The aim of this study was to evaluate the real-world persistence, effectiveness, and safety of secukinumab in adult patients with moderate-to-severe psoriasis in two different hospitals.
MethodsRetrospective cohort study that used registries and medical records from 2 different hospitals (February 2015–March 2024). Adults with moderate-to-severe psoriasis who initiated secukinumab treatment were identified and followed-up until March 2024, or disenrollment. Baseline demographic and clinical characteristics studied included sex, age at diagnosis, weight, prior failed treatments, duration of treatment and psoriasis area severity index (PASI) score. Adherence was measured using medication possession ratio (MPR); patients with MPR ≥ 80% were considered adherent. Persistence, effectiveness, safety, and dosage regimen of secukinumab were collected. Kaplan–Meier analysis was used to estimate secukinumab persistence using 1-year intervals.
ResultsA total of 88 patients with moderate-to-severe psoriasis were included, of whom 45 (51.1%) had not received prior biological treatment. Baseline PASI score was 15.0 ± 2.9 and patients received 1.4 ± 0.8 prior biological treatments. The most common previous biological treatments included anti-TNFα (60.5%) and ustekinumab (20.9%). 34 (38.6%) patients discontinued secukinumab treatment due to the following reasons.
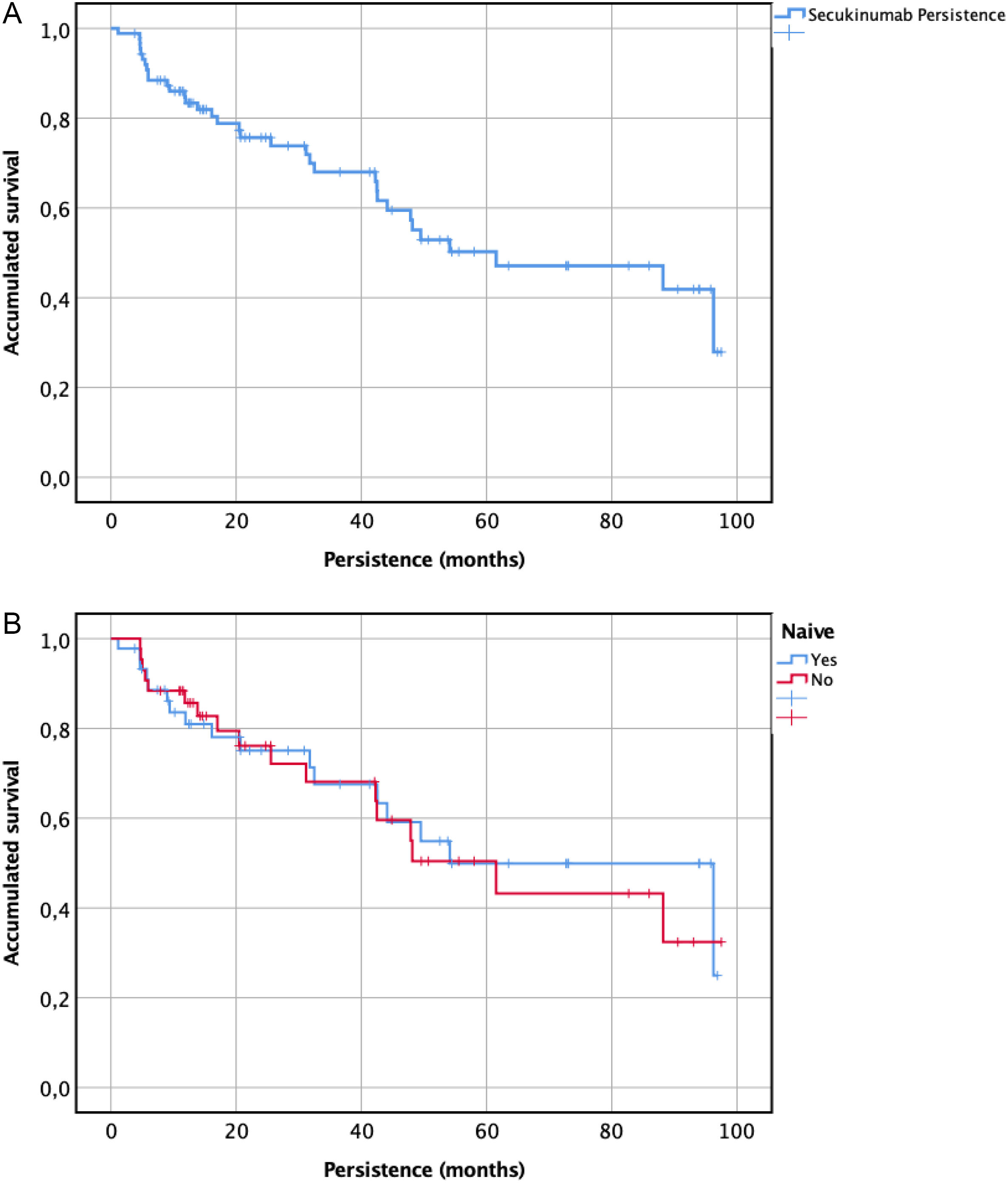
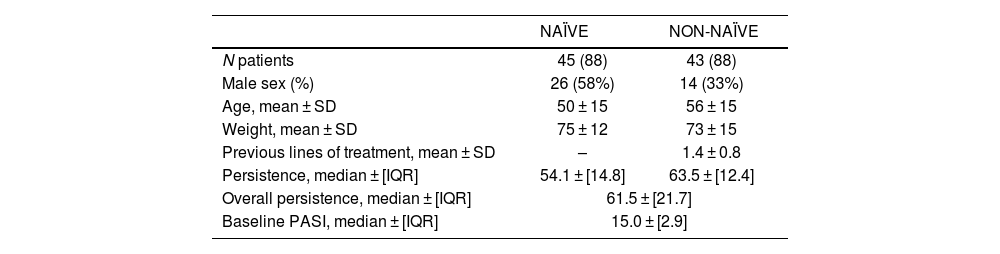
