Sacituzumab govitecan is an antineoplastic therapy composed of a monoclonal antibody directed to the Trop2 antigen, conjugated to SN-38, an active metabolite of irinotecan that inhibits topoisomerase I. It is indicated for the treatment of metastatic triple-negative breast cancer in patients who have received at least two prior lines of treatment, with at least one in the metastatic context. SN-38 is eliminated by glucuronidation mediated by uridine diphosphate-glucuronosyltransferase-1A1 (UGT1A1) enzymes, present in the liver. Mutations in the UGT1A1 gene decrease the expression of these enzymes, which increases the concentration of SN-38 and, consequently, increases the toxicity of the drug, especially in the form of neutropenia and diarrhea. This study aims to analyze the relationship between UGT1A1 gene polymorphisms and toxicity associated with treatment with sacituzumab govitecan, in addition to reviewing the usefulness of genetic screening prior to starting therapy.
MethodsA non-systematic literature review was conducted on the impact of UGT1A1 gene polymorphisms on the safety of sacituzumab govitecan treatment in patients with triple-negative breast cancer. The search included primary and secondary literature sources and communications from oncology conferences.
ResultsPatients treated with sacituzumab govitecan with the UGT1A1*28/*28 mutated genotype are more likely to experience grade more than 3 hematologic adverse events: neutropenia (approximate incidence of 60% compared to 40% for 1/*1 and 1/*28 genotypes), febrile neutropenia (18% homozygotes vs. 5% heterozygotes and 3% wild-type), grade more than 3 anemia (15% vs. 6% and 4%, respectively); as well as grade more than 3 diarrhea (24% vs. 13% and 6%, respectively). Additionally, treatment discontinuation rates are higher in *28/*28 individuals (6% compared to 1% heterozygotes and 2% wild-type).
ConclusionsPatients homozygous for the UGT1A1*28 allele are at significantly increased risk of developing serious adverse events. Despite the clear relationship between UGT1A1 polymorphisms and sacituzumab-govitecan toxicity, the review suggests that there is insufficient consensus on the need for systematic genetic screening. However, the findings indicate that such screening could be useful for identifying patients at risk and personalizing sacituzumab govitecan therapy.
Sacituzumab govitecan es una terapia antineoplásica compuesta por un anticuerpo monoclonal dirigido al antígeno Trop2, conjugado con SN-38, un metabolito activo de irinotecán que inhibe la topoisomerasa I. Está indicado para el tratamiento del cáncer de mama triple negativo metastásico en pacientes que han recibido al menos dos líneas de tratamiento previas, con al menos una en contexto metastásico. El SN-38 se elimina mediante glucuronización mediada por las enzimas uridindifosfato-glucuronosiltransferasas-1A1 (UGT1A1), presentes en el hígado. Mutaciones en el gen UGT1A1 disminuyen la expresión de estas enzimas, lo que eleva la concentración de SN-38 y, en consecuencia, se incrementa la toxicidad del fármaco, especialmente en forma de neutropenia y diarrea. Este estudio tiene como objetivo analizar la relación entre los polimorfismos del gen UGT1A1 y la toxicidad asociada al tratamiento con sacituzumab govitecan, además de revisar la utilidad del cribado genético previo al inicio de la terapia.
MétodosSe realizó una revisión bibliográfica no sistemática sobre el impacto de los polimorfismos del gen UGT1A1 en la seguridad del tratamiento con sacituzumab govitecan en pacientes con cáncer de mama triple negativo. La búsqueda incluyó fuentes bibliográficas primarias, secundarias y comunicaciones a congresos de oncología.
ResultadosLas pacientes tratadas con sacituzumab govitecan con el genotipo mutado UGT1A1*28/*28 tienen una mayor probabilidad de padecer efectos adversos hematológicos de grado ≥ 3: neutropenia (incidencia aproximada del 60% respecto al 40% de los genotipos 1/*1 y 1/*28), neutropenia febril (18% homocigotos vs. 5% heterocigotos y 3% wild-type), anemia grado ≥ 3 (15% vs. 6% y 4%, respectivamente); así como diarrea grado ≥ 3 (24% vs. 13% y 6%, respectivamente). Además, las tasas de discontinuación de tratamiento son mayores en individuos *28/*28 (6% respecto al 1% heterocigotos y 2% wild-type).
