Hepatocellular carcinoma is the most common and aggressive liver and biliary tumour. Hepatic chemoembolisation with doxorubicin-loaded DC Beads® is a local therapy for patients with localised nodes, which are not suitable for surgery. The objective of this study is to describe the clinical situations in which this procedure has been used and its early toxicity.
MethodsRetrospective descriptive study of patients treated with doxorubicin-loaded DC Beads® undergoing hepatic chemoembolisation from October 2006 until July 2009. Data were taken from the Farhos Oncología® programme and clinical histories.
ResultsTwenty-two patients were treated during the study period, 15 men and 6 women, with an average age of 66 years. This technique was used for patients diagnosed with unresectable liver cancer. Out of the patient total, 6 were on the liver transplant waiting list. Patients were assessed using the Child–Pugh score: 15 patients in group A, 5 in group B and 1 in group C; and according to Okuda staging system: 14 were in group I, 6 in group II and 1 in group III. The most common toxicity was post-chemoembolisation in 16 patients, who were treated with symptomatic medication.
DiscussionUsing doxorubicin-loaded microspherical DC Beads® during transarterial chemoembolisation has been adapted to use with scientific evidence and tolerated by all patients. Incidences during administration were mild and were resolved with symptomatic medication.
El carcinoma hepatocelular es el más común y agresivo del grupo de tumores hepatobiliares. La quimioembolización hepática con partículas DC Bead® cargadas de doxorrubicina es un tipo de terapia local para pacientes con nódulos localizados, no susceptibles de cirugía. El objetivo de este estudio es describir las situaciones clínicas en las que se ha utilizado este procedimiento y su toxicidad temprana.
MétodosEstudio descriptivo retrospectivo de los pacientes tratados con partículas DC Bead® cargadas de doxorrubicina en quimioembolización hepática desde octubre de 2006 hasta julio de 2009. Los datos se obtuvieron del programa Farhos Oncología® y las historias clínicas.
ResultadosDurante el periodo de estudio fueron tratados 21 pacientes, 15 hombres y 6 mujeres con una mediana de edad de 66 años. El diagnóstico que motivó la utilización de la técnica fue hepatocarcinoma no resecable. Del total de pacientes, 6 se encontraban en lista de espera para trasplante hepático. Los pacientes fueron clasificados según el sistema Child–Pugh: 15 pacientes en el grupo A, 5 en el grupo B y uno en el C, y según el Sistema Okuda: 14 pertenecían al grupo I, 6 al grupo II y uno al grupo III. La toxicidad más frecuente fue la aparición de síndrome posquimioembolización en 16 pacientes, que se resolvió con medicación sintomática.
DiscusiónLa utilización de doxorrubicina cargada en microesferas DC Bead® en quimioembolización transarterial se ha ajustado a usos con evidencias científicas y ha sido bien tolerado en todos los pacientes. Las incidencias durante la administración fueron leves y se resolvieron con medicación sintomática.
Hepatocellular carcinoma (HCC) is the most common of liver and biliary tumours. It is a very aggressive tumour, and is the fourth leading cause of cancer-related death in the world, and the third most common cancer in men, with 500000–1000000 deaths/year.1
Incidence depends on sex, race and geographical area. Sub-Saharan Africa and Southeast Asia have the highest incidence. Spain has intermediate incidence and mortality rates for this type of cancer, with a gross rate of 10–11/100000 inhabitants/year.2 The probability for 5-year survival after diagnosis does not reach 10%.2
HCC-related mortality is higher in men. In 2007, 1921 men and 659 women died in Spain due to primary liver cancer.3
The most important clinical risk factor for HCC development is hepatic cirrhosis, and the most common aetiologies are hepatitis B (HBV)- and/or C (HCV)-induced chronic infections and chronic alcoholism.4–7 Hepatitis B is the main cause of HCC in Asia and Africa, while it is hepatitis C in Europe, Japan and North America. There are other less common risk factors related to HCC development, such as hereditary haemochromatosis or carcinogenic agents, such as aflatoxins.
