High-risk medications (HRMs) are those with a high probability of causing severe or fatal harm when errors occur during their use. Enhancing safety in the management of HRMs is a priority for healthcare authorities. This study presents a multidisciplinary program designed to optimize the safe use of HRMs in adult inpatients at a Level 5 hospital.
ObjectivesThe objectives of the program are: a) To adapt national and international standards on HRM safety to the local setting. b) To define the competencies and responsibilities of the professionals involved. c) To increase the engagement of stakeholders in HRM safety. d) To establish indicators to evaluate the interventions.
MethodsThe program was developed in three phases: 1) Initial situation analysis: Assessment using the Institute for Safe Medication Practices’ self-assessment questionnaire on medication safety. 2) Protocol development: Creation of a local protocol based on a literature review and multidisciplinary consensus. 3) Action plan implementation: Dissemination, monitoring, and periodic updates of the protocol.
ResultsThe developed protocol included seven general measures, 29 specific measures, and five indicators to evaluate its impact. After the first year of implementation: 71.5% of HRMs were stored in high-security locations within automated dispensing systems, 71.36% of HRM prescriptions were validated within the first 24 hours and a total of 4,366 pharmaceutical interventions were performed.
Additional measures implemented included: Tall Man Lettering across all information systems, automated alerts for maximum doses in prescribing systems and alerts prompting independent double-checking during HRM dispensing.
ConclusionsThis program is an effective and transferable model for improving HRM safety in complex hospital settings and fostering a safety culture. Future initiatives should focus on developing a national dashboard with standardized indicators to enable inter-hospital comparisons and identify areas for improvement.
se denominan medicamentos de alto riesgo (MAR) aquellos que tienen probabilidad elevada de causar daños graves o mortales cuando se produce un error durante su utilización. Mejorar la seguridad en el manejo de los MAR es una meta prioritaria para las autoridades sanitarias. Este estudio presenta un programa multidisciplinar diseñado para optimizar el uso seguro de los MAR en pacientes adultos hospitalizados en un hospital de clase 5.
Objetivoslos objetivos del programa son: a) adaptar los estándares nacionales e internacionales sobre seguridad de los MAR al entorno local. b) Definir las competencias y responsabilidades de los profesionales implicados. c) Incrementar la implicación de los agentes involucrados en la seguridad de los MAR. d) Establecer los indicadores para evaluar las intervenciones.
Métodosel programa se desarrolló en 3 fases: 1) Análisis de situación inicial: evaluación mediante el cuestionario de autoevaluación del Instituto para el uso seguro de medicamentos sobre seguridad en el uso de medicamentos. 2) Elaboración del protocolo: creación de un protocolo local basado en una revisión bibliográfica y consenso multidisciplinar. 3) Ejecución del plan de acción: difusión, seguimiento y actualización periódica del protocolo.
Resultadosel protocolo desarrollado incluyó 7 medidas generales, 29 específicas y 5 indicadores para evaluar su impacto. Entre los logros destacados, tras el primer año de instauración del protocolo, se encontró que el 71,5% de los MAR fueron almacenados en ubicaciones de alta seguridad en los sistemas automatizados de dispensación, el 71,36% de las prescripciones de MAR fueron validadas en las primeras 24 horas, y se realizaron 4.366 intervenciones farmacéuticas. Además, se implementaron medidas como el Tall Man Lettering en todos los sistemas de información, alertas automatizadas para dosis máximas en los sistemas de prescripción y alertas sobre la necesidad en la administración de doble chequeo independiente durante la dispensación de MAR.
Conclusioneseste programa es un modelo eficaz y transferible a otros centros para mejorar la seguridad en el manejo de MAR en entornos hospitalarios complejos y fomentar una cultura de seguridad. Futuras iniciativas deberían centrarse en desarrollar un cuadro de mandos con indicadores estandarizados a nivel nacional para poder comparar entre centros y detectar áreas de mejora.
Safety in the use of medicines has become a priority of health systems worldwide. Medication errors (MEs) result in the occurrence of adverse events in patients, have a psychological impact on the healthcare professionals involved, and may ultimately cause a loss of trust in institutions. According to the World Health Organization (WHO), MEs cause at least one death every day and injure approximately 1.3 million people annually in the United States.1 Specifically, high-alert medications (HAMs) have become a priority issue for international, national and regional health authorities.