ConclusionesLas pacientes homocigotas para el alelo UGT1A1*28 presentan un riesgo significativamente mayor de desarrollar efectos adversos graves. A pesar de la relación evidente entre los polimorfismos UGT1A1 y la toxicidad de sacituzumab govitecan, la revisión sugiere que no hay consenso suficiente sobre la necesidad de realizar un cribado genético sistemático. Sin embargo, los hallazgos indican que este tipo de cribado podría ser útil para la identificación de pacientes en riesgo y personalizar la terapia con sacituzumab govitecan.
Breast cancer (BC) accounts for nearly 30% of all new female cancer cases, representing the most common cancer in women1. BC encompasses a group of neoplastic diseases with differing molecular characteristics. Triple-negative breast cancer (TNBC) constitutes 15 to 20% of BC cases2. This subtype is associated with a poorer diagnosis, as compared to other breast neoplasms. Its distinctive molecular profile is characterized by the absence of expression of estrogen receptors (ERs), progestogen receptors (PRs), and epidermal growth factor 2 (HER2) in tumor cells, which limits therapeutic options2.
To date, the treatments of choice for TNBC include standard chemotherapy schemes (CTX), with low response rates and poor disease control. The first targeted therapies were based on poly-adenosine diphosphate (ADP)-ribose-polymerase (PARP) inhibitors in patients harboring germline mutations in RCA1/2 (olaparib), with promising results. Subsequently, immunotherapy based on blockers of PD-L1 binding to the programmed death-receptor 1 (PD-1), atezolizumab and pembrolizumab, used in combination with CTX in advanced PD-L1-positive tumors, became the treatment of choice. However, the use of these two therapies is restricted to carriers of germline BCRA mutations or PD-L1-positive tumors. For this reason, research efforts are ongoing in pursuit of therapies that are effective in most TNBC patients3.
The FDA (U.S. Food and Drug Administration) in 2020 and the EMA (European Medicines Agency) in 2021 approved the indication of sacituzumab govitecan (SG) for the treatment of metastatic TNBC (mTNBC) in patients having received at least two previous lines of treatment, one of them for metastatic disease4. This indication is based on the results of the ASCENT study5, a randomized, open-label, multicentric, phase 3 clinical trial assessing the efficacy and safety of SG in patients with unresectable/metastatic TNBC with ≥2 previous systemic treatments. In this study, SG was compared to CTX alone at the investigator's discretion (eribulin, vinorelbine, capecitabine or gemcitabine), with a median progression-free survival (PFS) of 5.6 months for the SG arm versus 1.7 months for the CTX arm (HR 0.41; 95%CI 0.32–0.52; p < 0.001). Overall survival rates were 12.1 and 6.7 months, respectively (HR 0.48; 95%CI 0.38–0.59; p < 0.001).
Additionally, SG was recently granted EMA approval for metastatic hormone receptor-positive (RH+) breast cancer, HER2-negative BC previously treated with endocrine therapy and ≥2 previous additional systemic therapies for advanced disease. However, this indication has not yet been approved for coverage by the Spanish National Health System4.
SG is an antibody-drug conjugate (ADC) of a humanized anti-Trop2 monoclonal antibody linked to SN-38 (the active metabolite of irinotecan), with the latter having antineoplastic activity. Its mechanism of action mimics that of other marketed conjugates: when SG binds to Trop-2, the conjugate internalizes into cancer cells and releases SN-38. This agent inhibits topoisomerase 1, thereby causing DNA damage and inducing apoptosis and cell death6.
Its active metabolite, SN-38, is cleared from the body through glucuronidation mediated by hepatic uridine diphosphate (UDP) glucuronosyltransferase 1A1 (UGT1A1). Mutations in the UGT1A1, UGT1A7 and UGT1A9 genes may reduce the expression of these enzymes, leading to the elevation of SN-38 concentrations, which increases toxicity in the form of neutropenia and diarrhea6,7. The expression of this protein is reduced in homozygous and heterozygous carriers of the UGT1A1*28 and UGT1A1*6 alleles. As a result, glucuronidation is impaired, leading to a higher risk of toxicity, as compared to homozygous carriers of the UGT1A1*1 allele. In the Spanish population, the incidence of this homozygous mutated genotype is 9% versus 51% for the heterozygous genotype. Some UGT1A polymorphisms also modify SN-38 metabolism, although with a lower clinical relevance8.
