To identify and promote initiatives aimed at improving the management by hospital pharmacists of patients with congenital coagulopathies in the Spanish healthcare context.
MethodA series of initiatives to improve the care of patients with congenital coagulopathies were identified, evaluated, and prioritized by a panel of hospital pharmacists. Prioritization was based on an assessment of each initiative's impact and feasibility on a scale of 1 to 5. Once initiatives were prioritized, those assigned the highest priority were grouped into three action areas.
ResultsSeven areas of activity were identified in which the role of hospital pharmacists is key for the management of patients with congenital coagulopathies: coordination with the healthcare team; drug evaluation and selection; dispensing; patient information and education; pharmacotherapeutic follow-up; research and innovation in the field of congenital coagulopathies; and capacity-building and training of hospital pharmacists. Fifteen initiatives were considered a priority, with an average impact score ≥ 3.8 and a feasibility score ≥ 3.2. A total of, 29.4% of the prioritized initiatives corresponded to healthcare, 23.5% to patient information and education, 11.8% to drug evaluation and selection, 11.8% to pharmacotherapeutic monitoring, 11.8% to cross-sectional initiatives, 5.9% to dispensing and 5.9% to research and innovation in the field of congenital coagulopathies: In contrast, initiatives related to capacity-building and training were not prioritized.
ConclusionsThree main action areas were proposed based on the initiatives identified as high priority for the management of patients with congenital coagulopathies by a panel of 16 hospital pharmacists. Action areas revolved around specific activities that hospital pharmacy departments can undertake to contribute to improving the healthcare situation in Spain.
Identificar e impulsar iniciativas orientadas a la mejora del manejo de los pacientes con coagulopatías congénitas por parte de farmacia hospitalaria en el contexto sanitario español.
MétodoSe identificaron, evaluaron y priorizaron, por parte de un panel de farmacéuticos especialistas en farmacia hospitalaria, iniciativas para la mejora de la atención a los pacientes con coagulopatías congénitas. La priorización se realizó en base a la valoración de su impacto y factibilidad en una escala del 1 al 5. Una vez obtenida la priorización de las iniciativas, las de mayor puntuación se agruparon en tres grandes líneas de actuación.
ResultadosSe identificaron siete áreas de actividad en las que el papel de los farmacéuticos especialistas en farmacia hospitalaria resulta clave para el manejo del paciente con coagulopatías congénitas: coordinación con el equipo asistencial de pacientes con coagulopatías congénitas; evaluación y selección de medicamentos; dispensación; información y formación al paciente; seguimiento farmacoterapéutico; investigación e innovación en estas patologías; formación y capacitación continuada del farmacéutico especialista en farmacia hospitalaria. Se consideraron prioritarias 15 iniciativas por tener una puntuación media de impacto ≥ 3,8 y factibilidad ≥ 3,2. Así, el 29,4% de las iniciativas priorizadas pertenecen al ámbito asistencial, el 23,5% a información y formación al paciente, el 11,8% a evaluación y selección de medicamentos, el 11,8% al seguimiento farmacoterapéutico, el 11,8% a iniciativas transversales, el 5,9% a dispensación y el 5,9% a investigación e innovación en el campo de las coagulopatías congénitas, mientras que las iniciativas referentes a la formación y capacitación a profesionales no resultaron priorizadas.
ConclusionesSe han propuesto tres grandes líneas de actuación basadas en las iniciativas identificadas como altamente prioritarias por un panel de 16 expertos farmacéuticos especialistas en farmacia hospitalaria para el manejo de pacientes con coagulopatías congénitas. Estas iniciativas se basan en acciones concretas y pueden llevarse a cabo desde los servicios de farmacia hospitalaria, por lo que se cree que podrán llegar a tener un impacto real en el contexto sanitario español.
Congenital coagulopathies (CCs) are a series of conditions characterized by bleeding disorders resulting from blood clotting alterations. They are complex difficult-to-manage chronic conditions, the most significant of which in terms of both prevalence and severity are hemophilia A and B and von Willebrand's disease1.