HAMs are medications with a higher potential for causing severe or life-threatening harm when administered improperly. Healthcare authorities and scientific societies have given priority to HAM safety best practices to prevent medication errors and minimize potential damage to patients. The concept of ‘high-alert medications’ emerged from the need for defining target medicines on which efforts should be focused. The identification of priority medicines facilitates the development of interventions for improving patient safety, in view of the high complexity of medication use processes and the large number of medications available.2
The third WHO global patient safety challenge “Medication Without Harm” urged its members to focus their patient safety efforts on three priority areas, including high-risk situations, which involve HAMs.3
The Spanish Ministry of Health, in collaboration with the autonomous communities, promoted the implementation of safety practices for HAMs through the Patient Safety Strategy of the National Health System (2015–2020 period). This Strategy is aimed at fostering the implementation of HAM-related safety practices and includes recommendations to establish specific interventions to prevent the most frequent errors in the use of these medications.4
Given the relevance of HAMs for patient safety, a multidisciplinary Program was designed to pursue a comprehensive approach to the use of HAMs in adult patients hospitalized in a class-5 hospital.5 This Program was developed within the framework of a local patient safety Program coordinated by the hospital pharmacists, where physicians and the nursing staff of different specialties and areas were actively involved.
The purpose of this publication is to share our experience in the implementation of safety practices for the use of HAMs in the hospital setting.
The objectives of our project included (i) adapting widely accepted national and international standards and best practices for the safe use of HAMs; (ii) developing a protocol that establishes the duties and responsibilities of the professionals involved; (iii) promoting the engagement of all the stakeholders involved in the safe use of medicines; and (iv) selecting a set of assessment items to identify areas for improvement.
MethodsThe project was divided into three phases. In the first phase, an analysis of baseline HAM-related safety practices was performed. Secondly, a local safety protocol was designed for these medications. Finally, a plan of action was developed for the dissemination and monitoring of the protocol.
State of affairsIn March 2022, the Pharmacy Safety Task Group, in coordination with the hospital Patient Safety Committee and the Risk Management Committee, agreed to prioritize interventions for a safe use of HAMs. The baseline situation was analyzed using a self-assessment questionnaire to evaluate safety HAM practices in hospitals of the Institute for Safe Medication Practices (ISMP).6 The analysis was performed by a multidisciplinary team including members of the Hospital Pharmacy, the Quality Assurance Unit, the Managing Board, and physicians and nurses from different hospital specialties (oncology, intensive care, pediatrics, internal medicine, pediatric surgery and urology).
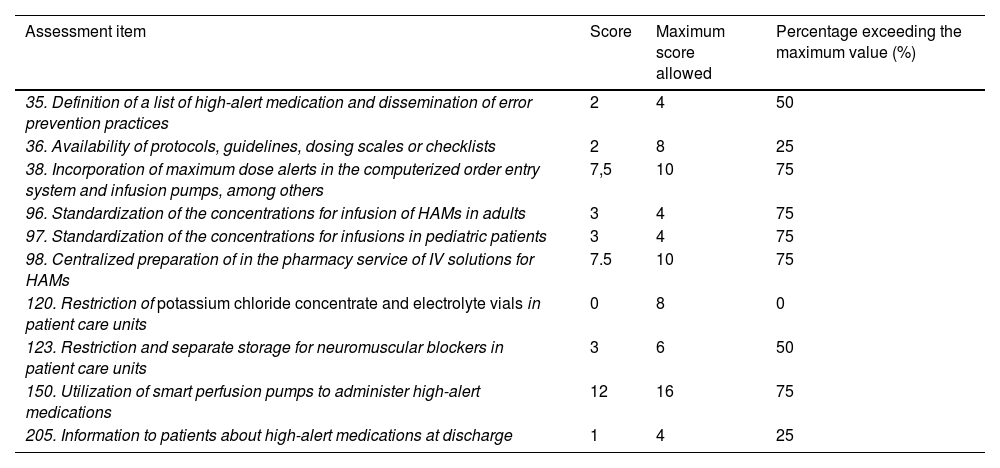
The items with a score < 50% were prioritized.