A summary of allelic UGT1A1 variants and a description of their phenotypic activity are provided in Table 1. UGT1A1 diplotypes and their phenotypes6,9 are shown in Table 2.
It is worth noting that the FDA contraindicates the concomitant use of this drug with UGT1A1 inhibitors owing to the risk of SN-38 accumulation, which could increase toxicity. Likewise, the AEMPS recommends a cautious use of this agent. However, in general, UGT1A1 screening prior to treatment initiation is not recommended by any regulatory agency. In contrast, the guidelines of the FDA and other agencies provide specific recommendations for irinotecan. Thus, the authorities recommend adjusting dosage according to the UGT1A1 phenotype, especially when administered at doses >180 mg/m2 for the treatment of metastatic colorectal cancer10–13. This difference is relevant, as a SG dose generates a SN-38 concentration of 90 ng/ml, whereas irinotecan 350 mg/m2 (significantly higher than the dose administered in colorectal cancer) only induces a concentration of 56 ng/ml14,15.
A review was conducted to assess SG toxicity based on an analysis of the evidence available on the influence of UGT1A polymorphisms on SG exposure and toxicity in patients with mTNBC. The results of this review will contribute to determining the need for genotyping prior to treatment initiation.
MethodsA non-systematic literature review was performed to assess the toxicity of SG and assess the impact of UGT1A mutations on treatment exposure and safety. Additionally, a review was conducted for evidence available on the need for screening for genetic polymorphisms prior to initiation of SG therapy.
A systematic review could not be conducted, since the articles selected used descriptive statistics for comparative analysis of AEs across different genotypes. Moreover, heterogeneous results were provided.
A literature search was performed on PubMed, Embase, Scopus, Web of Science, Cochrane and Epistemonikos without any time limit as of 2024, including primary and secondary literature sources. The search also included communications to the main medical oncology conferences, such as the American Society of Clinical Oncology (ASCO) and European Society for Clinical Oncology (ESMO) providing relevant results that had not yet been published in the literature.
Search terms included «UGT1A1 polymorphism AND Sacituzumab Govitecan», «Glucuronosyltransferase pharmacogenomics AND Sacituzumab govitecan», «security AND Sacituzumab govitecan», «Sacituzumab govitecan AND toxicity AND UGT1A1»
ResultsA total of 38 articles were retrieved from the databases mentioned above. Following preliminary screening, duplicates and articles not meeting inclusion criteria were excluded. Finally, five studies were selected. Fig. 1 shows the flow chart of article selection.
A review was performed of the evidence available on the influence of UGT1A polymorphisms in SG safety16.
Influence of UGT1A1 polymorphisms in the toxicity of sacituzumab govitecanSG-related toxicity is the same as that associated with irinotecan, which has been examined in a variety of drug development studies.
The most frequent adverse events (AEs) reported in the IMMU-132-01 study17 in TNBC patients who received SG included nausea (67%); neutropenia (64%); diarrhea (62%); fatigue (55%); and anemia (50%). Ten percent of study participants developed grade ≥3 AEs, with anemia and neutropenia being the most common. As many as 32% of patients with severe AEs required hospitalization, 7% due to febrile neutropenia; 6% for vomiting; 4% for diarrhea, and 3% for dyspnea. Treatment was discontinued permanently due to toxicity in three patients (2.8%)8.
The main AEs reported in the ASCENT study5 included neutropenia (64% in the SG group vs. 43% in the standard CTX group); diarrhea (59% vs. 12%, respectively); nausea (57% vs. 26%); alopecia (46% vs. 16%); and fatigue (45% vs. 30%) by that order. In total, 51% of patients treated with SG developed Grade ≥3 neutropenia vs. 33% in the CTX group. A higher proportion of patients in the SG group received granulocyte colony-stimulating factors (G-CSF) as compared to the CTX group (49% vs. 23%). Dose was interrupted in 61% of cases in the SG group, vs. 33% in the CTX group.
