In some patients, acute respiratory distress syndrome (ARDS) leads to life-threatening refractory hypoxemia developing. Physicians may consider hypoxemic rescue therapies in an attempt to improve oxygenation in these patients while on conventional mechanical ventilation support. Use of inhaled nitric oxide (iNO) in ARDS is one of the most widely-studied pharmacological interventions over the past two decades. Its efficacy was examined in several randomized clinical trials and has undergone meta-analyses. Although iNO treatment was associated with improved oxygenation, researchers unfortunately never demonstrated a concomitant decrease in mortality or any improved outcome. Hence the current evidence suggests that iNO should not be routinely used in patients with ARDS however may be considered as adjunct therapy to tentatively improve oxygenation while other therapies are being considered in patients with severely hypoxemic ARDS.
This review focuses on the therapeutic use of iNO in adult ARDS patients. We set out some recommendations for its use as rescue therapy against refractory hypoxemia.
En algunos pacientes, el síndrome de distrés respiratorio agudo (SDRA) provoca el desarrollo de una hipoxemia refractaria que compromete la vida. En este contexto pueden considerarse terapias de rescate en un intento de mejorar la oxigenación mientras los pacientes permanecen en ventilación mecánica. El uso de óxido nítrico inhalado (NOi) en el SDRA ha sido una de las terapias farmacológicas más estudiadas en las últimas dos décadas. Diversos ensayos clínicos y metaanálisis han evaluado su eficacia, y aunque se ha demostrado un aumento en la oxigenación, no se ha podido demostrar un descenso en la mortalidad o una mejora en el pronóstico. La evidencia actual sugiere que aunque el NOi no debe usarse de forma rutinaria en pacientes con SDRA, puede considerarse su uso para mejorar la oxigenación en pacientes severamente hipoxémicos.
Esta revisión examina la aplicación terapéutica del NOi en pacientes adultos con SDRA. Se propone un esquema con diversas recomendaciones para su uso como terapia de rescate frente a la hipoxemia refractaria.
Acute respiratory distress syndrome (ARDS) is defined by acute-onset hypoxemia (PaO2/FiO2 ratio ≤ 300 mm Hg) in conjunction with the presence of bilateral pulmonary infiltrates not due to cardiac insufficiency or hydrostatic edema.
This hypoxemia occurs as a result of an alteration in the ventilation/perfusion ratio, due both to alveolar inflammation and alteration of pulmonary vascular reactivity, which sometimes becomes refractory, when persistent respiratory insufficiency is maintained under pneumoprotective measures with PaO2/FiO2 < 100 mm Hg or plateau pressure > 30 cm H2O21.
Despite the advances in managing ADRS patients, their mortality rate still continues to be high. Since pneumoprotective ventilation revolutionized the ventilation strategy for ARDS patients, numerous therapies have been studied for correcting the hypoxemia, and although many of these interventions improve arterial oxygenation, unfortunately very few are associated with a benefit relating to survival1.
Since the role of nitric oxide (NO) in vascular biology was first discovered, its administration in inhaled form was incorporated into the treatment of ARDS due to the belief that selective pulmonary vasodilation in the ventilated alveoli would improve gas exchange and hence the prognosis of these patients. Its efficacy has been evaluated by numerous randomized clinical trials, and it has undergone several meta-analyses, having demonstrated its effect on the transient increase in arterial oxygenation, albeit no clinically-relevant benefit has been demonstrated in prognosis parameters (i.e. survival rate or ventilator-free days). Nevertheless, inhaled nitric oxide (iNO) continues to be used in critical adult ARDS patients, and although its routine use is not recommended, its use as a rescue therapy in patients with severe refractory hypoxemia seems reasonable.
The objective of this review is the clinical evaluation of the role of iNO in ARDS, by setting out the currently-available evidence, the usage-related controversies concerning and the current recommendations for use. A scheme is detailed for its use as a rescue therapy against refractory hypoxemia, which includes aspects such as the indications for administration and withdrawal, dosage and monitoring.
MethodologyA non-systematic search was conducted for articles in the PubMed/MEDLINE base, confined to the English and Spanish languages, without any time limit, using the MeSH terms: “Respiratory Distress Syndrome, “Adult/therapy” and “Nitric Oxide”. The articles most relevant with regard to their relationship to the aforesaid topic were selected. A manual search was also conducted in the references of the articles selected.