Older age and male sex are two HCC predictive factors in cirrhotic patients, although patients with a healthy liver may also develop HCC.5
The prevalence of HCC increases as liver function deteriorates. As a result, HCC is detected in 5% of patients with compensated hepatic cirrhosis; this percentage increases to 15% in patients who are admitted to hospital due to bleeding oesophageal varices and/or decompensated liver disease.5
There are several scales or scores that assess liver function, evaluate prognosis and/or predict survival of patients with chronic liver disease, particularly those with HCC.1,8 These scales help to decide what type of treatment must be performed on a given patient.
The Child–Pugh score assesses the liver function in patients with chronic liver disease, especially cirrhosis, dividing them into three classes, depending on the probability of survival: Child–Pugh A includes patients with mild compensated cirrhosis; Child–Pugh B includes patients with moderate cirrhosis; and Child–Pugh C includes those with severe cirrhosis.9,10
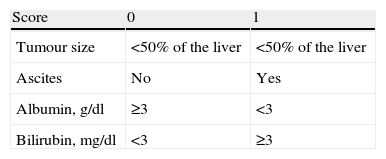
When a patient develops liver cancer, staging involves different aspects to assess the prognosis or predict survival. The Okuda staging system classifies patients in 3 stages, depending on the survival probability.1
Another important aspect that must be assessed is the patient's functional state. The Eastern Cooperative Oncology Group's (ECOG)11 quality of life scale is often used.
When the disease is at an advanced stage, there is no satisfactory treatment, as the tumour is resistant to chemotherapy agents. At present, there is an oral treatment, sorafenib, which taken as a monotherapy increases the median overall survival and median time to progression.12,13
When a patient presents with localised HCC, surgery is chosen as a curative treatment. Partial hepatectomy and liver transplant are among the surgical procedures that can be used. Partial hepatectomy is the treatment of choice for early stage cancer patients with a good liver function (Child–Pugh A) and no evidence of portal hypertension.9,10 It is indicated for HCC patients who have a single, small node (<5cm) or ≤3 nodes (≤3cm) within a segment, without vascular involvement. In these patients, 5-year overall survival rates have reached 50%–70%.
Liver transplant can be a curative treatment for patients with moderate-severe cirrhosis (Child–Pugh B–C), although it is also an option for patients with Child–Pugh A. It would be indicated in patients with unresectable cancer, which meet the UNOS criteria10: tumour ≤5cm diameter, or 2–3 tumours ≤3cm diameter, with no evidence of macrovascular involvement, lack of remote metastasis and with no contraindications for surgery.5,10,14
Patients suitable for local treatment are those who do not meet the requirements for surgical resection or liver transplant (given the extension of the tumour, reduced liver function or the patient's functional state) and who do not present with disseminated or metastatic disease.9,15 Its use as a bridge therapy until the liver transplant has also been described.10,14
The objective of local therapies is to induce tumour necrosis. They are normally performed using laparoscopic techniques or a percutaneous approach, and there are 2 types: ablation and embolisation.
Ablation is achieved by directly exposing the tumour to chemical substances (ethanol or acetic acid) or changes in temperature (radio frequency, microwave, and cryoablation). Ablation has proven to be more effective for small nodes (≤3cm) and used in combination with embolisation for lesions between 3 and 5cm.5,10,14
In general, transarterial embolisation (TAE) is a technique based on selective occlusion of the liver artery/ies that feed the tumour. When cytostatic drugs are perfused, this technique is called transarterial chemoembolisation (TACE). Radioembolisation is TAE with microspheres charged with yttrium-90. The objective is to selectively administer high doses of radiation on the liver tumour/s.5,16
The result is ischaemia and necrosis, with the subsequent cell death and possible tumour size reduction.5,10
Embolisation particles are made of a gelatin sponge, polyvinyl alcohol or polyacrylamide particles.17,18 Chemotherapy agents are used for TACE, such as doxorubicin-lipiodol emulsion to promote intratumoural retention.9,17,18 Llovet et al.19 were the first authors to show that TACE significantly improves survival, comparing it with the best support treatment.
