To present a new dexamethasone mouthwash formulation and analyze its effectiveness and safety among patients receiving stomatitis-producing antineoplastic agents.
MethodProspective observational study conducted in a university hospital between March 2017 and November 2019. Consecutive patients starting everolimus were enrolled. Patients were instructed to rinse dexamethasone mouthwash formulation twice daily until discontinuation of everolimus. A second cohort of patients with existing stomatitis induced by high probability of producing stomatitis chemotherapy therapies was also recruited to assess treatment effectiveness. Effectiveness and safety of dexamethasone mouthwash formulation was assessed.
ResultsDexamethasone mouthwash formulation was prescribed in nine patients as prophylaxis. Six patients were diagnosed with breast cancer, two with neuroendocrine tumor and one with renal cell carcinoma. Four patients developed mild stomatitis (grade 1-2) and three patients discontinued everolimus due to other treatment-related adverse events. In addition, dexamethasone mouthwash formulation was prescribed as treatment in five patients with existing stomatitis. All patients achieved a significant reduction in the severity of stomatitis after starting the dexamethasone mouthwash formulation. In both cohorts, dexamethasone mouthwash formulation was well tolerated and neither dose reduction nor discontinuation related to stomatitis was required.
ConclusionsDexamethasone mouthwash formulation could be considered as a suitable alternative for stomatitis management.
Describir una nueva formulación de enjuague bucal con dexametasona y analizar su efectividad y seguridad en pacientes que reciben agentes antineoplásicos que producen estomatitis.
MétodoEstudio observacional prospectivo realizado en un hospital universitario entre marzo de 2017 y noviembre de 2019. Se incluyeron los pacientes que iniciaron everolimus. El tratamiento consistió en enjuagar con solución oral de dexametasona dos veces al día hasta la interrupción del tratamiento con everolimus. Se reclutó una segunda cohorte de pacientes con estomatitis inducida por otros agentes antineoplásicos con alta probabilidad de provocar estomatitis. Se evaluó la efectividad y seguridad del enjuague bucal con dexametasona.
ResultadosSe reclutaron nueve pacientes en profilaxis con formulación de enjuague bucal con dexametasona; seis pacientes presentaban un diagnóstico de cáncer de mama, dos de tumor neuroendocrino y uno de carcinoma renal. Cuatro pacientes desarrollaron estomatitis leve (grado 1-2) y tres pacientes descontinuaron everolimus por otros eventos adversos relacionados con el tratamiento. Se prescribió enjuague bucal con dexametasona en cinco pacientes con estomatitis existente como tratamiento. Todos los pacientes lograron una reducción significativa de la gravedad de la estomatitis tras iniciar el enjuague bucal con dexametasona. En general, el nuevo enjuague bucal con dexametasona fue bien tolerado y no se requirieron reducción de dosis ni interrupción debido a estomatitis.
ConclusionesLa nueva formulación de enjuague bucal con dexametasona podría considerarse una alternativa adecuada para el manejo de la estomatitis.
Stomatitis is a common adverse effect of chemotherapy consisting on ulcerative and painful lesions at the oral cavity that can prevent oral intake and may be also a source of secondary infections1 causing low adherence and quality of life, leading to dose reduction or treatment discontinuation, potentially affecting survival2.
The prophylactic use of an alcohol-free dexamethasone mouthwash reduced the incidence and severity of stomatitis in patients with breast cancer receiving everolimus3. Patients swished four times daily a US commercially available alcohol-free dexamethasone 0.1 mg/mL solution. The incidence of G2 or higher of stomatitis at 8 weeks compared to BOLERO-2 study was 2.4% vs. 33% and the incidence of all grades was 21.2% vs. 67%3. Adverse events associated with the dexamethasone mouthwash were minimal with only two patients presenting oral candidiasis3.
The hypothesis that everolimus associated stomatitis may arise from an inflammatory process4,5 suggests that the use of steroid-based mouthwashes might be effective6,7.
However, the main drawback of this strategy is the lack of either commercially available dexamethasone mouthwash or standard compounded-formulation in Europe. In addition, there is no further evidence of the use of dexamethasone in other everolimus indications or stomatitis induced agents.