The TROPiCS-02 phase-3 clinical trial13 included 543 patients with metastatic RH+ and HER2- breast cancer. Of them, 268 received SG and 271 received standard CTX alone (eribulin, vinorelbine, capecitabine or gemcitabine). The most common AEs in the SG arm, as compared to the CTX arm included neutropenia (70% vs. 54%); diarrhea (57% vs. 17%); and nausea (55% vs. 31%). In total, 74% of patients treated with SG developed grade ≥3 toxicity vs. 60% of the CTX arm, including grade ≥3 neutropenia (51% vs. 38%). During treatment, G-CSF was administered to 54% of SG patients vs. 34% of CTX patients.
An evaluation was conducted of the safety data obtained in the phase-3 TROPHY-U-01 study14. However, this study involved patients with metastatic urothelial cancer (mUC) at progression following platin-based chemotherapy in combination with checkpoint inhibitors. The TROPHY-U-01 study is a single-cohort clinical trial assessing SG treatment that included a total of 113 patients with mUC who received a SG dose of 10 mg/kg at days 1 and 8 of the 21-day cycle (the same as for TNBC). The most common AEs reported included diarrhea (65%); nausea (60%); fatigue (52%); alopecia (47%); neutropenia (46%) and anemia (33%). Grade ≥3 AEs included neutropenia (35%); anemia (14%); diarrhea (10%) and febrile neutropenia (10%). Neutropenia was treated with dose reductions or interruptions. Thirty percent of patients received G-CSF as support treatment (18% from Cycle 1, and the remainder in subsequent cycles).
In the pivotal studies mentioned above, UGT1A1 genotyping was performed and safety was assessed for each variant.
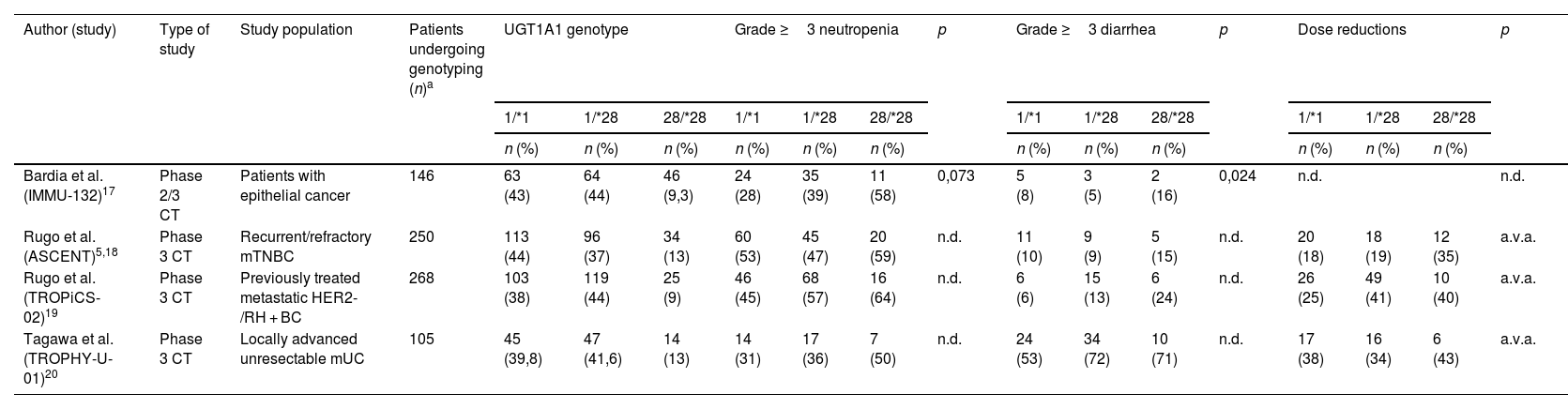
Table 3 classifies the patients included in drug development studies by genotype prevalence.