Although TACE is a well-tolerated technique, some complications may arise such as acute portal vein thrombosis, cholecystitis, myelosuppression or post-chemoembolisation syndrome.5,9,14,17,20 The mortality rate associated with this technique is <5%, and the most severe cases have been due to arteriovenous fistulae in the tumour.10
A new TACE variant consists in using DC Beads®. They comprise a range of hydrogel microspheres that are biocompatible, hydrophilic, non-resorbable, precisely calibrated and capable of loading doxorubicin or other cytostatic agents,17,21 which are slowly released once deposited in the tumoural capillary bed. This new technique aims to minimise the quantity of free drug and the possible systemic effects.17,18
The DC Bead® particles are produced from polyvinyl alcohol and are available in different sizes, to be adapted in the blood vessel that is to be obliterated.18,21
Using doxorubicin-loaded DC Beads® for transarterial chemoembolisation is novel and studies published on the matter have obtained good results.18,22,23 Therefore, the objectives of this study are:
- 1.
To describe the clinical situations that doxorubicin-loaded DC Beads® have been used in for hepatic chemoembolisation, in a referral hospital.
- 2.
Describe early procedure-related toxicity.
We conducted a descriptive, retrospective study of the patients treated with transarterial chemoembolisation using doxorubicin-loaded DC Beads®.
We included all patients undergoing this procedure since it was first used in the hospital: from October 2006 to July 2009. Patients had been indicated chemoembolisation by doctors specialising in surgery, (digestive system) internal medicine, and vascular radiology techniques. All patients provided their informed consent.
Data were taken from the Farhos Oncología® programme and clinical records.
The following variables were recorded: age, sex, diagnosis, medical history, underlying liver disease (virus- or ethanol-induced cirrhosis, hepatitis, etc.), laboratory parameters, number and size of lesions, ECOG score, doxorubicin dose administered, medication used during chemoembolisation, and onset of adverse effects.
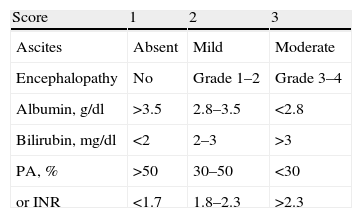
For each patient we calculated liver function in accordance with the Child–Pugh score (Table 1), and stage and prognosis in accordance with the Okuda staging system (Table 2).
Child–Pugh Score and Estimated Survival.
| Score | 1 | 2 | 3 |
| Ascites | Absent | Mild | Moderate |
| Encephalopathy | No | Grade 1–2 | Grade 3–4 |
| Albumin, g/dl | >3.5 | 2.8–3.5 | <2.8 |
| Bilirubin, mg/dl | <2 | 2–3 | >3 |
| PA, % | >50 | 30–50 | <30 |
| or INR | <1.7 | 1.8–2.3 | >2.3 |
| Stage | Score | 1-Year Survival | 2-Year Survival |
| Child–Pugh A | 5–6 | 100% | 85% |
| Child–Pugh B | 7–9 | 80% | 60% |
| Child–Pugh C | 10–15 | 45% | 35% |
PA: prothrombin activity; and INR: international normalised ratio.
For every procedure, the pharmacy department prepared 4ml of 300–500μm beads loaded with 150mg of doxorubicin (maximum recommended dose),18,21,24 mixed with iodixanol contrast. We used the quantity needed to obliterate the arterial bed of the treated tumours, until circulation in its afferent arteries stopped.
ResultsDuring the study period, 22 patients were scheduled to undergo the procedure. One patient had to be withdrawn from the treatment as high-grade portal vein fistulae were discovered prior to chemoembolisation. Twenty-one patients received treatment (15 men and 6 women), being an average of 66 years old (range 38–82 years).
All patients were previously diagnosed with HCC. None of the patients presented with remote metastasis or lymph node involvement.
Transplant was indicated for 6 patients (28.6%), which was used as a bridge therapy while the patient was waiting for a liver donor. The remaining 15 patients had been indicated this technique as a palliative measure, given that they were not eligible for surgery. Three of these 15 patients were staged once more after chemoembolisation and underwent surgical resection.