Our objective was to describe a novel dexamethasone mouthwash formulation (DMF) and to assess its effectiveness and safety preventing stomatitis among patients on everolimus. DMF was also evaluated as stomatitis treatment due to high probability of producing stomatitis chemotherapy therapies (HPSCT).
MethodsProspective observational study conducted in a university hospital between March 2017 and November 2019. Consecutive patients starting everolimus regardless their indications were enrolled. A second patient cohort on HPSCT (afatinib and fluorouracil) with grade 2 or 3 of stomatitis was also recruited at the discretion of the investigator to assess treatment effectiveness. The study protocol was approved by the ethics committee of our institution.
Patients under the age of 18, with allergy to dexamethasone or excipients were excluded.
Data collected from the patients’ electronic medical record included demographical, clinical and regarding efficacy/safety of DMF. Also, data concerning cancer treatment, diagnosis, DMF onset and treatment duration were recorded.
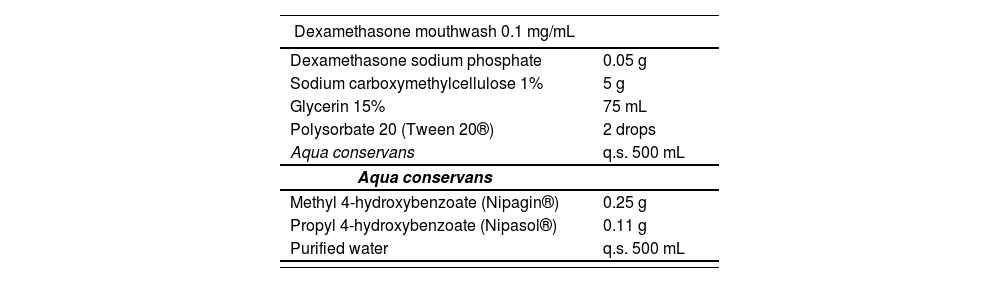
The proposed formula composition is shown in table 1. In preventive cohort, DMF was initiated early in antineoplastic treatment. Unlike SWISH trial3 and to improve adherence in a real-world situation, patients were instructed to rinse DMF only twice daily that lasted during antineoplastic treatment. The treatment cohort initiated DMF at the discretion of the investigator. In this case, it was allowed to use twice or 4-times daily at the discretion of the investigator.
Dexamethasone-based mouthwash composition
| Dexamethasone mouthwash 0.1 mg/mL | |
|---|---|
| Dexamethasone sodium phosphate | 0.05 g |
| Sodium carboxymethylcellulose 1% | 5 g |
| Glycerin 15% | 75 mL |
| Polysorbate 20 (Tween 20®) | 2 drops |
| Aqua conservans | q.s. 500 mL |
| Aqua conservans | |
| Methyl 4-hydroxybenzoate (Nipagin®) | 0.25 g |
| Propyl 4-hydroxybenzoate (Nipasol®) | 0.11 g |
| Purified water | q.s. 500 mL |
In preventive cohort, primary endpoint was the stomatitis incidence evaluated by the oncologist after DMF beginning. The incidences of all-grade and specific grade of stomatitis were summarised by counts and percentages, for the entire treatment duration.
Secondary endpoints included duration of DMF, antineoplastic treatment discontinuation or dose reduction because of stomatitis and other AE. Assessment of DMF compliance was based on medication records and interviews at pharmacy pick-up visits and was assessed by the Morisky Medication Adherence Scale8,9.
Adverse events (AE) were graded according the Common Terminology Criteria for Adverse Events (CTCAE) version 5.08. The efficacy and safety values were compared with those described in the pivotal everolimus (Afinitor®) clinical trials.
For descriptive analysis, quantitative variables were described through median and interquartile range (IQR) and qualitative variables were described through frequencies table (number and percentage). STATA version 15.1 (StataCorp, College Station, TX, USA) was used for statistical analysis.