Nitric oxide biologyNO is nitrogen monoxide, one of the nitrogen oxides in conjunction with nitrogen dioxide (NO2), nitrogen tetroxide (N2O4) and nitrogen protoxide (N2O), the last of which has anesthetic properties. Under atmospheric conditions, nitric oxide is a gas which is produced in combustion processes and comprises part of air pollution, it having been considered merely a toxic substance until its role in the regulation of animal physiology was discovered. In the human body, it is synthesized in the vascular endothelium from L-arginine amino acid by means of an enzyme called nitric oxide synthase (NOS), of which several types of different cells in the organism in addition to the vascular endothelium has been characterized and identified2. It is an unstable molecule with a very short average lifetime (3-5 seconds) and is highly lipophilic, giving it the special ability to pass through membranes. When it is administered via the inhaled route, it passes through the alveolar epithelial cell barrier and into the smooth muscle cells, where it directly stimulates the guanylyl cyclase enzyme, creating cyclic guanosine monophosphate (cGMP), which is the mediator in smooth muscle relaxation and vascular dilation2,3.
In turn, NO spreads through the endothelial cell toward the vessel lumen, where it quite avidly combines with the hemoglobin and is inactivated, forming meta-hemoglobin, which is reduced by the meta-hemoglobin reductase of the erythrocytes4. iNO flows solely in the well-ventilated regions of the lung, and this inactivation on spreading to the blood is what makes it possible to cause selective pulmonary vasodilation without causing systemic vasodilation. Another two main reactions stem from the NO reacting with the oxygen in the blood forming the toxic nitrogen dioxide (NO2) molecule and from the reaction with the pl asma proteins to form S-nitrosothoioles or thionitrites with vasodilating properties and possible extra-pulmonary effects2-4.
A modulation of bronchial tone with iNO has also been observed, although even in large doses (80 parts per million (ppm) the bronchodilating response is less than that of inhaling a standard beta-2 agonist5. iNO can also have other pulmonary effects (pro-inflammatory or anti-inflammatory properties) and extra-pulmonary effects, although its clinical relevance must be investigated2-4.