Fifteen patients (10 men and 5 women) had a history of cirrhosis, which had originated from chronic alcohol use in 5 of the men. No previous data were available for 3 of the patients.
Eleven patients had a history of hepatitis: 5 due to HCV (2 men and 3 women), 5 men were infected with HBV and one patient's aetiology was not indicated.
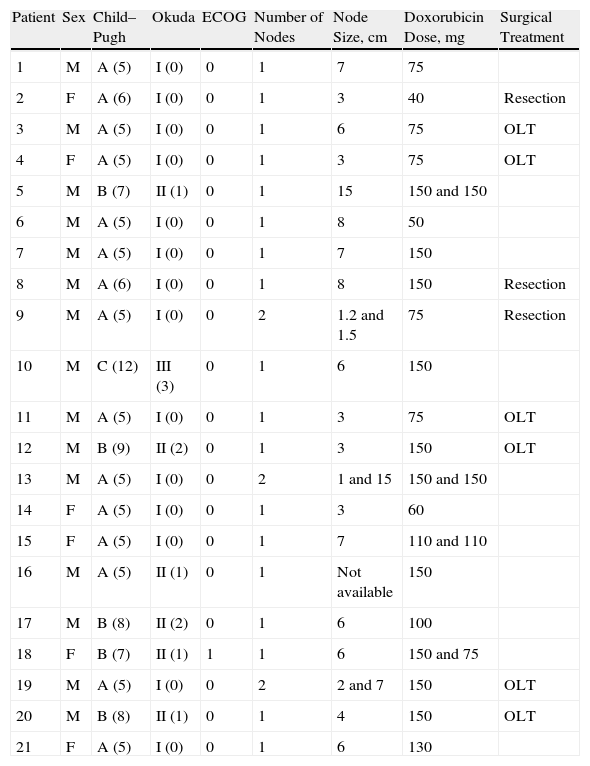
The laboratory results and other relevant clinical data, obtained before the chemoembolisation procedure, were used to calculate the patients’ general condition and staging (Table 3). The albumin levels ranged from 1.9mg/dl to 4mg/dl, bilirubin levels from 0.3mg/dl to 7.6mg/dl, and prothrombin activity from 45% to 100%. Four patients presented with ascites (2 mild and 2 moderate) and only one presented with mild encephalopathy.
General Health Scales, Staging, Number and Size of Nodes, Doxorubicin Dose Administered, and Surgical Treatment After Chemoembolisation.
| Patient | Sex | Child–Pugh | Okuda | ECOG | Number of Nodes | Node Size, cm | Doxorubicin Dose, mg | Surgical Treatment |
| 1 | M | A (5) | I (0) | 0 | 1 | 7 | 75 | |
| 2 | F | A (6) | I (0) | 0 | 1 | 3 | 40 | Resection |
| 3 | M | A (5) | I (0) | 0 | 1 | 6 | 75 | OLT |
| 4 | F | A (5) | I (0) | 0 | 1 | 3 | 75 | OLT |
| 5 | M | B (7) | II (1) | 0 | 1 | 15 | 150 and 150 | |
| 6 | M | A (5) | I (0) | 0 | 1 | 8 | 50 | |
| 7 | M | A (5) | I (0) | 0 | 1 | 7 | 150 | |
| 8 | M | A (6) | I (0) | 0 | 1 | 8 | 150 | Resection |
| 9 | M | A (5) | I (0) | 0 | 2 | 1.2 and 1.5 | 75 | Resection |
| 10 | M | C (12) | III (3) | 0 | 1 | 6 | 150 | |
| 11 | M | A (5) | I (0) | 0 | 1 | 3 | 75 | OLT |
| 12 | M | B (9) | II (2) | 0 | 1 | 3 | 150 | OLT |
| 13 | M | A (5) | I (0) | 0 | 2 | 1 and 15 | 150 and 150 | |
| 14 | F | A (5) | I (0) | 0 | 1 | 3 | 60 | |
| 15 | F | A (5) | I (0) | 0 | 1 | 7 | 110 and 110 | |
| 16 | M | A (5) | II (1) | 0 | 1 | Not available | 150 | |
| 17 | M | B (8) | II (2) | 0 | 1 | 6 | 100 | |
| 18 | F | B (7) | II (1) | 1 | 1 | 6 | 150 and 75 | |
| 19 | M | A (5) | I (0) | 0 | 2 | 2 and 7 | 150 | OLT |
| 20 | M | B (8) | II (1) | 0 | 1 | 4 | 150 | OLT |
| 21 | F | A (5) | I (0) | 0 | 1 | 6 | 130 |
OLT: orthotopic liver transplant.