Genotype-based patient classification in each study and common adverse events.
| Author (study) | Type of study | Study population | Patients undergoing genotyping (n)a | UGT1A1 genotype | Grade ≥3 neutropenia | p | Grade ≥3 diarrhea | p | Dose reductions | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/*1 | 1/*28 | 28/*28 | 1/*1 | 1/*28 | 28/*28 | 1/*1 | 1/*28 | 28/*28 | 1/*1 | 1/*28 | 28/*28 | |||||||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |||||||
| Bardia et al. (IMMU-132)17 | Phase 2/3 CT | Patients with epithelial cancer | 146 | 63 (43) | 64 (44) | 46 (9,3) | 24 (28) | 35 (39) | 11 (58) | 0,073 | 5 (8) | 3 (5) | 2 (16) | 0,024 | n.d. | n.d. | ||
| Rugo et al. (ASCENT)5,18 | Phase 3 CT | Recurrent/refractory mTNBC | 250 | 113 (44) | 96 (37) | 34 (13) | 60 (53) | 45 (47) | 20 (59) | n.d. | 11 (10) | 9 (9) | 5 (15) | n.d. | 20 (18) | 18 (19) | 12 (35) | a.v.a. |
| Rugo et al. (TROPiCS-02)19 | Phase 3 CT | Previously treated metastatic HER2-/RH + BC | 268 | 103 (38) | 119 (44) | 25 (9) | 46 (45) | 68 (57) | 16 (64) | n.d. | 6 (6) | 15 (13) | 6 (24) | n.d. | 26 (25) | 49 (41) | 10 (40) | a.v.a. |
| Tagawa et al. (TROPHY-U-01)20 | Phase 3 CT | Locally advanced unresectable mUC | 105 | 45 (39,8) | 47 (41,6) | 14 (13) | 14 (31) | 17 (36) | 7 (50) | n.d. | 24 (53) | 34 (72) | 10 (71) | n.d. | 17 (38) | 16 (34) | 6 (43) | a.v.a. |
mTNBC: metastatic triple-negative breast cancer; mUC: metastatic urothelial cancer; a.n.a.: abbreviation not available.
In the IMMU-132 study, homozygous carriers of the UGT1A1*28 allele were at a higher risk of developing neutropenia, with incidence rates of 33, 38.3 and 60.9% for patients with genotypes 1/*1, 1/*28 and 28/*28, respectively. The same was observed in relation to grade ≥3 neutropenias, occurring in 28% (1/*1), 39% (1/*28) and 58% (28/*28) of patients retrospectively analyzed.
The results of the ASCENT study regarding SG safety were similar to those of the IMMU-132 study for carriers of UGT1A1*28 mutations. AEs led to dose reductions in 18, 19 and 35% of wild type heterozygous and homozygous carriers, respectively18.
Grade ≥3 AEs were more common in homozygous 28/*28 carriers, including grade ≥3 febrile neutropenia (3% wild-type, 5% heterozygous and 18% homozygous carriers), grade ≥3 anemia (4, 6 and 15%, respectively), or grade ≥3 diarrhea (10, 9 and 15%, respectively). In this study, treatment was interrupted in 61% of SG patients vs. 33% for CTX patients. Dose was reduced in 22 and 26% of patients, respectively. In relation to SG efficacy, PFS was longer in patients subjected to dose reductions or treatment interruptions, as compared to those who received the full dose. The median PFS for the SG group was 8.3 months vs. 2.9 months for the CTX group with dose reduction; and 4.6 vs. 1.5 months, respectively, without dose reductions. The median PFS was 5.7 months for the SG group vs. 2.7 months for the CTX group with treatment interruption. PFS was 4.2 vs. 1.6 months, respectively, when treatment had not been discontinued.
These results are consistent with the TROPiCS-0219 study, which reported a similar safety profile in patients undergoing genotyping. Rates of treatment discontinuation due to grade ≥3 AEs were more common in homozygous carriers of the *28 allele, as compared to heterozygous wild-type carriers (92, 75 and 67%, respectively). Grade ≥3 diarrhea was reported for 24, 13 and 6% of patients, respectively.
Similar results were obtained in patients with mUC (TROPHY-U-01 study)20. Grade ≥3 AEs such as neutropenia occurred in 31% of wild-type carriers; 36% of heterozygous carriers; and 50% of homozygous carriers. Grade ≥3 anemia was reported for 13, 19 and 29% of patients, respectively. Treatment was interrupted in 42, 43 and 71% of patients, and suspended in 7, 6 and 14% of patients. The study conducted by Wong et al. within routine clinical practice included 68 patients, of whom 25% were homozygous and 35% were heterozygous carriers. Treatment was suspended due to toxicity in a significantly higher proportion of *28/*28 allele carriers (HR 5.52, 95%CI 1.15–6.49, p = 0.039)21.