Design of a local protocolThe Pharmacy Safety Task Group conducted a systematic literature review to identify the safety practices most widely recommended by relevant quality assurance and patient safety agencies in relation to HAMs. PubMed and official WHO, ISMP and Spanish Ministry of Health documents were searched for relevant information. Search terms included «high-risk medications», «medication errors», «patient safety» and «safe medication practices». All articles published within the last ten years in English and Spanish were included. Priority was given to systematic literature reviews, clinical practice guidelines, and publications from relevant institutions.
A Task Group composed of healthcare professionals was commissioned to develop a local protocol for the safe administration of HAMs. The composition of the Task Group was as follows: three hospital pharmacists; the pharmacy supervisor; a specialist in anesthesiology and resuscitation; a surgery block supervisor; and a medical hospitalization supervisor.
The protocol was structured into:
- •
General measures
- •
Specific measures for high-alert units or medications and specific stages of the drug use process
- •
Items for assessing the efficacy and level of compliance with the measures adopted
- •
Dissemination strategy
- •
Updating strategy
- •
Annex (list of HAMs included in the local pharmacotherapy guide).
The Protocol was reviewed and approved by the Risk Management Unit and the Quality Assurance Unit in November 2022.
Plan of ActionA literature search was performed to establish the roadmap of the Pharmacy Safety Task Group. In 2023, priority was granted to the implementation of new practices, which would be included as milestones attained in the next protocol update.
ResultsThe scores obtained by our hospital in the self-assessment questionnaire for HAM-related items are shown in Table 1.
Scores obtained for the items related to high-alert medications on the ISMP Medication Safety Self-Assessment for Hospitals.
| Assessment item | Score | Maximum score allowed | Percentage exceeding the maximum value (%) |
|---|---|---|---|
| 35. Definition of a list of high-alert medication and dissemination of error prevention practices | 2 | 4 | 50 |
| 36. Availability of protocols, guidelines, dosing scales or checklists | 2 | 8 | 25 |
| 38. Incorporation of maximum dose alerts in the computerized order entry system and infusion pumps, among others | 7,5 | 10 | 75 |
| 96. Standardization of the concentrations for infusion of HAMs in adults | 3 | 4 | 75 |
| 97. Standardization of the concentrations for infusions in pediatric patients | 3 | 4 | 75 |
| 98. Centralized preparation of in the pharmacy service of IV solutions for HAMs | 7.5 | 10 | 75 |
| 120. Restriction of potassium chloride concentrate and electrolyte vials in patient care units | 0 | 8 | 0 |
| 123. Restriction and separate storage for neuromuscular blockers in patient care units | 3 | 6 | 50 |
| 150. Utilization of smart perfusion pumps to administer high-alert medications | 12 | 16 | 75 |
| 205. Information to patients about high-alert medications at discharge | 1 | 4 | 25 |
IV: intravenous.
The main bibliographic databases were searched for relevant papers on safety medication practices. Based on the results of the review, a local protocol was designed entitled Using high-alert medications safely.
A review of the following recommendations from relevant authorities was performed to identify best practices:
WHO: Medication safety in high-risk situations.3
Ministry of Health: Practices to improve HAM safety.7
ISMP: Medication Safety Self Assessment for Hospitals (version 2).6
ISMP: List of HAMs.8
Recommendations for the safe use of automated medication dispensing systems, prepared by the ISMP in collaboration with the TECNO task group of the Spanish Society of Hospital Pharmacy.9
The Protocol included seven general safety measures related to the use of HAMs:
- •
Establish a list of the HAMs available at the hospital (see Annex).
- •
Provide training and education to the healthcare professionals involved in the HAM administration process.
- •
Provide access to this Protocol and the HAM list on the hospital Intranet to all local professionals.
- •
Promote the reporting of detected errors to the Integrated Safety and Emergency Center (CISEM).
- •
Change the name of “look alike/sound alike” high-alert medications using highlighted Tall Man lettering10 on unit-dose packages, electronic prescriptions, automated dispensing cabinets (ADCs) and other automated systems for the administration of AHMs.
- •
Foster communication between the Hospital Pharmacy and other local clinical units by disseminating alerts and relevant drug-related information among supervisors via email; disseminate this information to all the professionals involved in the administration of these medications.
- •
Monitor the level of implementation of specific detailed measures in the protocol based on the use of indicators.