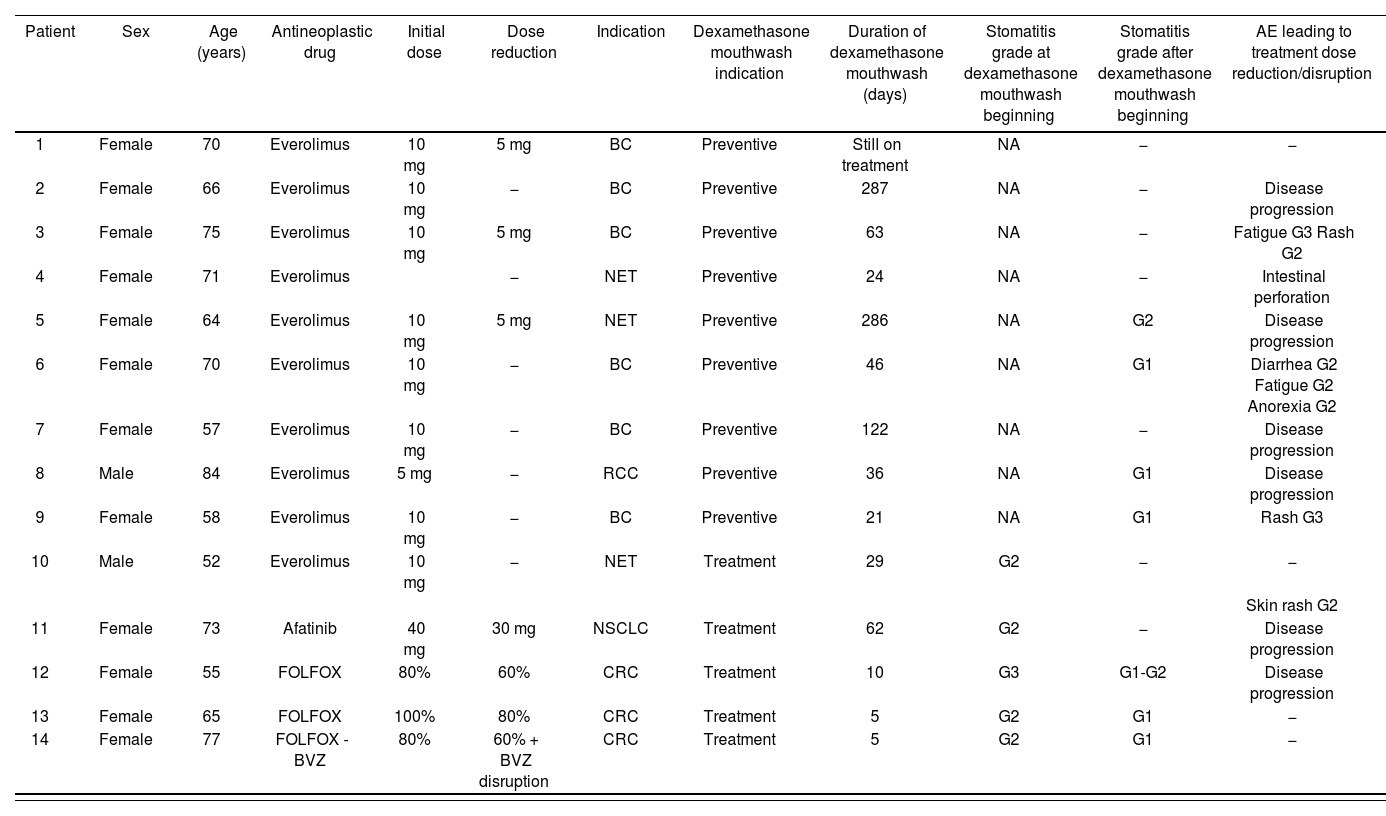
ResultsA total of fourteen patients were included in the study (Table 2).