Inhaled nitric oxide in ARDSHistoric perspective and current evidenceIn 1980, it was discovered that stimulating endothelial receptors with acetylcholine triggered the production of a substance which spread to the vascular smooth muscle cells and caused vasodilation, this substance having been termed the endothelium-derived relaxing factor (EDRF). Some years later, in 1987, this molecule was proven to be NO6,7, the authors of said studies having been the 1998 Nobel Prize for their discoveries of this molecule.
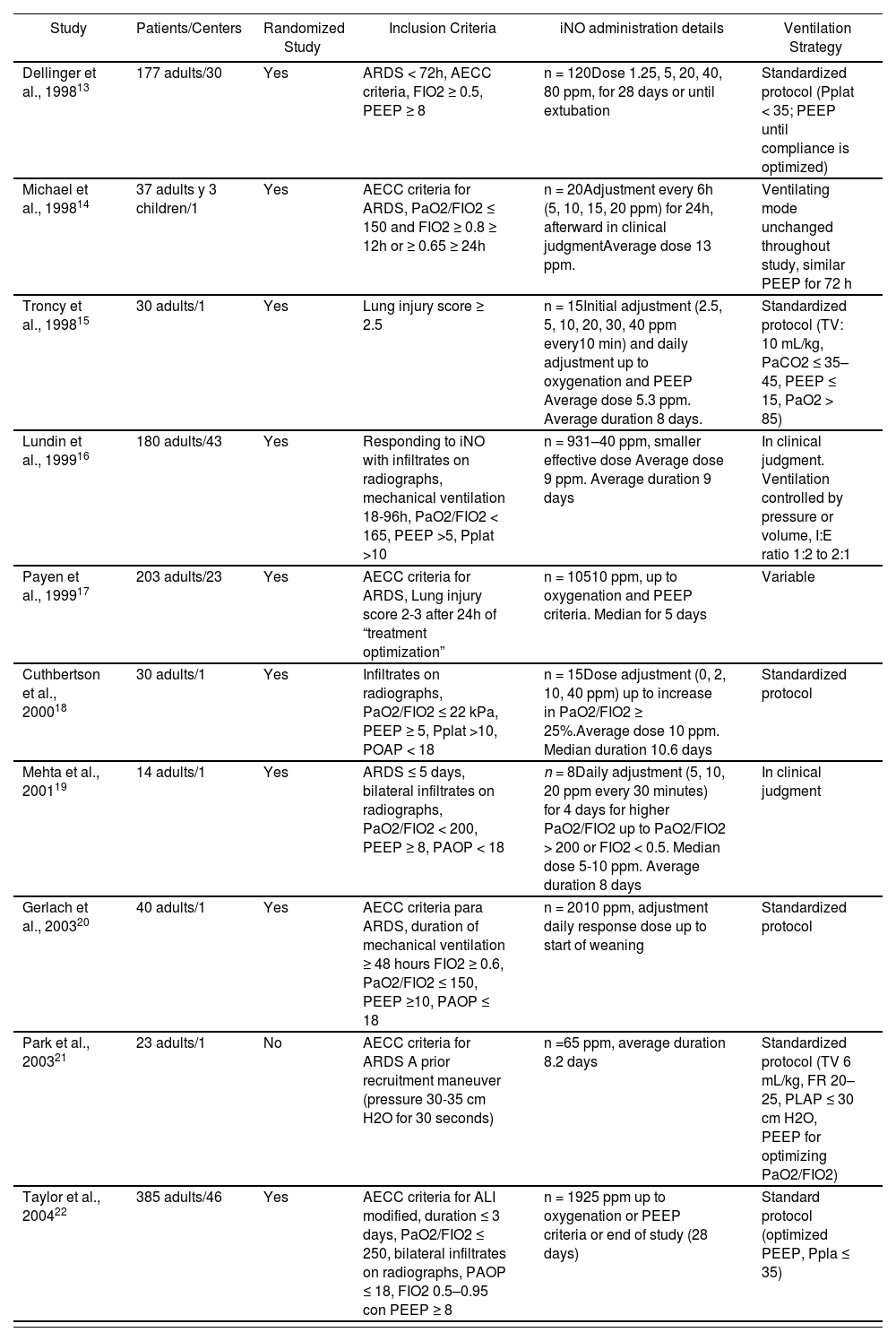
Following the promising results of studies on the use of iNO in animals and later in humans with pulmonary hypertension8, its use was broadened to ARDS patients. Rossaint et al9 were the first to study the effects of iNO in ARDS patients, having found there to be a reduction of the pulmonary arterial pressure and an increase of the oxygenation at doses of 18-36 ppm. Despite the limitations of their study, the findings justified the dissemination of numerous studies for evaluating iNO in ARDS, which were burgeoning in the 1990’s, demonstrating that when inhaled in small doses (5-80 ppm), it rapidly caused selective pulmonary vasodilation of the ventilated alveoli, hence entailing an improvement in the pulmonary hypertension and an increase in the arterial oxygenation10-12. These findings provided renewed encouragement to researchers such as Gerlach et al.31, who evaluated the response of severe ARDS patients to iNO by means of dose response curves. The results showed an improvement in the oxygenation and reduction in the use of extracorporeal membrane oxygenation in the iNO group, there however having been no differences in the length of time of the mechanical ventilation. Despite the belief that the improvement in the gas exchange would have a bearing on the prognosis of these patients, this encouraged different randomized clinical trials subsequently being conducted13-22 (Table 1). These studies demonstrated the inhalation of NO in ARDS to tentatively improve the arterial oxygenation, although they failed to confirm an improvement in the survival rate or in the morbidity of critical patients. Different meta-analyzes and systematic reviews have also confirmed the increase in oxygenation but nevertheless have not provided evidence of a reduction in the mortality or in the number of ventilator-free days23-30.