Recommendations for genotyping prior to treatment initiationAll pivotal trials revealed an association between UGT1A1 polymorphisms and SG safety. However, all authors highlight the need for further clinical trials to assess the safety of the treatment more thoroughly. As a result, genotyping prior to initiation of treatment is not currently recommended.
The Summary of Product Characteristics of SG (Trodelvy®) does not establish any dose adjustments according to the UGT1A1 genotype but only recommends close monitoring of carriers of UGT1A1 variants, including the UGT1A1*28 allele due to impaired enzymatic activity4. Despite this, UGT1A1 genotyping prior to initiation of SG treatment is not considered. In contrast, dose adjustments are recommended in the presence of grade 4 neutropenia for 7 or more days; grade 3–4 febrile neutropenia; or grade 3–4 neutropenia causing delayed dose administration. In these settings, secondary prophylaxis with G-CSF is recommended. In the event of a second episode of toxicity, it is recommended to reduce the SG dose by 25% and administer G-CSF. In case of a third episode of toxicity, the dose must be reduced by 50% and G-CSF must be administered. Finally, if a fourth episode of toxicity occurs, the treatment must be suspended. Non-hematological toxicities are managed with similar dose reductions.
Influence of UGT1A1 polymorphisms in sacituzumab govitecan exposureBased on the non-compartmental pharmacokynetic (PK) analysis of SG included in the IMMU-132-01 and IMMU-132-05 (ASCENT) studies, the volume of distribution of SG is 2.96 L, with a SG and unbound SN-38 half-life of 15.3 and 19.7 h, respectively.
Population-based pharmacokinetic models have been developed for SG based on data from 529 patients of the IMMU-132-01 and ASCENT studies, both for SG and unbound SN-38. The results reveal two-compartmental kynetics with first-order elimination for this drug22,23. No association was observed with any of the covariates included (age, sex, moderate–severe kidney failure, moderate–severe liver failure, albumin, ECOG status, type of tumor, Trop2 expression or UGT1A1 genotype). A study performed by the marketing laboratory to estimate exposure to SG and unbound SN-38 developed a PK model based on data from 237 patients from the IMMU-132-01, ASCENT and IMMU-132-06 studies. UGT1A1 genotype data were also included (31.5% *1/*1; 13.6% *1/*28 and 12.4% *28/*28)18. The estimated areas under the curve (AUC) for SG were 9.790, 9.481 and 9.370 mg.h/ml for wild-type, heterozygous and homozygous carriers, respectively. The estimated AUC for unbound SN-38 were 5.39; 5.25 and 4.82 mg.h/ml, respectively, for each variant. No significant differences were observed in exposure to SG and unbound SN-38 by the type of polymorphism. Therefore, the authors concluded that no dose adjustment was necessary for carriers of UGT1A1 polymorphisms10.