At present, there are around 400,000 people with hemophilia worldwide, of which nearly 3,000 live in Spain2. Approximately 80-85% of cases are type A hemophilia, which is associated to a deficiency in coagulation factor VIII; patients with type B hemophilia, where the deficient clotting factor is factor IX, account for the remaining 15-20%3. Both types of hemophilia are hereditary x-linked recessive conditions, where men suffer from the disease while women act as carriers of the affected gene4. The main symptom of these conditions is potentially life-threatening recurrent internal or external bleeds, which may be spontaneous or induced by slight trauma. Fifty percent of patients suffer from severe hemophilia A1. The most formidable complication caused by bleeding is hemarthrosis, a hemophilic arthropathy that may cause severe mobility restrictions. Moreover, patients may present with muscle hematomas as well as neurologic disorders, compartment syndrome, shortness of breath, hypovolemia and intracranial hemorrhage1.
Standard treatment of hemophilia is based mainly on replacement therapy, i.e., intravenous infusions of the deficient coagulation factor aimed at treating or preventing bleeds. Such intravenous infusions may be administered on demand or prophylactically and, in Spain, they can be self-administered by patients if they are 8 years of age or older. The amount of deficient coagulation factor to be infused and the frequency of administration depend on the type of hemophilia and the severity, type and location of the bleeds5. The most significant complication associated with replacement therapy is the development of inhibitors, which typically result in an increase in bleeding risk, reducing the patients’ quality of life and increasing their mortality2.
As mentioned above, another example of a CC is von Willebrand's disease, a hereditary autosomal recessive condition where a disruption in the gene coding for Von Willebrand factor (vWF) produces qualitative and/or quantitative alterations in the vWF. The prevalence of this condition is 1-3%1, and its most characteristic symptom is the appearance of mucocutaneous bleeds of variable intensity6. As with hemophilia, three levels of severity can be distinguished in von Willebrand's disease, depending on the plasma concentration of vWF. The most severe phenotype is the most unusual one7.
Treatment of von Willebrand's disease currently consists in the administration of antifibrinolytics and desmopressin, which trigger the release of the vWF that could have built up in endothelial cells. This treatment is not indicated in all patients, with recourse to exogenous replacement factor or other alternatives being necessary in cases where it cannot be administered1,8.
There are other CCs that result in a deficiency or alteration of other factors or proteins involved in hemostasis. It is not easy to fathom the real incidence of these CCs and replacement treatment of the deficient or altered factors is not always available1.
As CCs are hereditary and, for the time being, incurable chronic conditions, the people who suffer from them are forced to live with situations that negatively impact their quality of life. These situations are highly diverse and are usually related to three kinds of factors: biological factors (pain, complications derived from the treatments, comorbidities, multiple surgical procedures; prolonged hospital stays, etc.); psychological factors (pain, stress, feelings of guilt, depression, low perceived social support, poor adherence to treatment, etc.); and social factors (negative reactions by family members, lack of support networks, financial instability, difficulties in managing pediatric patients, difficulty to travel, etc.)9.
The main contribution of hospital pharmacists to patients, society and the health system consists in promoting the improvement of patients’ health outcomes by providing a pharmacological therapy adapted to their individual characteristics; optimizing the effectiveness, efficiency and safety of the treatments; making available all the information they need; and promoting clinical continuity across all levels of care10.
The purpose of this study was to identify and promote initiatives geared towards improving the way hospital pharmacy departments manage patients with CCs, thus contributing to obtaining the best health outcomes for these patients in a changing healthcare environment.
