a p value < 0.05. The SPSS v.25.0 software package was used to test the research hypotheses.
Ethical and legal considerationsThis observational study was approved by the Ethics Committee for Research into Medicines of the hospital where the research was conducted.
ResultsThe study sample included eight patients on treatment with atezolizumab [mean age: 55.6 years (range: 53.3-67.4)], 19 patients treated with nivolumab [mean age: 66.3 years (range: 59.4-73.5)] and 16 patients on pembrolizumab [mean age:59.1 years (range: 54.5-65.9)] (Table 1).
Median values for OS and PFS are reflected in figure 1. Median OS was not reached for atezolizumab or pembrolizumab.
With regard to atezolizumab, no statistically significant differences were found between median OS and PFS values and any of the analyzed variables.
As far as nivolumab is concerned, median PFS and OS values were statistically higher in patients with 0-1 metastasis than in those with ≥ 2 metastasis. Median PFS was 16.6 months (95%CI 7.2-26)] in the former group and 0.8 months (95%CI 0-6) in the latter [p = 0.004; HR = 5.5 (95%CI 1.5-20)]. The median OS was not reached for patients with 0-1 metastasis but was 0.8 months (95%CI 0-5.9] for those with ≥ 2 metastasis [p = 0.002; HR = 6.4 (95%CI 1.7-24.7)]. Although not statistically significant, a certain trend was observed towards a longer OS in patients with non-squamous cell NSCLC [(p = 0.278; HR = 2.3 (95%CI 0.5-11.4)].
PFS and OS values were statistically improved in ECOG 0-1 patients on pembrolizumab. PFS in these patients was 11.8 months (95%CI 6.4-17.2) as compared with 1 month (95%CI 0.1-1.8) in ECOG ≥ 2 patients [(p = 0.002; HR = 0.097 (95%CI 0.02-0.6)]. As regards OS, the median for ECOG 0-1 was not reached but was 1 month (95%CI 0.1-1.8) for ECOG ≥ 2 patients [p = 0.002; HR = 0.097 (95%CI 0.02-0.6)]. Although no statistically significant OS differences were found in terms of the time elapsed since the previous treatment, a favorable trend was observed when the period was ≥ 6 months [p = 0.208; HR = 2.5 (95%CI 0.6-11.4)].
SafetyA total of 87.5%, 94.7% and 75% of patients treated with atezolizumab, nivolumab, and pembrolizumab, respectively, experienced adverse events of some kind. The most common adverse events with atezolizumab were respiratory infection (4; 50%) and asthenia (3; 37.5%). Adverse events with nivolumab, included asthenia (12; 63.2%), anemia (7; 36.8%), arthralgia (4; 21%) and, importantly, two cases of vitiligo, related with a long-term response to treatment. Finally, pembrolizumab was associated with asthenia (5; 31.3%) and itching (5; 31.3%). As far as grade ≥ 3 events are concerned, patients on atezolizumab experienced two (hyperthermia and hepatotoxicity), those on pembrolizumab four (asthenia, itching, diarrhea, and nephrotoxicity), and those on nivolumab five (hyperthermia, elevated liver enzymes, and three cases of asthenia). As regards deferrals or discontinuations of treatment, visits to the emergency room and/or hospital admissions, they occurred in 25% of patients treated with atezolizumab and pembrolizumab, and in 42.1% of those on nivolumab.
DiscussionOn the whole, the effectiveness observed in our study for the three drugs under analysis was higher than in the clinical trials published in the literature.
Specifically, the median PFS recorded in our study for patients treated with atezolizumab, nivolumab and pembrolizumab (Figure 1) was higher than median PFS values observed in pivotal clinical trials: 2.8 months (95%CI 2-6.3) for atezolizumab4; 2.33 months (95%CI 2.1-3.3) for nivolumab in non-squamous cell NSCLC1 and 3.48 months (95%CI 2.1-4.9) in squamous-cell NSCLC2; and 3.9 months (95%CI 3.1-4.1) for pembrolizumab3.
These differences can partly be explained by the different methods used to evaluate the tumor's response. Indeed, while clinical trials generally use the RECIST 1.1. criteria (Response Evaluation Criteria In Solid Tumors)6, the most widely used criteria in clinical practice are the immune-related response criteria (IrRC)7, where any increases in lesion size caused by necrosis or hemorrhage are not regarded as indicative of progression of the disease and where stabilization of the disease is considered as PFS, and thus an activity marker. Another factor that may explain these differences has to do with the time at which response is evaluated. While in clinical trials imaging tests were conducted every six weeks, in our study the interval ranged between 9 and 16 weeks.
Median OS was not reached for atezolizumab or pembrolizumab; median OS for nivolumab (Figure 1) was higher than the values obtained in pivotal studies for squamous-cell NSCLC [9.2 months (95%CI 7.3-13.3)] and similar to those for non-squamous cell NSCLC [12,2 months (95%CI 9.7-15)]. The distribution of pulmonary histology in our study did not warrant this difference given that it was precisely patients with squamous-cell NSCLC who obtained lower OS values in clinical trials (68.4% in our study).
No differences were found between this study and pivotal clinical trials in terms of median patient age, percentage of patients ≥ 70 years, ECOG score, and/or percentage of stage IV NSCLC patients that could explain these differences.
Our PFS and OS results were not consistent across all patient subgroups. Values were significantly higher in ECOG 0-1 patients treated with pembrolizumab, which is in line with the findings of other published studies8,9.
As regards nivolumab, both medians were statistically significantly higher for patients with 0-1 metastasis, which coincides with the results published by Garde et al.10.
The frequency and characteristics of adverse events were similar to those in the literature.
Two of the patients treated with nivolumab developed vitiligo. One of them was still being treated three years after the onset of symptoms. In the other, treatment had to be discontinued as a result of grade 3 nephrotoxicity. Four months after discontinuation, the patient developed facial vitiligo, which has progressively worsened. Nearly two years later, the disease remains radiologically stable. The significance of these findings lies in the fact that very few published reports describe the development of vitiligo in NSCLC patients treated with immune therapy11–13, all of them related with a long-term treatment response.
The main limitations of this study are related to the small patient sample and to its single-centric and retrospective nature.
The analyzed sample showed that the effectiveness of nivolumab is lower in patients with ≥ 2 metastases, and that the effectiveness of pembrolizumab is lower in ECOG 2 patients. Development of vitiligo as an adverse event seems to be related to a more durable response to treatment.
FundingNo funding.
AcknowledgementsThe authors would like to thank the preventive medicine service of the Alicante General University Hospital for their invaluable help with the statistical analysis of data.
Conflict of interestNo conflict of interest
Presentation at congressesThe results of the present study were presented in a summarized version at the 13th Meeting of the Spanish Lung Cancer Group, held in Valencia in November 2019.
Contribution to the scientific literature
The significance of this study lies in the fact that it contributes real-life data on the use of immune checkpoint inhibitors. It is well known that the conditions under which drugs are used in clinical practice are less restrictive than those governing their use in the context of the clinical trials that resulted in their approval. As a result, effectiveness and safety-related results obtained in clinical practice tend to be different from those published in clinical trials. Considering these facts in the light of the high cost of treatment with these kinds of inhibitors emphasizes the need to obtain data on their use in clinical practice.
The results obtained in this study provide a real-life picture of what it means to treat lung cancer with these drugs in our hospital. Together with the results obtained by other authors in other centers, our data will undoubtedly contribute to a better understanding of how immune checkpoint inhibitors are used in real life, which is indispensable for adopting healthcare policy strategies in the realm of lung cancer.