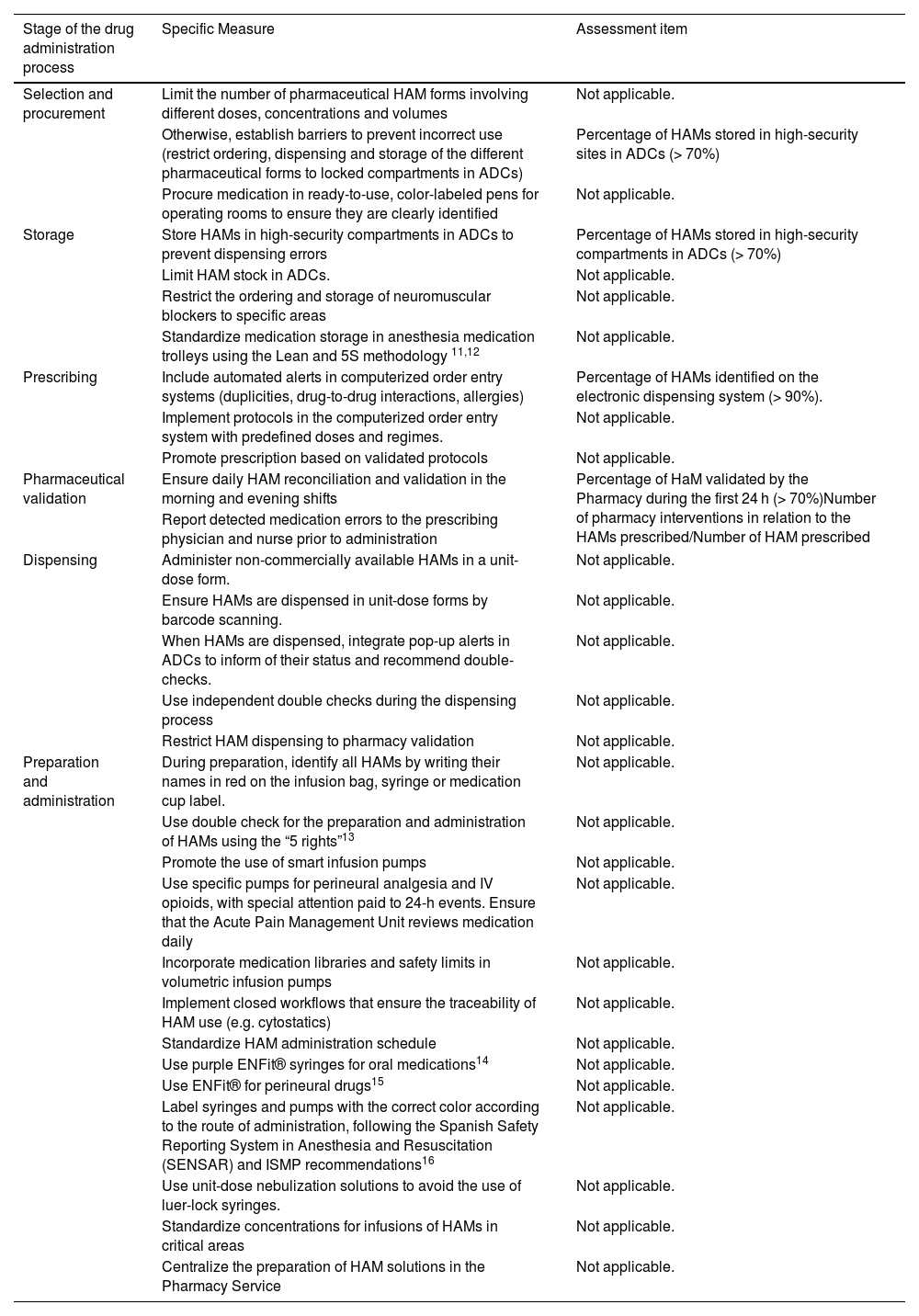
The Protocol includes 30 specific measures to be implemented at all stages of the medication use process (Table 2).