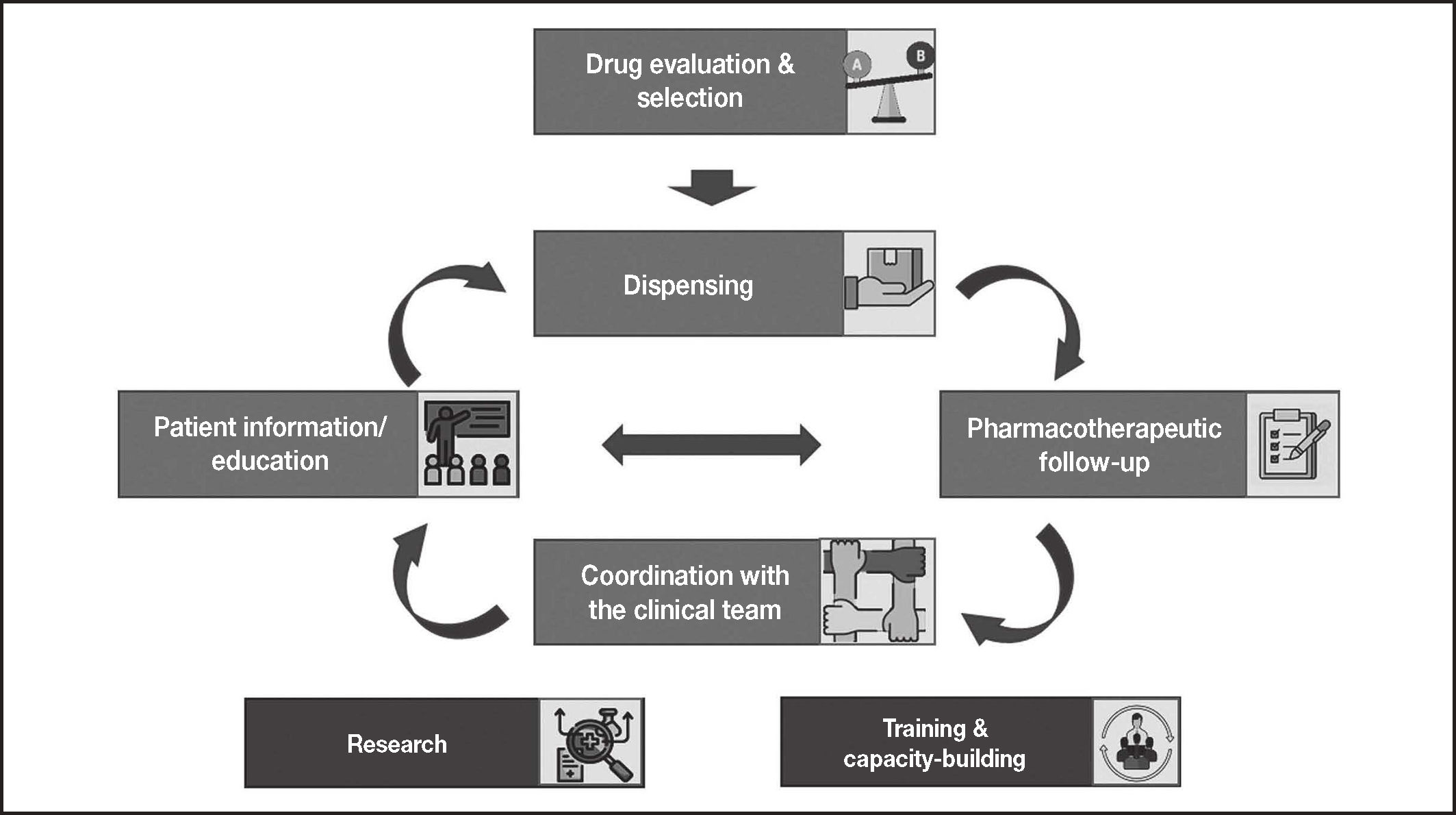
MethodsThe authors came together in a coordinating committee charged with identifying the most significant milestones along the journey travelled by patients with CCs, particularly hemophilia, where hospital pharmacists (HPs) have a role to play (Figure 1). The Committee was also responsible for identifying the key actions performed by HPs as well as the main needs patients may have at each stage along their journey.
Subsequently, a group of 16 HPs was created, all of them experienced in the management and treatment of patients with CCs, to identify and undertake initiatives aimed at improving the standard of care provided to these patients. To this effect, a structured pre-read was sent to each HP to promote individual reflection. At a later stage, a debate was held on the key attributes of HPs and the challenges they faced, and on the current needs of patients with CCs. Particular emphasis was laid on the areas where HPs could add the greatest value to the management of these patients and on the importance of ensuring proper coordination between the work of HPs and that of the other members of the clinical team. Taking these challenges and opportunities into consideration, a series of initiatives were identified that should be implemented to improve the standard of care provided by hospital pharmacy departments to these patients (Table 1).