Characteristics of the main clinical trials on inhaled nitric oxide (iNO) in adult patients with acute lung injury (ALI) and acute respiratory distress syndrome (ARDS)
| Study | Patients/Centers | Randomized Study | Inclusion Criteria | iNO administration details | Ventilation Strategy |
|---|---|---|---|---|---|
| Dellinger et al., 199813 | 177 adults/30 | Yes | ARDS < 72h, AECC criteria, FIO2 ≥ 0.5, PEEP ≥ 8 | n = 120Dose 1.25, 5, 20, 40, 80 ppm, for 28 days or until extubation | Standardized protocol (Pplat < 35; PEEP until compliance is optimized) |
| Michael et al., 199814 | 37 adults y 3 children/1 | Yes | AECC criteria for ARDS, PaO2/FIO2 ≤ 150 and FIO2 ≥ 0.8 ≥ 12h or ≥ 0.65 ≥ 24h | n = 20Adjustment every 6h (5, 10, 15, 20 ppm) for 24h, afterward in clinical judgmentAverage dose 13 ppm. | Ventilating mode unchanged throughout study, similar PEEP for 72 h |
| Troncy et al., 199815 | 30 adults/1 | Yes | Lung injury score ≥ 2.5 | n = 15Initial adjustment (2.5, 5, 10, 20, 30, 40 ppm every10 min) and daily adjustment up to oxygenation and PEEP Average dose 5.3 ppm. Average duration 8 days. | Standardized protocol (TV: 10 mL/kg, PaCO2 ≤ 35–45, PEEP ≤ 15, PaO2 > 85) |
| Lundin et al., 199916 | 180 adults/43 | Yes | Responding to iNO with infiltrates on radiographs, mechanical ventilation 18-96h, PaO2/FIO2 < 165, PEEP >5, Pplat >10 | n = 931–40 ppm, smaller effective dose Average dose 9 ppm. Average duration 9 days | In clinical judgment. Ventilation controlled by pressure or volume, I:E ratio 1:2 to 2:1 |
| Payen et al., 199917 | 203 adults/23 | Yes | AECC criteria for ARDS, Lung injury score 2-3 after 24h of “treatment optimization” | n = 10510 ppm, up to oxygenation and PEEP criteria. Median for 5 days | Variable |
| Cuthbertson et al., 200018 | 30 adults/1 | Yes | Infiltrates on radiographs, PaO2/FIO2 ≤ 22 kPa, PEEP ≥ 5, Pplat >10, POAP < 18 | n = 15Dose adjustment (0, 2, 10, 40 ppm) up to increase in PaO2/FIO2 ≥ 25%.Average dose 10 ppm. Median duration 10.6 days | Standardized protocol |
| Mehta et al., 200119 | 14 adults/1 | Yes | ARDS ≤ 5 days, bilateral infiltrates on radiographs, PaO2/FIO2 < 200, PEEP ≥ 8, PAOP < 18 | n = 8Daily adjustment (5, 10, 20 ppm every 30 minutes) for 4 days for higher PaO2/FIO2 up to PaO2/FIO2 > 200 or FIO2 < 0.5. Median dose 5-10 ppm. Average duration 8 days | In clinical judgment |
| Gerlach et al., 200320 | 40 adults/1 | Yes | AECC criteria para ARDS, duration of mechanical ventilation ≥ 48 hours FIO2 ≥ 0.6, PaO2/FIO2 ≤ 150, PEEP ≥10, PAOP ≤ 18 | n = 2010 ppm, adjustment daily response dose up to start of weaning | Standardized protocol |
| Park et al., 200321 | 23 adults/1 | No | AECC criteria for ARDS A prior recruitment maneuver (pressure 30-35 cm H2O for 30 seconds) | n =65 ppm, average duration 8.2 days | Standardized protocol (TV 6 mL/kg, FR 20–25, PLAP ≤ 30 cm H2O, PEEP for optimizing PaO2/FIO2) |
| Taylor et al., 200422 | 385 adults/46 | Yes | AECC criteria for ALI modified, duration ≤ 3 days, PaO2/FIO2 ≤ 250, bilateral infiltrates on radiographs, PAOP ≤ 18, FIO2 0.5–0.95 con PEEP ≥ 8 | n = 1925 ppm up to oxygenation or PEEP criteria or end of study (28 days) | Standard protocol (optimized PEEP, Ppla ≤ 35) |
ALI: Acute Lung Injury. Pplat: Pressure plateau or mean airway pressure. AECC: American European Consensus Conference. POAP: Pulmonary Artery Occlusion Pressure (in cm H2O). PEEP: Positive End-Expiratory Pressure (in cm H2O). PaO2/FiO2: Ratio of partial pressure arterial oxygen and fraction of inspired oxygen (in mm Hg). TV: Tidal Volume. RR: Respiratory Rate.