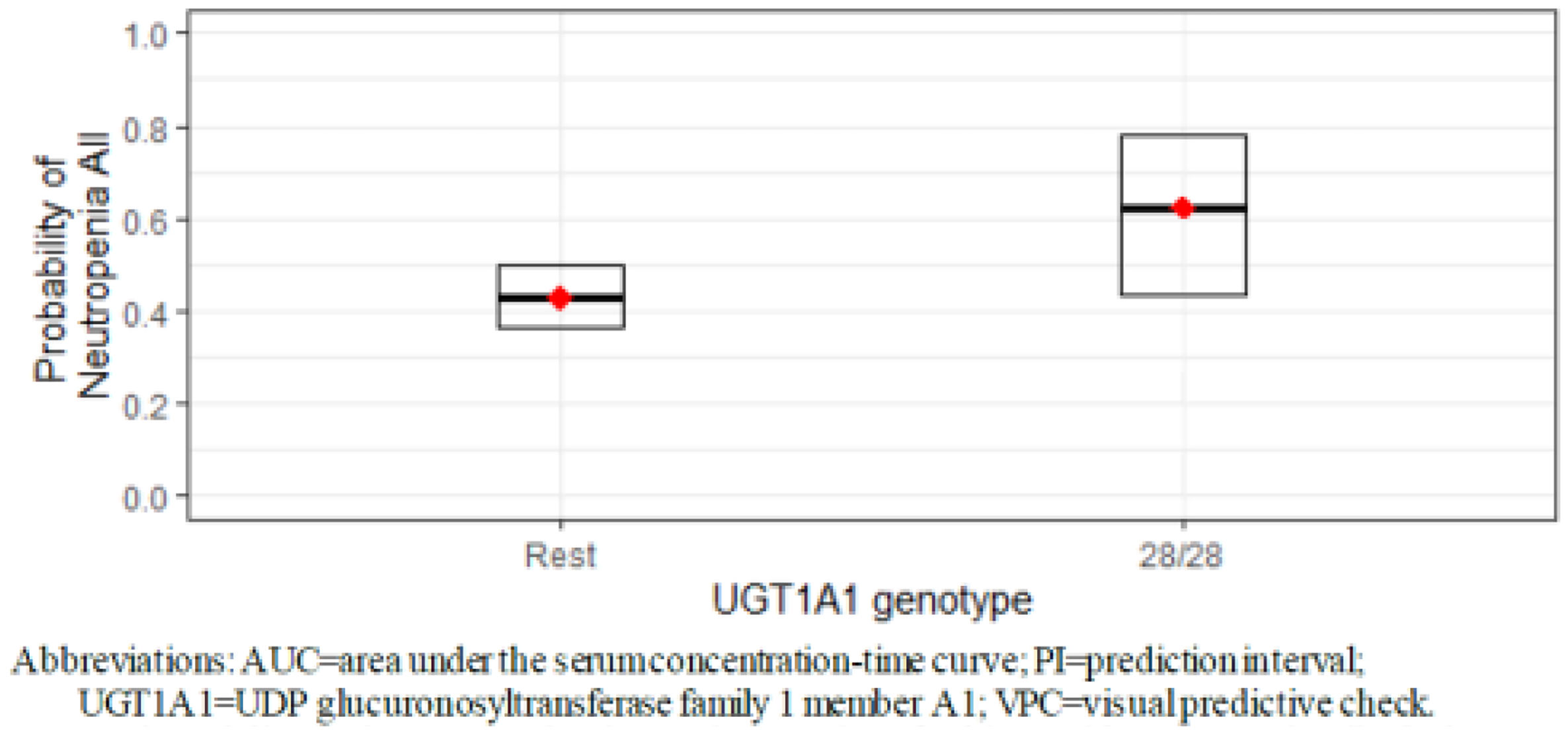
However, the ASCENT study revealed a higher risk for developing neutropenia as the AUC and maximum SG concentration (Cmax) increased in carriers of the UGT1A1 28/*28 genotype (OR > 1)5 (Fig. 2).
Exposure-safety correlation was assessed for patients with metastatic TNBC and RH+, HER2− breast cancer from the ASCENT, IMMU-132-01 and IMMU-132-09 studies, including a total of 569 patients with safety and pharmacokynetic estimates available. Mean SG concentration, (CAVGSG), Cmax and AUC were estimated during the first cycle of treatment for SG, unbound SN-39 and total antibodies. Then, the potential correlation between these parameters and the most common AEs was assessed. A statistically significant correlation was observed between elevated levels of CAVGSG and a higher probability of developing any-grade neutropenia (OR 1.39 HF 95%CI 1.33–1.45). The patients with the UGT1A1*28/*28genotype were more likely to develop any-grade neutropenia and grade 3–4 neutropenia, as compared to patients without the UGT1A1*28/*28 genotype10 (Fig. 3).
DiscussionThe main limitation of this review is the low number of publications assessing the presence of UGT1A1 polymorphisms in patients treated with SG. Although the most relevant clinical trials provide some data, the information available is primarily descriptive and lacks statistically significant values.
SG for the treatment of mTNBC is an alternative targeted therapy to standard chemotherapy, having shown improved overall survival rates in TNBC (12.1 months vs. 6.7 months, HR 0.48, 95%CI 0.38–0.59), with modest results in metastatic RH + HER2- breast cancer (14.5 vs. 11.2 months, HR 0.79; 95%CI 0.65–0.96). However, SG therapy is not exempt from AEs, the ones reported for irinotecan, including hematological toxicity (neutropenia and anemia) and gastrointestinal toxicity (nausea, vomiting and diarrhea). These AEs not only affect the quality of life of patients but also compromise treatment efficacy, as they result in delayed dose administration, as shown in the TROPiCS-02 study; dose reductions (24% of cases); or treatment suspension (7%)13. SG toxicity is directly related to SN-38, which is eliminated through glucuronidation by the UDP-glucuronosyltransferase enzyme (UGT1A1). In patients with impaired glucuronidation, such as carriers of the UGT1A1 gene, exposure to SN-38 increases, resulting in a significantly higher hematological and gastroenterological toxicity.