Initiatives proposed by SHPs
| Domain | Initiative |
|---|---|
| 1. COORDINATION WITH THE HEALTHCARE TEAM | 1.1 To define integrated clinical processes for the treatment of coagulopathies that also involve primary care and emergency units. |
| 1.2 To establish a clinical and pharmacotherapeutic follow-up coordination committee that comprises all the different units involved in in the care of patients with congenital coagulopathies. The committee should also carry out a pharmacoeconomic follow-up. | |
| 1.3 To define and establish enhanced communication channels between hematologists and HPs to promote harmonized decision-making and avoid conveying patients the same information more than once. | |
| 1.4 SEFH should liaise with FEDHEMO in preparing a guide containing joint hematologic/pharmacologic recommendations on the economic and effectiveness factors related to coagulopathies directed at both healthcare providers and patients. | |
| 1.5 To establish surgical planning protocols involving HPs. | |
| 2. DRUG EVALUATION AND SELECTION | 2.1 In line with the procedure followed with other hospital-based drugs, standardized procedures should be established to evaluate the drugs used to treat congenital coagulopathies including their review by pharmacy committees. |
| 2.2 To identify the safer and most effective and convenient therapeutic strategies for each patient on the basis of a comparison of the therapeutic alternatives available in the market. | |
| 2.3 To convene multidisciplinary drug evaluation and selection meetings involving hospital management. | |
| 2.4 To incorporate the patients' point of view in all decisions made about their treatment. | |
| 2.5 To incorporate both patient-reported outcomes and the patients themselves in evaluation and decision-making procedures. | |
| 3. DISPENSING | 3.1 To establish hospital pharmacy-led home dispensing systems geared towards patients who may require this service. |
| 3.2 To establish systems that provide an accurate or an approximate view of the medication stocks held by patients at home so as to manage the available resources, schedule procurement of new drugs and plan for exceptional situations (surgeries, accidents, etc.). | |
| 3.3 Develop automatic alert and appointment systems to remind patients that they need to restock their medications or made an appointment with the hospital pharmacy department in charge of dispensing them (e.g., through mobile devices). | |