The increase in oxygenation substantiated with the iNO has not meant an improvement in prognosis in clinically-relevant terms such as the survival rate. Some authors assert that these results obtained in small-scale, low-powered clinical trials may have significant limitations, and their results may serve better for giving rise to rather than confirming hypotheses4. In fact, the greatest criticism of these studies is that they do not distinguish between severe and moderate ARDS, in the belief that the greatest benefit is achieved in patients with severe hypoxemia and that this sub-group has been poorly represented in the clinical trials32,33. However, a meta-analysis30 recently examined the effect which iNO has on mortality by sub-groups defined by the degree of severity of the hypoxemia for 9 clinical trials and 1,142 patients, no benefit having been found according to the severity of the group. The analysis of sub-groups showed that the therapy with iNO does not reduce the mortality in patients with baseline PaO2/FiO2 ≤ 100 mm Hg (RR 1.01 [95% CI, 0.78-1.32]; p = 0.93; n = 329; 6 trials) or those with a baseline PaO2/FiO2 > 100 mm Hg (RR 1.12 [95% CI, 0.89-1.42]; p = 0.33; n = 740; 7 trials). Nor was any PaO2/FiO2 threshold (increments of 10 mm Hg within 70-200 mm Hg range) identified in which the mortality of the patients treated with iNO was lower than in the controls. One limitation of this study, according to the authors, is that it included solely 329 patients in the severe ARDS sub-group, which limits the statistical power for detecting actual differences in mortality among sub-groups.
Other limitations of these reviews are due to the lack of homogeneity of the patients included in one trial and another, due in part, up to relatively a few years ago, to there being no universal definition of ARDS to improve the coherence between research and clinical practice. Furthermore, there is a lack of homogeneity in the indication and in the treatment, with different methods for administering iNO and differing doses from one trial to another3, which complicates their comparison for drawing any significant conclusions (Table 1). Furthermore, it is currently known that the ventilating strategy employed for managing a patient with acute respiratory insufficiency has a significant bearing on the evolution of the disease, although this finding was discovered after many of these studies had been conducted, in which the patients were ventilated with a tidal volume above the recommended volume of 6 ml kg-1. The slight improvements in oxygenation due to the iNO may be masked by the deleterious effects of a non-protective ventilating strategy used in most trials, which limits neither the tidal volume nor the airway pressure. A protective ventilation within the context of the use of iNO might perhaps have provided more positive results as far as prognosis-related parameters are concerned4,34.
On the other hand, the mortality of ARDS patients is related more to events such as sepsis or multiple-organ dysfunction than to hypoxemia per se35. The fact that the improvement in oxygenation substantiated with the use of iNO has not meant an improvement in the mortality rate may be influenced for this reason4. Furthermore, most of the treatments researched for ARDS are focused on short-term prognosis parameters, such as mortality. However, the patients who survive ARDS may have long-range pulmonary sequelae, such as obstructive, restrictive disorders and alterations in gas exchange, which may compromise their quality of life. The actual impact which iNO may have on these parameters is unknown36. In 2004, Taylor et al.22 evaluated the efficacy of small doses of iNO (5 ppm) in 385 critical patients with moderate/severe lung damage (PaO2/FiO2 ≤ 250) from 46 hospitals, finding a transient increase in the PaO2 without any benefit on the mortality or ventilator-free days. A later follow-up carried out as part of the original study however showed better values in functional respiratory tests at 6 months among the ARDS patients who had been treated with iNO than those treated with placebo36. Nevertheless, the validity of the study is limited by the loss of follow-up of most of the survivors and the lack of information on smoking. The effects of iNO on long-term pulmonary function of ARDS patients and hence their morbidity and quality of life are still as yet to be determined. Nevertheless, the improvement in oxygenation demonstrated with the use of iNO could suffice in itself to justify iNO being used in some severely hypoxemic ARDS patients34.
Use and current recommendationsFollowing the burgeoning number of articles published concerning iNO in the mid-1990’s, the use of therapy employing iNO began expanding to different disorders such as persistent neonatal pulmonary hypertension, pediatric and adult heart surgery and ARDS34. In fact, at the end of the 1990’s, a working group on using iNO in the ICU of the European Society of Intensive Care Medicine (ESICM) explored the clinical practice of the use of iNO in the ICU by means of a questionnaire which was answered by 310 physicians from 21 countries37. More than 60% of said physicians reported using iNO therapy (63.2%), the specialists among whom were mainly intensive care specialists, pediatricians and anesthesiologists.