The most relevant scientific societies and clinical pharmacogenetics working groups have established recommendations for UGT1A1 genotyping prior to initiation of irinotecan therapy, due to the toxicity of the active metabolite SN-3824,25. The Dutch Pharmacogenetics Working Group-DPWG determined that UGT1A1 genotyping was essential to ensuring safety in patients who are initiating irinotecan therapy26. According to the DPWG, genotyping should be performed prior to treatment initiation, irrespectively of the dose. Hence, homozygous *28 carriers must initiate irinotecan at 70% of the standard dose; then, the dose can be uptitrated according to the neutrophil count and clinical tolerance.
The French National Network of Pharmacogenetics-RNPGx establishes that all patients initiating treatment with irinotecan at doses >240 mg/m2 must undergo genotyping2 but contraindicates the administration of such high doses to *28/*28 carriers27. For the latter, the RNPGx recommends reducing the dose by 25–30%. Finally, the RNPGx considers it advisable to perform genotyping in patients receiving doses of 180–230 mg/m2.
The National Comprehensive Cancer Network Guidelines (NCCN) do not recommend UGT1A1 genotyping prior to initiation of irinotecan, as they consider that patients will require a dose reduction regardless of the result28. The Consensus ESMO guidelines10 establish that screening for UGT1A1 polymorphisms is an option for patients who are initiating irinotecan at a dose of ≥180 mg/m2.
In relation to SG, regulatory agencies (FDA, EMEA, HS-Canada) do not recommend UGT1A1 genotyping prior to treatment initiation, as they consider that AEs will be managed the same in all patients. When genotyping is performed, carriers of the *28 allele will require close monitoring for the occurrence of potential AEs.
Our results indicate that carriers of the UGT1A1*28/*28 diplotype receiving SG therapy may be at a higher risk of experiencing grade ≥3 AEs, such as neutropenia (with an incidence of near 60%, vs. 40% in patients with other diplotypes); febrile neutropenia (18% in homozygous vs. 5% in heterozygous carriers; and 3% in wild-type) patients, grade ≥3 anemia (15% vs 6 vs 4%, respectively) and grade diarrhea ≥3 (24% vs. 13 vs. 6%). Accordingly, these patients may be at a higher risk for hospitalization and require support treatments such as G-CSF. Apart from influencing patient safety, these AEs may interfere with treatment efficacy due to treatment interruptions, delays, and suspensions29.
In conclusion, considering the evidence available, screening for UGT1A1 polymorphisms is recommended for all patients with BC prior to initiation of SG treatment. Systematic reduction of the dose should be considered prior to treatment initiation in homozygous carriers of the *28 allele. Additionally, these patients should be subjected to close monitoring to prevent the occurrence of severe AEs. Future studies, probably promoted by independent scientific societies, will support our recommendations as they will improve the efficacy of SG and the quality of life of patients with mTNBC.
CRediT authorship contribution statementEva María Legido Perdices: Writing – original draft, Validation, Methodology, Formal analysis. Fernando do Pazo Oubiña: Validation, Investigation, Data curation. Elena Prado Mel: Validation, Formal analysis, Data curation. Marta Miarons Font: Validation, Formal analysis, Data curation. Betel Del Rosario García: Writing – original draft, Validation, Formal analysis, Conceptualization. Fernando Gutiérrez Nicolás: Writing – original draft, Validation, Formal analysis, Conceptualization.
AuthorshipAll authors contributed to the literature search. Eva Legido, Fernando do Pazo, Elena Prado and Marta Miarons contributed to peer reading, analysis and data extraction. Eva Legido, Fernando Gutiérrez and Betel del Rosario contributed to the drafting of the manuscript. All authors were involved in successive reviews until the final review.
FundingThe authors did not receive any funding for the preparation of this manuscript.
We thank M. José Giménez Santos and Pedro Muñoz Alcañiz, responsible for the Library of the Hospital Arnau de Vilanova-Llíria, for their contribution to the literature search.