| 4. PATIENT INFORMATION AND EDUCATION |
|
| 5. PHARMACOTHERAPEUTIC FOLLOW-UP |
|
| 6. RESEARCH AND INNOVATION |
|
| 7. TRAINING AND CAPACITY-BUILDING |
|
| 8. CROSS-SECTIONAL INITIATIVES |
|
FEDHEMO: Spanish Hemophilia Federalion; HP: hospital pharmacist; SEFH: Spanish Society of Hospital Pharmacists.
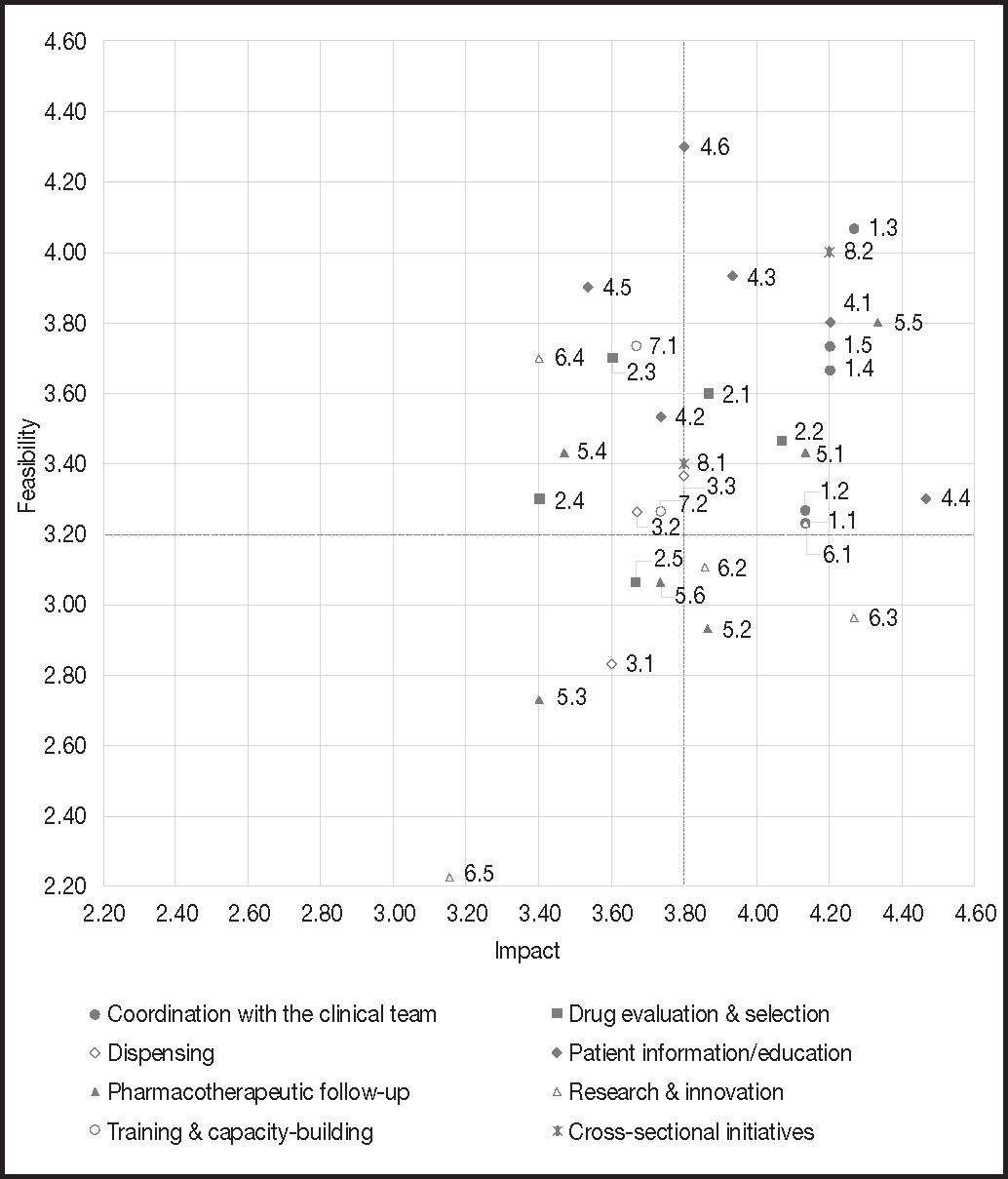
These consensus-based initiatives were prioritized by the 16 HPs individually according to their impact and feasibility. The initiatives’ impact, i.e., their potential to ensure that the HP's intervention would result in an improvement in the patients’ status, was scored on a scale from 1 (initiatives with the lowest impact) to 5 (those with the highest impact). Feasibility scores were the mean of the scores for two items. On the one hand, the resources needed, i.e., the financial or spatial resources required to implement the different initiatives identified (scores for this item were assigned on a scale from 1 to 5, with higher scores corresponding to higher levels of feasibility); and, on the other hand, decision-making, i.e., the complexity or difficulty inherent in making the decisions required for the implementation of each initiative (scores were assigned on a scale from 1 to 5, with higher scores corresponding to higher levels of feasibility).
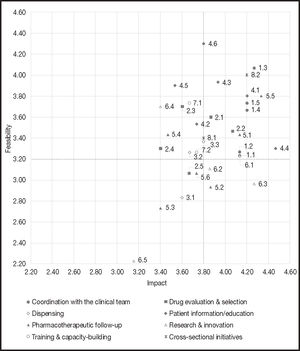
The scores given by each HP were collected and aggregated for the analysis. The mean score assigned by each individual for both impact and feasibility was also recorded (Figure 2).
Once all the initiatives were prioritized, the key action areas to be addressed were identified, all of them including top priority initiatives in terms of their impact and feasibility.
ResultsFirst of all, the coordinating committee identified the areas where the role played by hospital pharmacists was believed to be key to the management of patients with CCs. As shown in Figure 1, these areas are as follows: coordination with the other members of the clinical team in charge of taking care of these patients (hematologists, nursing staff, emergency staff, trauma surgeons, rehab physicians, etc.); drug evaluation and selection; dispensing; patient information and education; pharmacotherapeutic follow-up; research and innovation in the field of CCs; and capacity-building and training of the hospital pharmacy staff.