However, in ARDS patients, its clinical and prognosis-related repercussion has not met with the anticipated degree of success. In 2005, a group of experts organized by the EISCM and by the European Society of Cardiothoracic Anesthesiologists set out some recommendations regarding iNO therapy in adults within the perioperative and critical care realm. In the specific case of ARDS, they mention that its routine use cannot be recommended, although its use as a rescue treatment in patients with severe refractory hypoxemia is considered reasonable32. The recent publication of the results of a meta-analysis which seem to reject the belief that the greatest benefit is achieved in patients with severe hypoxemia30 continue fostering the idea of relegating the use of iNO in patients with ARDS to extreme situations1,30.
The treatment indications approved by Spain’s Medicines Agency for the use of iNO are the treatment of newborns born with more than 34 weeks of gestation who have hypoxic respiratory insufficiency associated with clinical or echocardiography evidence of pulmonary hypertension, and the treatment of perioperative and postoperative pulmonary hypertension in heart surgery38. There is currently no indication approved for its use in adult ARDS patients, the use thereof hence requiring informed consent (Fig. 2). Despite the foregoing, it continues to be used extensively (off-label indication) as a rescue therapy against refractory hypoxemia. For example, it was used in over 20% of the patients included in a large-scale clinical trial published in 2010 concerning the use of neuromuscular relaxants in ARDS patients39. Similarly, it was used as a treatment during the 2009 influenza-A (H1N1) virus pandemic in 32% of the patients with refractory hypoxemia prior to the administration of ECMO in Australia and New Zealand40 and as rescue therapy in up to 14% of critical patients in Canada41, although scarcely any use was documented in Spain and some series from Latin America42.
Based on the foregoing, inhaled nitric oxide therapy seems reasonable as a medication for compassionate use as an off-label therapy in ARDS patients who present severe refractory hypoxemia.
Combined treatmentiNO improves the ventilation/perfusion ratio in the aerated pulmonary regions, as a result of which the use of recruitment maneuvers to get previously-atelectatic terminal alveoli opened up improves their usefulness with a synergetic effect on the increase of the PaO2(43) These recruitment maneuvers include the use of appropriate PEEP (positive end-expiratory pressure) (making it possible to turn patients not responding to iNO into responding patients)44, ventilation in prone position45 and high-frequency ventilation46. Although the repercussion of this synergism on the prognosis of ARDS patients has not been evaluated, the importance of the maneuvers affording the possibility of preventing atelectasis is relevant to allowing iNO to improve the gas exchange by means of selective pulmonary vasodilation in the largest possible number of aerated alveoli.
As far as the use of catecholamines is concerned, it has been hypothesized that septic patients may have an inadequate response to iNO due to the influence of the endogenous and exogenous catecholamines on the pulmonary vasculature, although some studies have revealed that the sepsis condition does not modify the response to the iNO in PaO2/FiO214, and that the effect of the iNO is not influenced by the administration of vasopressors such as noradrenalin47.
DosageIn adults, for the treatment of the pulmonary hypertension associated with heart surgery, the initial dose of iNO recommended on technical data sheet is of 20 ppm of inhaled gas. This dose can be increased up to 40 ppm as a maximum dose if the minimum dose has not caused sufficient clinical effects38. However, in the case of ARDS patients, the dosage is a source of controversy. Within this context, different studies have delved into the response in the oxygenation following the administration of different iNO concentrations. The results differ from one another, such that the response observed (increase in the oxygenation or decrease in the pulmonary arterial pressure) varies widely with different doses and treatment times evaluated11,20,31,48-52.
The maximum benefit on the oxygenation has been documented with doses of 0.1 – 2 ppm48,49, and with doses of under 20 ppm31 or under 40 ppm11,50. On the other hand, a worsening of oxygenation has also been substantiated with doses above 20 ppm52 or even 10 ppm20,31. Nor is the relationship between the dose of iNO and the response in the oxygenation (dose response curve) concordant, in contrast to small-scale trials in which both a dose-dependent effect48,49 and a non-dose-dependent effect has been described with the improvement in the variable oxygenation11,50 or with interpatient differences51,52. Gerlach et al.20 found there to be a progressive shifting of the dose-response curve toward the left in ARDS patients who were given iNO continuously for several days. A small percentage of ARDS patients are non-responders to iNO on not increasing the oxygenation relevantly with doses of up to 20 ppm52.