Description of patients' features
| Patient | Sex | Age (years) | Antineoplastic drug | Initial dose | Dose reduction | Indication | Dexamethasone mouthwash indication | Duration of dexamethasone mouthwash (days) | Stomatitis grade at dexamethasone mouthwash beginning | Stomatitis grade after dexamethasone mouthwash beginning | AE leading to treatment dose reduction/disruption |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 70 | Everolimus | 10 mg | 5 mg | BC | Preventive | Still on treatment | NA | − | − |
| 2 | Female | 66 | Everolimus | 10 mg | − | BC | Preventive | 287 | NA | − | Disease progression |
| 3 | Female | 75 | Everolimus | 10 mg | 5 mg | BC | Preventive | 63 | NA | − | Fatigue G3 Rash G2 |
| 4 | Female | 71 | Everolimus | − | NET | Preventive | 24 | NA | − | Intestinal perforation | |
| 5 | Female | 64 | Everolimus | 10 mg | 5 mg | NET | Preventive | 286 | NA | G2 | Disease progression |
| 6 | Female | 70 | Everolimus | 10 mg | − | BC | Preventive | 46 | NA | G1 | Diarrhea G2 Fatigue G2 Anorexia G2 |
| 7 | Female | 57 | Everolimus | 10 mg | − | BC | Preventive | 122 | NA | − | Disease progression |
| 8 | Male | 84 | Everolimus | 5 mg | − | RCC | Preventive | 36 | NA | G1 | Disease progression |
| 9 | Female | 58 | Everolimus | 10 mg | − | BC | Preventive | 21 | NA | G1 | Rash G3 |
| 10 | Male | 52 | Everolimus | 10 mg | − | NET | Treatment | 29 | G2 | − | − |
| Skin rash G2 | |||||||||||
| 11 | Female | 73 | Afatinib | 40 mg | 30 mg | NSCLC | Treatment | 62 | G2 | − | Disease progression |
| 12 | Female | 55 | FOLFOX | 80% | 60% | CRC | Treatment | 10 | G3 | G1-G2 | Disease progression |
| 13 | Female | 65 | FOLFOX | 100% | 80% | CRC | Treatment | 5 | G2 | G1 | − |
| 14 | Female | 77 | FOLFOX - BVZ | 80% | 60% + BVZ disruption | CRC | Treatment | 5 | G2 | G1 | − |
BC: breast cancer; CRC: colorectal cancer; FOLFOX: folinic acid + fluorouracil + oxaliplatin scheme; FOLFOX-BVZ: folinic acid + fluorouracil + oxaliplatin + bevacizumab; NET: neuroendocrine tumour; NSCLC: non-small cell lung cancer; RCC: renal cell carcinoma.
Nine patients were collected. Patient and treatment features are summarised in table 2. The patients comprised eight women and one man, and median of age was 70 years old (IQR: 7). Six patients were diagnosed with breast cancer (BC), two with neuroendocrine tumor (NET) and one with renal cell carcinoma (RCC).
The primary endpoint of grade 2 or worse stomatitis occurred in one (11%) of nine patients.
Three patients needed everolimus dose reduction (median time to dose reduction was 55 days, IQR: 124). Four out nine patients developed mild stomatitis (grade 1-2). Three of nine patients discontinued everolimus due to other treatment-related AE, commonly fatigue and rash (Table 2).
The mouthwash was well tolerated with no disturbing effect. Adherence to DMF was 100%.
Two patients diagnosed with BC required dose reduction and treatment discontinuation was either due to progression disease (PD) or AE, in two and three patients respectively. It is remarkable that one patient is still on treatment without stomatitis after three years.
Patients with NET had a great range of everolimus treatment duration (3 weeks to 7 months). In both patients, treatment discontinuation also occurred owing to PD or AE.
Finally, the only RCC patient started with 5 mg everolimus due to hepatic disease. Five weeks later, patient developed PD.
In our cohort, no patient presented candidiasis due to DMF use.
DMF treatment cohortFive patients were treated with DMF, four women and one man, and median of age was 65 years old (IQR 18) (Table 2).
Dose interruptions/reductions were required in all patients excluding the one on everolimus. Interesantly, all patients achieved a severity grade stomatitis reduction after DMF therapy.
One patient was diagnosed with NET, another with NSCLC and three with colorectal cancer (CRC).
First patient initiated everolimus for NET and was discontinued due to G2 stomatitis one month later. After 2 weeks of DMF, stomatitis was reduced to G1 and everolimus was reintroduced. Then, stomatitis was resolved.
Second patient, with NSCLC, initiated afatinib. A reduction to 30 mg was required due to G2 stomatitis despite presenting partial response. Finally, afatinib was discontinued. After stomatitis resolution (G1) and PD evidence, 30 mg-afatinib was restarted with DMF. One month later, after total resolution of stomatitis an increase of afatinib to 40 mg was prescribed. No further stomatitis was observed, and more remarkably a partial response was evidenced. Six months after, afatinib treatment was reduced due G2 rash until PD.
First patient with a CRC and FOLFOX (leucovorin + fluorouracil + oxaliplatin) developed G3 stomatitis after 11 cycles. After bicarbonate and hyaluronate mouthwash without improvement, a dose reduction plus DMF was indicated resulting in a mild stomatitis improvement. Two cycles later, severe stomatitis reappeared and treatment was switched.