Within each of these areas, participating HPs discussed first of all the key actions and specific functions they performed vis-a vis patients with CCs to ensure that the best health outcomes could be achieved. That was a starting point that provided a reflection framework to identify the initiatives required to further improve the standard of care provided. This process led to identification of 34 initiatives, which were grouped under 8 domains (Table 1).
A prioritization matrix was put together (Figure 2) to represent the different initiatives based on their impact and feasibility. The matrix therefore categorized initiatives into top priority initiatives (high impact and high feasibility), intermediate priority initiatives (high impact and low feasibility, or vice versa) and low priority initiatives (low impact and low feasibility).
Of the 34 initiatives proposed, 33 obtained a score equal to or higher than 2.5 in terms of impact and feasibility. A total of 15 actions were defined as top priority, i.e., those whose score was equal to or higher than the mean overall score (3.8 for impact and 3.2 for feasibility).
As regards actions considered a priority, most of them pertained to the clinical domain (29.4%), followed by the patient information and education domain (23.5%). For their part, actions under the drug evaluation and selection, pharmacotherapeutic follow-up and cross-sectional initiative domains accounted for 11.8% of initiatives considered a priority, respectively. Lastly, the initiatives under the dispensing and the research and innovation domains accounted for 5.9% of top priority actions, respectively.
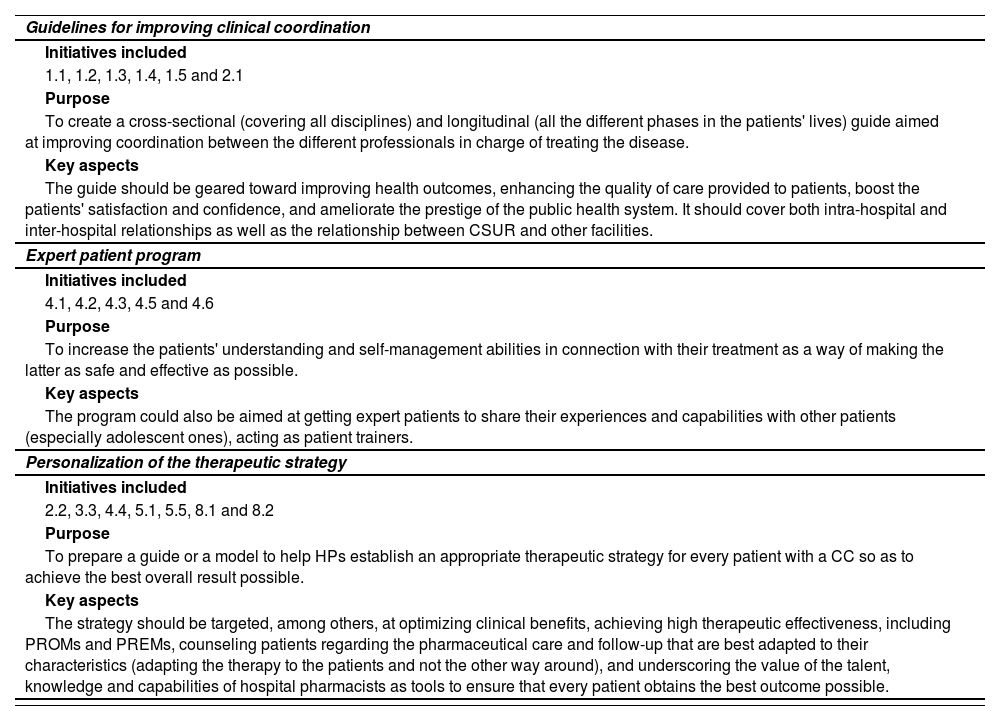
During the second phase, three overall priority action areas were defined (Table 2), which encompassed the highest priority initiatives. These priority action areas were: 1) Preparation of a guide aimed at improving clinical coordination (intra- and inter-hospital communication and coordination); 2) design of an expert patient program; and 3) personalization of the therapeutic strategy.