Furthermore, the parameter which must be taken into consideration as a favorable response is not made clear in literature, ranging from a 15% reduction in the FiO214, a higher than 10% increase3 or 20% increase13 in pO2 or at least a 20% increase in the PaO2/FiO233. Nevertheless, taking into account its fast-acting quality, this clinically-significant improvement in the oxygenation must be noticeable within the first hour of the therapy in order to justify continuing its use33. This lack of concordance in seeking the optimum iNO dosage in ARDS patients is patent in a recently-published meta-analysis evaluating the effect of iNO on in-hospital mortality of severe ARDS patients30. The nine clinical trials analyzed show variability in the doses of iNO used. Four trials used a set dose of 521,22 and 1017,20 ppm, one trial randomized patients to different does (1.25-80 ppm)13, the rest of the studies having used smaller doses, thus achieving a response in the oxygenation (average dose of 5.315, 916, 1314 or 5-1019 ppm).
It is therefore complicated to draw any conclusions regarding the ideal dose of iNO in ARDS patients, which should be adjusted daily in each patient33, the minimum effective dose being administered by means of slow-paced reductions, provide that the systemic arterial oxygenation continues to be appropriate with reach reduction32,53.
SafetyAdministering therapeutic doses of iNO seems safe in terms of NO2 and toxic methemoglobinemia forming. There is no direct evidence of direct iNO toxicity or severe side-effects at clinically-relevant doses27-29. Nevertheless, some safety precautions must be taken, and the administering technique must minimize the amount of NO2 administered to the patient and the environmental exposure to the healthcare workers32,49.
MethemoglobinIn healthy volunteers, inhaling iNO in much larger than therapeutic doses (up to 128 ppm) was not associated with clinically-significant methemoglobinemia levels (over 5%), the elevation of the maximum levels at 3.5 hours after starting the iNO having been substantiated54. In critical ARDS patients, methemoglobin levels of higher than 5% can be detected with high concentrations of iNO (40 and 80 ppm), severe methemoglobinemia being extremely rare, which has not be found to be the case with therapeutic doses (< 20 ppm) in Cochrane reviews27-29. Using iNO must be avoided in patients with a methemoglobin reductase deficit, it being necessary to monitor the baseline methemoglobinemia and at 4-6 hours after starting the therapy as well as on a daily basis, the dose being lowered in the event of finding a methemoglobinemia < 5%32,38,49. The methemoglobinemia which does not cease to exist after cutting back on or halting the therapy or which is compromising oxygenation can be treated with vitamin C, N-acetylcysteine, tocopherol, methylene blue or exchange transfusion, depending on the clinical situation38.
Nitrogen dioxide (NO2)NO oxidizes in the presence of oxygen to form NO2, a highly toxic gas, levels higher than 2 ppm of which can increase alveolar permeability, and which can cause severe lung damage at levels above 10 ppm3. The NO to NO2 conversion rate is directly proportional to the NO concentration, to the O2 concentration and to the length of time NO and O2 are in contact with one another, it therefore being necessary for iNO to be administered using a continuous or synchronized release system with the inspiratory outlet near the patient’s circuit (on the inspiratory limb) and monitored distally from the point of administration32,55. In one clinical trial, an increase was found to exist in the NO2 concentrations in three patients who had received concentrations higher than 80 ppm over the course of several days’ time13. Nevertheless, no increased risk of NO2 formation was found to exist with doses lower than 80 ppm27-29.
In long-term treatments, is it recommended to reduce the iNO concentration to 10 ppm or less in order to reduce exposure to the potentially toxic NO232. According to the technical data sheet the highest exposure limit (average exposure) to NO on the part of the personnel determined by the labor legislation is of 25 ppm for 8 hours (30 mg m3-1) in most countries, the respective limit for NO2 being 2-3 ppm (4-6 mg m3-1)38.
Renal insufficiencyTwo meta-analyses showed an increased risk of renal dysfunction with the use of iNO27,29. Nevertheless, the authors proper state there not to have been any generally-accepted classification such as RIFLE or AKIN which would have increased the validity of the results, and that this must be interpreted cautiously on the basis of the fact that the result stems from a post hoc analysis and it’s potentially being biased on publishing as a result of no renal function data having been obtained in some of the trials analyzed.