Specific measures for a safe use of high-alert medications (HAMs) and assessment items.
| Stage of the drug administration process | Specific Measure | Assessment item |
|---|---|---|
| Selection and procurement | Limit the number of pharmaceutical HAM forms involving different doses, concentrations and volumes | Not applicable. |
| Otherwise, establish barriers to prevent incorrect use (restrict ordering, dispensing and storage of the different pharmaceutical forms to locked compartments in ADCs) | Percentage of HAMs stored in high-security sites in ADCs (> 70%) | |
| Procure medication in ready-to-use, color-labeled pens for operating rooms to ensure they are clearly identified | Not applicable. | |
| Storage | Store HAMs in high-security compartments in ADCs to prevent dispensing errors | Percentage of HAMs stored in high-security compartments in ADCs (> 70%) |
| Limit HAM stock in ADCs. | Not applicable. | |
| Restrict the ordering and storage of neuromuscular blockers to specific areas | Not applicable. | |
| Standardize medication storage in anesthesia medication trolleys using the Lean and 5S methodology 11,12 | Not applicable. | |
| Prescribing | Include automated alerts in computerized order entry systems (duplicities, drug-to-drug interactions, allergies) | Percentage of HAMs identified on the electronic dispensing system (> 90%). |
| Implement protocols in the computerized order entry system with predefined doses and regimes. | Not applicable. | |
| Promote prescription based on validated protocols | Not applicable. | |
| Pharmaceutical validation | Ensure daily HAM reconciliation and validation in the morning and evening shifts | Percentage of HaM validated by the Pharmacy during the first 24 h (> 70%)Number of pharmacy interventions in relation to the HAMs prescribed/Number of HAM prescribed |
| Report detected medication errors to the prescribing physician and nurse prior to administration | ||
| Dispensing | Administer non-commercially available HAMs in a unit-dose form. | Not applicable. |
| Ensure HAMs are dispensed in unit-dose forms by barcode scanning. | Not applicable. | |
| When HAMs are dispensed, integrate pop-up alerts in ADCs to inform of their status and recommend double-checks. | Not applicable. | |
| Use independent double checks during the dispensing process | Not applicable. | |
| Restrict HAM dispensing to pharmacy validation | Not applicable. | |
| Preparation and administration | During preparation, identify all HAMs by writing their names in red on the infusion bag, syringe or medication cup label. | Not applicable. |
| Use double check for the preparation and administration of HAMs using the “5 rights”13 | Not applicable. | |
| Promote the use of smart infusion pumps | Not applicable. | |
| Use specific pumps for perineural analgesia and IV opioids, with special attention paid to 24-h events. Ensure that the Acute Pain Management Unit reviews medication daily | Not applicable. | |
| Incorporate medication libraries and safety limits in volumetric infusion pumps | Not applicable. | |
| Implement closed workflows that ensure the traceability of HAM use (e.g. cytostatics) | Not applicable. | |
| Standardize HAM administration schedule | Not applicable. | |
| Use purple ENFit® syringes for oral medications14 | Not applicable. | |
| Use ENFit® for perineural drugs15 | Not applicable. | |
| Label syringes and pumps with the correct color according to the route of administration, following the Spanish Safety Reporting System in Anesthesia and Resuscitation (SENSAR) and ISMP recommendations16 | Not applicable. | |
| Use unit-dose nebulization solutions to avoid the use of luer-lock syringes. | Not applicable. | |
| Standardize concentrations for infusions of HAMs in critical areas | Not applicable. | |
| Centralize the preparation of HAM solutions in the Pharmacy Service | Not applicable. |
ISMP: Institute for Safe Medication Practices; HAMs: High-alert medications.
Five items were established to assess the level of compliance with some of the measures. Items were selected by consensus considering their suitability for automated computation. Table 2 shows the specific measure that is evaluated with each item. The indicators selected included:
- 1.
Availability of an updated protocol (maximum validity of 2 years) for a safe use of HAMs including a list of ingredients (Yes/No).
- 2.
Number of HAMs stored in high- or maximum-security locations/total number of HAMs in the ADC, except for thermolabile medications (>70%).
- 3.
Number of medications labeled as HAMs in the computerized order entry system and the ADC/number of true HAMs (>90%).
- 4.
Number of pharmaceutically validated HAMs/number of HAMs prescribed via the electronic prescribing system (>70%).
- 5.
Number of pharmacy interventions in relation to the HAMs prescribed/Number of HAM prescribed (without a reference value).
It was agreed to update the Protocol biannually and perform item monitoring annually.