Patients had between 1 and 9 nodes, and they varied in size, from 1cm to 15cm. Table 3 describes the embolised nodes (1–2 per patient) and their size.
Four patients (19%) needed 2 sessions to completely embolise the lesion, given that it was large in size or difficult to access. As a result, 25 hepatic chemoembolisations were performed during the study. The doxorubicin dose administered in each procedure is shown in Table 3.
Table 3 also shows the patients who underwent a liver transplant or surgical resection after chemoembolisation, during the study period.
For all cases, chemoembolisation was performed with local anaesthetic, and the vital signs were regularly controlled.
All patients received antibiotic prophylaxis with 1g of ceftriaxone.
Below are the incidences that occurred during the 25 chemoembolisations, which required administration of adjuvant drugs:
- -
Arterial spasms (in small vessels) occurred during 9 procedures while the catheter was being positioned, which made it impossible to continue the technique. 200μg of intravenous nitroglycerin was used as a peripheral vasodilator.
- -
On 7 occasions the patient presented with agitation during the intervention, requiring administration of 1mg of intravenous midazolam.
- -
Six patients felt pain, and analgesia included: 3.3mg of subcutaneous morphine was used in 3 cases; 2g of intravenous metamizol in 2 cases and 30mg of intravenous ketorolac in the remaining patients.
- -
Sublingual nifedipine was administered in 2 cases for increased arterial pressure.
- -
Only one patient presented with nausea which improved after administering 4mg of intravenous ondansetron.
In total, 14 of the 21 patients in the study (66.7%) needed complementary medication to be administered during the procedure.
During the following 24–48h, 16 patients (76.2%) presented with symptoms compatible with post-embolisation syndrome:
- -
Abdominal pain in the treatment site for 13 cases (62%), which eased with metamizol.
- -
Fever over 38.5°C in 10 cases (48%), which improved with paracetamol.
- -
Nausea and vomiting in 2 cases (10%), which were alleviated with metoclopramide.
There was one case in which a cutaneous exanthema developed, noting an erythematous rash on the abdomen. The patient was administered corticosteroids and systemic anti-histamines.
DiscussionLiver cancer is one of the most common and aggressive liver and biliary cancers at present, and the mortality rate associated with it is increasing in developed countries.1,4 Our findings show a predominance in older men (71%; average age 66 years) who have received TACE. This coincides with the data published on incidence and prevalence of liver cancer2–4 and with the characteristics of the patients included in other studies using similar techniques.22,23
The risk factor for our study population was established cirrhosis (79%), on many occasions due to chronic alcohol use. Previous studies have estimated that between 60% and 80% of HCC patients present with cirrhosis as the underlying disease and this percentage reaches 80%–90% when the histological data came from necropsias.4 Fifty-eight percent had a HCV- or HBV-induced infection. Fattovich et al.4 reported this association in 25%–75% patients (HCV) and 10%–55% (HBV).
Within the scope of this study, doxorubicin-loaded DC Beads® chemoembolisation has always been indicated for localised and partially unresectable HCC.24 This is a TACE indication widely established in the clinical guidelines.10 It was used as a bridge therapy for 6 patients, while they were awaiting their liver transplant. This use is based on data published by different authors.10,14,16,25,26 Millonig et al.25 showed a higher survival rate following liver transplant in some patients who reached a complete or partial tumoural response to previous chemoembolisation treatment.