CoagulopathyAlthough alteration of hemorrhaging time with the use of iNO has been documented56, as well as an attenuation of platelet aggregation in patients with ARDS (which did not change the bleeding time even with iNO fractions above 100 ppm)57, the data in adult humans is contradictory, no increase in the risk of bleeding or in hemorrhaging events having been found in recent meta-analyses and Cochrane reviews27-29.
Rebound phenomenonWithdrawing iNO suddenly should be avoided32, given that a rebound phenomenon has been found to exist in some patients with acute pulmonary hypertension, hemodynamic collapse and worsening oxygenation58, which is due to a reversible inhibition of the endothelial NOS by the iNO3. Withdrawal must therefore be gradual, at least every 12 hours, once the oxygenation has improved and the patient is stable, with a low dose of iNO (5 ppm). The dose should then be progressively reduced to 1 ppm over a period of 6-12 hours, to then be maintained for 30 minutes, continuously monitoring the arterial tension, heart rate and the O2 sat, to then finally disconnect the system38 (Fig. 1).
OthersAlthough no interaction studies have been conducted, a clinically-significant interaction with NO donor substances (local anesthetics, nitroprussiate, nitroglycerin, etc.) or with other vasodilators which act through the GMPc or AMPc systems which must be used cautiously cannot be ruled out completely38.
The treatment with iNO can elevate the transpulmonary gradient in certain situations and worsen cardiac insufficiency in situations of left-right blood shunting, it therefore having to be used cautiously in these patients and in those with deteriorated left ventricular function and an elevated baseline pulmonary capillary pressure4,32,38.
Proposed scheme of useBased on current evidence, the routine use of iNO in adult ARDS patients is not recommended. The use thereof should be considered under certain circumstances in which the patient has a high risk of death or damage due to hypoxemia despite other available treatments30,32,34. The benefit in increasing the oxygenation can provide valuable time necessary for remedying the process which caused the damage in order to optimize the ventilation strategy or establish other treatment modalities such an extracorporeal membrane oxygenation. Furthermore, there are certain patients with severe refractory hypoxemia who could also benefit from an increase in oxygenation by means of treatments such as iNO, in which some therapies which improve the gas exchange (PEEP, prone position) cannot be used as a result of being contraindicated or involving excessive risk (i.e. intracranial hypertension or instability of the cervical spine). The use thereof for this indication, as a rescue therapy against refractory hypoxemia in optimally-ventilated adult ARDS patients requires informed consent (Fig. 2).
Fig. 1 provides a scheme of recommendations for using iNO in ARDS patients with severe refractory hypoxemia which includes aspects such as the indications for administration and withdrawal, dosage and monitoring. The implementation of a protocol for use and withdrawal can reduce the direct costs associated with iNO use59.
Prospects of future researchThe prospects are not good of finding future clinical trials evaluating iNO therapy in ARDS30. Although numerous pharmacological therapies are currently continuing to be researched for the treatment of ARDS patients, in the case of iNO, in view of the evidence available showing no benefit on parameters such as mortality or duration of mechanical ventilation23-30, its high cost (markedly higher following its approval as a pharmaceutical product and its patenting by the industry)60, the possible associated risk of renal dysfunction27,29, the number of patients with severe hypoxemia being small to detect an effective treatment30, and the existence of therapeutic alternatives which have clearly shown clinical benefits such as protective ventilation or ventilation in prone position1, it is unlikely that future clinical trials will be conducted evaluating iNO dosage and duration strategies in severely hypoxemic patients, if it is not in conjunction with other interventions which have clearly shown a benefit on ARDS30,34.
ConclusionsIn ARDS patients, iNO causes on-the-spot pulmonary vasodilation by improving arterial oxygenation, although an improvement in the survival rate or in the morbidity of critical patients has not been demonstrated. Although its routine use cannot be recommended, iNO still continues to be used as a safe option, and its administration is reasonable as a rescue treatment in patients with severe refractory hypoxemia.
FundingNo funding
AcknowledgementsNone
Conflicts of interestNo conflict of interest
- Inicio
- Todos los contenidos
- Publique su artículo
- Acerca de la revista
- Métricas