The milestones achieved by the Medication Safety Task Group throughout 2023 included:
- •
Identification of HAMs in electronic prescriptions with the standard symbol established by the Spanish Ministry of Health and the ISMP.
- •
Modification of the name of “look alike/sound alike” HAMs by adopting Tall Man lettering in unit-dose packages, electronic prescriptions, ADCs and other automated systems related to medication use.
- •
Inclusion of an alert in ADCs on the need for an independent double check every time a HAM is administered to a patient.
- •
Integration of maximum dose alerts for HAMs in electronic prescriptions. A total of 5361 alerts were included for 213 active ingredients. Alerts reported the maximum daily dose per administration and weight, for the different age ranges (newborns, infants, preschoolers, schoolchildren, adolescents, adults and older adults).
- •
Calculation of the items suggested, with 71.51% of HAMs stored in high-security AD compartments; 100% of HAMs identified as such in electronic prescriptions; 71.36% of HAMs validated within the first 24 h following prescription; and 4366 pharmacy interventions related to HAMs (with 72.3% having been accepted).
A comprehensive HAM use Program was developed in a class-5 hospital. The Protocol was developed taking into account the items for improvement identified through the ISMP self-assessment questionnaire. The areas for improvement identified included: design and dissemination of a HAM list; availability of protocols, guidelines, dosing scales or checklists; and restricted access to neuromuscular blockers and potassium chloride concentrate vials. To address these areas, the Protocol included labeling HAMs as high-alert medications in information systems, using Tall Man lettering, and storing HAMs in high-security ADC compartments.
One of our main achievements was the adaptation of the safety practices recommended by agencies such as the WHO to the characteristics of our hospital. Later, after our Program was implemented, in 2023, the Ministry of Health published its Safety HAM Practices which included most of the measures adopted in our hospital.17 In 2023, a survey was conducted to assess the level of implementation of the Ministry's Safety HAM Practices. As compared to other participating hospitals, our hospital had a lower score in some items (such as HAMs list dissemination or restriction of potassium chloride concentrate ampoule/vial stock), which indicates that some measures need to be adopted or intensified to prioritize these items.18
Collaboration between the Pharmacy Department, information systems and technology equipment made it possible to include alerts in computerized order entry systems and ADCs, thereby facilitating HAM traceability and ultimately minimizing errors. The participation of multidisciplinary teams, added to institutional support, was key to the success of the Program, which promoted a safety culture.
One of the strengths of this project lies on the design and use of items that enable the continuous evaluation of the Program. These items will help identify areas for improvement as the hospital environment changes and new technologies become available.
One of the limitations of this project is that protocols and operating procedures need to be periodically updated and reviewed. This is necessary to guarantee their validity and ensure compliance with the standards or recommendations of patient safety agencies. Another limitation was that removal of concentrated potassium chloride vials from wards was not included in the first phase. The reason was that adopting this measure would have delayed the implementation of the other measures. A variety of strategies are currently under consideration, such as the standardization of fluid replacement therapy to facilitate the integration of this measure in a near future.
This unprecedented experience in the implementation of safety practices throughout the whole HAM administration process in the hospital setting is applicable to other centers. The strategic development of a national standardized dashboard would ensure the homogeneity and comparability of data across centers. Additionally, this tool would help identify best practices and areas for improvement.
In conclusion, the Program demonstrated to be an effective, applicable tool for ensuring patient safety in relation to the use of HAMs in a complex hospital setting. The implementation of this comprehensive, multidisciplinary approach represents a significant step forward in reducing high-alert medication errors in the hospital setting.
CRediT authorship contribution statementCanales-Siguero Maria Dolores: Writing – original draft, Methodology, Conceptualization. Caro Teller Jose Manuel: Supervision, Project administration, Methodology, Data curation, Conceptualization. Pablos Bravo Siria: Validation, Supervision, Project administration, Methodology, Conceptualization. Quintana Estelles Delicias: Visualization, Project administration, Methodology, Conceptualization. Gomis Muñoz Pilar: Supervision, Resources, Project administration, Conceptualization. Tejido Sanchéz Angel: Visualization, Project administration, Methodology, Conceptualization. Ferrari Piquero Jose Miguel: Visualization, Validation, Supervision, Resources, Project administration, Methodology, Conceptualization.
FundingThe authors did not receive any funding for the preparation of this manuscript.
The authors declare no conflict of interest.