Action areas proposed to improve management of patients with congenital coagulopathies by hospital pharmacists
| Guidelines for improving clinical coordination |
| Initiatives included |
| 1.1, 1.2, 1.3, 1.4, 1.5 and 2.1 |
| Purpose |
| To create a cross-sectional (covering all disciplines) and longitudinal (all the different phases in the patients' lives) guide aimed at improving coordination between the different professionals in charge of treating the disease. |
| Key aspects |
| The guide should be geared toward improving health outcomes, enhancing the quality of care provided to patients, boost the patients' satisfaction and confidence, and ameliorate the prestige of the public health system. It should cover both intra-hospital and inter-hospital relationships as well as the relationship between CSUR and other facilities. |
| Expert patient program |
| Initiatives included |
| 4.1, 4.2, 4.3, 4.5 and 4.6 |
| Purpose |
| To increase the patients' understanding and self-management abilities in connection with their treatment as a way of making the latter as safe and effective as possible. |
| Key aspects |
| The program could also be aimed at getting expert patients to share their experiences and capabilities with other patients (especially adolescent ones), acting as patient trainers. |
| Personalization of the therapeutic strategy |
| Initiatives included |
| 2.2, 3.3, 4.4, 5.1, 5.5, 8.1 and 8.2 |
| Purpose |
| To prepare a guide or a model to help HPs establish an appropriate therapeutic strategy for every patient with a CC so as to achieve the best overall result possible. |
| Key aspects |
| The strategy should be targeted, among others, at optimizing clinical benefits, achieving high therapeutic effectiveness, including PROMs and PREMs, counseling patients regarding the pharmaceutical care and follow-up that are best adapted to their characteristics (adapting the therapy to the patients and not the other way around), and underscoring the value of the talent, knowledge and capabilities of hospital pharmacists as tools to ensure that every patient obtains the best outcome possible. |
CCs: congenital coagulopathies; CSUR: Centers, Services and Reference Units of the National Health System; HP: hospital pharmacist; PREM: patient-reported experience; PROM: patient-reported outcome measure.
CCs are complex conditions that have a significant impact on patients not only from a phsyiopathological point of view throughout all the different stages of their lives, but also from a psychological and social standpoint. For that reason, it is vital to identify the areas within the management of these chronic conditions where healthcare providers can add value to their patients, ensuring at all times that they cater for their needs.
HPs are playing an increasingly significant role in the management of patients, not least those with CCs. Although the approach to patients with CCs was traditionally limited to third-level hospitals, a growing number of less specialized facilities are now treating those patients, which means that the preparation, coordination and continuous improvement of the HPs from different hospitals is key to successfully approach their condition and optimize their health outcomes and quality of life. The prioritization carried out as part of this study together with the action areas proposed have been influenced by a changing reality.
In this respect, the three overall action areas resulting from our analysis were identified and described with a view to aligning and grouping together the most impactful and feasible initiatives in a practical easy-to-implement manner, making it possible for HPs to cater for the patients’ needs. The proposed action areas are in line with and have been designed to confirm to the CMO pharmaceutical care model, aimed at providing pharmaceutical care with consideration to individual patient needs (capacity), aligning each patient's short, medium and long-term goals in collaboration with other healthcare providers (motivation), and responding to the patients’ needs in real time through the new information technologies (opportunity)”.
The development of a guide intended to improve clinical coordination would help convey the experiences of hospitals with large numbers of patients with CCs to smaller facilities, fostering and formalizing intra- and inter-hospital communication and coordination and establishing communication channels between HPs, hematologists and other healthcare providers. The guide would also benefit patients as it would improve their experience of the clinical process, aid in homogenizing the information and the messages that they receive from the different professionals involved and improve pharmacotherapeutic follow-up. It would also improve communication between healthcare providers, increase the visibility of HPs and make it easier to understand their role in the multidisciplinary team. Moreover, a global view of the patient would be adopted and there would be an increase in knowledge and education. As regards the health system, the benefits would consist in an optimization of resources, which would increase efficacy, lead to better health outcomes and greater patient satisfaction, and enhance the prestige of the public health system.
Expert patient programs have been successfully implemented for other conditions, where they have proved their usefulness. Given the complex nature of CCs and the strict treatment regimens patients must follow, initiatives involving information and education activities are of the essence. According to the experts, the benefits of these programs include an increased commitment of patients with their pharmacotherapeutic goals; more informed decision-making through empowerment and capacity-building; an increase in adherence; treatment optimization and an ensuing reduction in the number of bleeds; and an increase in safety and quality of life.