Second patient with CRC and FOLFOX, presented G2 stomatitis after first cycle. Consequently, fluorouracil dose was reduced and DMF was indicated four times daily during the first 5 days of the cycle. Finally, after several dose reductions, fluorouracil bolus was disrupted at fourth cycle due bad general tolerance to the treatment, including stomatitis.
Last patient with CRC started FOLFOX-bevacizumab. Besides other AE, G1 stomatitis appeared from first cycle and several dose reductions were performed. After 14 cycles stomatitis raised to G2, then DMF was indicated four times daily the first 5 days of the cycle. Stomatitis was reduced to G1 requiring lidocaine to reduce oral pain.
DiscussionTo our knowledge, this is the first study describing an alternative DMF for patients receiving HPSCT. This study also shows long-term DMF effectiveness and safety.
Our protocol differs from SWISH trial recommending DMF twice daily in the preventive cohort to improve adherence10, rising to a 100%, since the 95% compliance rate reported by SWISH may not reflect real true-to-life proportions3. Additionally, DMF was administered throughout antineoplastic treatment in the preventive cohort to avoid stomatitis recurrence.
In the preventive cohort, the incidence of grade 2 or worse stomatitis was lower than in the BOLERO-2 pivotal trial despite the reduced number of patients included (one (11%) of nine patients compared with 159 (33%) of 482 patients in BOLERO-2 study). DMF reduced the proportion of all-grade stomatitis by 27% and grade 2 or worse stomatitis by 59% compared with BOLERO-2 by 8 weeks.
Therefore, as in the SWISH study (the incidence of G2 or higher of stomatitis at 8 weeks was 2.4% and the incidence of all grades was 21.2%), a reduction in the occurrence of stomatitis was observed.
The most frequently AE leading to dose reductions/disruptions were consistent with those reported previously: pneumonitis, dyspnoea and fatigue2. Moreover, the major reason for discontinuation was PD.
Although not all patients improved on DMF treatment, no dose reduction/discontinuation was required because of stomatitis. Significantly, there were no adverse effects related to DMF, since no patient developed oral candidiasis, despite longer duration of DMF (median of duration 76.6 [98.3] days).
Finally, it was shown that DMF might be applicable to other indications with same effectiveness and safety results and with a longer duration of use than previously reported.
The most frequent AE of afatinib are rash, diarrhoea, stomatitis, nausea and anorexia11. Notably, despite no previous literature about DMF effectiveness in afatinib-induced stomatitis, this was resolved allowing full dose treatment resulting in another partial response after a PD during afatinib discontinuation. Despite our limited experience, DMF could be considered for patients on afatinib presenting stomatitis.
Drugs that affect DNA synthesis, such as 5-fluorouracil, are associated with incidences of oral mucositis close to 40-60%12. Several studies have shown that application of cryotherapy before, during, and up to 30 minutes after 5-fluorouracil bolus administration significantly reduces stomatitis12. DMF was employed on patients with severe 5-fluororuracil induced stomatitis refractory to other treatments. Unfortunately, results on these patients were not optimal. One possible explanation is that fluorouracil interferes in DNA synthesis, causing cell death, while everolimus and afatinib inhibit different cellular growth mechanism.
Study limitations include the low number of patients and the lack of randomization and controlled arm. However, is the first real-life study assessing efficacy and toxicity of DMF in different indications of everolimus and other HPSCT.
On the whole, DMF may be a suitable alternative to commercial product for stomatitis. Further research with more patients is needed to provide unequivocally evidence about efficacy of preventing and treating stomatitis with this DMF.
FundingNo funding.
Conflict of interestsNo conflict of interests.
Contribution to the scientific literature
On the basis of the SWISH trial, the use of prophylactic dexamethasone mouthwash is an option for the prevention of everolimus induced oral mucositis. However, there is a lack of commercially available mouthwash outside the United States. This study describes a new formulation of dexamethasone mouthwash and provides additional information regarding dosage frequency, other agents, and indications.
The use of a novel twice daily dexamethasone mouthwash formulation should be considered a potential option to prevent and treat the occurrence of everolimus-induced oral regardless its indication and possibly due to other antineoplastic agents.