In accordance with different recommendations10,18 liver cancer patients eligible for embolisation techniques are those who in addition to having an unresectable cancer, do not have extra-hepatic involvement, have good liver function (Child–Pugh: A or B), good prognosis (Okuda: 1–2) and good general health (ECOG: 0–1). The National Comprehensive Cancer Network (NCCN) recommendations10 add that patients with hepatic nodes >5cm should be eligible for this therapy. In this study, most patients met the criteria and had single nodes over 5cm. Patients included are similar to those in the Llovet et al.19 study, with the exception that our study included a patient who had bilirubin levels >5mg/ml. This level was an exclusion criterion in the Llovet et al. study and is an important contraindication for chemoembolisation according to the NCCN. These differences could be due to the indication for pre-transplant maintenance, which meant that patients with other characteristics could have been included. For example, a patient with a Child–Pugh C score and patients with several smaller nodes.
Using TACE with DC Beads® is supported by several authors’ results, which were reviewed by Kettenbach et al.18 and Marelli et al.17 More recent studies21,22,27 have compared this technique with conventional TACE, obtaining favourable results for chemoembolisation with loaded-beads, both for response21,27 and survival rates.22 Malagari et al.28 conducted a prospective, randomised study which was recently published, comparing DC Beads® chemoembolisation with traditional embolisation. They observed an increase in complete responses, fewer relapses and a higher progression-free median time in the first group.
The drugs used during the procedure were another data that the study recorded. The systematic use of antibiotic prophylaxis and local anaesthetic matches data reported in other studies.25,28 The studies consulted do not usually describe incidences or the drugs used during the procedure, but the Malagari et al. study28 (2010) does comment on the use of antibiotic prophylaxis and corticosteroids, gastric protectors, and antiemetics. Analgesics were also used to control pain and fever from the post-embolisation syndrome. In our case, it seems relevant that 67% of patients needed support medication: nitroglycerin as an arterial vasodilator, midazolam as a sedative, analgesics and antiemetics. It would be interesting to find this incidence in more studies and compare the drugs used.
It has been reported that the procedure with DC Beads® is safer than the conventional TACE, probably due to the hepatic parenchyma toxicity associated with lipiodol administration, and mainly, given that doxorubicin is released much more slowly from the beads, reaching much lower systemic levels.18,21,27
In our study, the toxicity profile of the technique during the early stage was good. Mild adverse effects were reported for 76% of patients as abdominal pain, fever and nausea. All these adverse effects were within the symptomatology of post-chemoembolisation syndrome, which is widely documented5,9,14,17,19,21,28 and they eased with symptomatic medication. The incidence of this syndrome varied from 25% described by Lammer et al.27 and 40%–85% reported by Wigmore et al.,20 to 80%–100% in the Malagari et al.28,29 studies. This was considered the result of hepatic necrosis, caused by arterial occlusion and cytotoxic action of doxorubicin.
Among the less common adverse reaction is the cutaneous exanthema, which developed from an erythematous rash on the abdomen and improved with systemic corticosteroids and anti-histamines. Two studies published by Malagari et al.28,29 describe similar cases. It is normally a delayed cutaneous anaphylactic reaction, frequently reported with iodinated contrasts, especially iodixanol, and also doxorubicin.
One of this study's limitations is that we did not interview the patients but data were taken from clinical records, which could result in an underestimation, i.e., we may have detected fewer symptoms.
To conclude, the use of doxorubicin-loaded DC Beads® has been adjusted to indications based on scientific evidence and has mostly followed the recommendations featured in clinical guidelines. Furthermore, it has been tolerated well in all patients and incidence rates during administration were mild and resolved with symptomatic drugs.
Conflict of InterestThe authors affirm that they have no conflicts of interest.
Please cite this article as: Muros-Ortega M, et al. Experiencia de uso de partículas DC Bead® cargadas con doxorrubicina en quimioembolización hepática. Farm Hosp. 2011; 35(4):172–179.