Lastly, in order to improve safety, effectiveness and adherence, a suggestion mas made to further personalize therapeutic strategies by combining the different drug evaluation and selection, dispensing, patient information and education, pharmacotherapeutic follow-up and cross-sectional initiatives identified. It was thus concluded that developing a guide or model that made it easier for HPs to establish the most appropriate therapeutic strategy for each patient with a CC could be extremely beneficial as it would improve overall health outcomes and significantly increase the patients’ quality of life.
Preparation of a guide or a model that makes it easier for HPs to personalize the therapeutic strategy and the care of patients would go a long way towards ensuring that patients receive a treatment adapted to their clinical situation, lifestyle and expectations that allows them to live their lives to the full thanks to a therapy tailored to their needs. HPs would also be better off as they would be provided with an overall view of each patient, which would help them make more informed decisions and increase their effectiveness at preventing complications, thus enhancing patient satisfaction. Furthermore, making the guide generally available would facilitate the sharing of the information provided with other professionals.
This analysis has identified and prioritized a series of actions into three global priority action areas which, if addressed, could contribute to improving the way patients with CCs, particularly those with hemophilia, are currently approached. The actions proposed, all of them amenable to implementation in the Spanish health system, could make it possible for HPs to exert a real impact on the lives of their patients.
FundingThis project benefited from a grant from CSL-Behring within the framework of a project identified with code 108858.
AcknowledgementsExpert panel:
Víctor Jiménez Yuste: Head of the Hematology Department. La Paz University Hospital, Madrid.
Ramiro Núñez Vázquez: Hematology Department. Virgen del Rocio University Hospital, Seville.
José Manuel Martínez Sesmero: Head of the Pharmacy Department. San Carlos Clinical Hospital, Madrid. Head of Research of the Spanish Society of Hospital Pharmacists.
María Reyes Abad Sazatornil: Head of the Pharmacy Department. Miguel Servet University Hospital, Zaragoza.
Alberto Espuny Miró: Pharmacy Department. Virgen de La Arrixaca Clinical University Hospital, Murcia.
Rosa Farré Riba: Head of the Pharmacy Department. Sant Joan de Déu Hospital, Barcelona.
Alberto Jiménez Morales: Head of the Pharmacy Department. Virgen de las Nieves University Hospital, Granada.
Juan Carlos Juárez Giménez: Plarmacy Department. Vall d'Hebron University Hospital, Barcelona.
María Antonia Mangues Bafalluy: Head of the Pharmacy Department, Santa Creu i Sant Pau Hospital, Barcelona.
Isabel Martín Herranz: Head of the Pharmacy Department, A Coruña University Hospital Complex, A Coruña.
Juan Eduardo Megías Vericat: Pharmacy Department. La Fe University Hospital, Valencia.
Victoria Morales León: Head of the Pharmacy Department. Dr. Negrín University Hospital, Gran Canaria.
María Ángeles Ocaña Gómez: Pharmacy Department. Nuestra. Señora. de Candelaria University Hospital, Santa Cruz de Tenerife.
Antonio Palomero Massanet: Pharmacy Department. Son Espases University Hospital, Palma de Mallorca.
Jesús Prada Lobato: Pharmacy Department. Río Ortega University Hospital, Valladolid.
Pablo Quintero García: Pharmacy Department. Virgen del Rocío University Hospital, Seville.
Juan Selva Otaolarruchi: Pharmacy Department. Alicante General University Hospital, Alicante.
Contribution to the scientific literature
This is the first study to dwell on the critical activities performed by hospital pharmacists in connection with patients with congenital coagulopathies and to put forward a series of initiatives to improve the way these patients are managed based on the novel CMO (capacity, motivation, opportunity) model.
The study identified specific actions that could contribute to improving the way patients with congenital coagulopathies, specifically those with hemophilia, are approached by hospital pharmacists. If implemented in the Spanish health system, the initiatives proposed could exert a positive impact on patients’ lives.
Early Access date (04/07/2022).