19 (21.5%) due to a lack of effectiveness, 8 (9.2%), due to achieve only a partial response, and 7 (7.9%) due to adverse effects. Secukinumab persistence was 61.5 ± [21.7] months for all patients. When performing a subgroup analysis, non-naïve patients obtained a persistence of 63.5 ± [12.4] months followed by 54.1 ± [14.8] months for naïve patients (p = .804). Secukinumab persistence at 1 year, 2 years, and 3 years was 72.7%, 51.1%, and 39.8%, respectively.
ConclusionsSecukinumab demonstrated persistence in more than 70% of patients with moderate to severe psoriasis after the first year of treatment.
Evaluar la persistencia, efectividad y seguridad en el práctica clínica real de secukinumab en pacientes adultos con psoriasis de moderada a grave en un análisis multicéntrico.
Métodosestudio de cohorte retrospectivo que utilizó registros e historias clínicas de 2 hospitales diferentes (febrero de 2015 a marzo de 2024). Se identificaron adultos con psoriasis moderada a grave que iniciaron tratamiento con secukinumab y se les hizo un seguimiento hasta marzo de 2024 o interrupción del tratamiento. Las características demográficas y clínicas basales estudiadas incluyeron sexo, edad al diagnóstico, índice de gravedad del área de psoriasis (PASI), tratamiento previo y comorbilidades. La adherencia se midió mediante el índice de posesión de medicamentos (MPR, por sus siglas en inglés); se consideró que los pacientes con MPR ≥ 80% eran adherentes. Se determinó la persistencia, tasa de retención, efectividad, seguridad y pauta posológica de secukinumab. Se utilizó el análisis de Kaplan–Meier para estimar la persistencia de secukinumab a uno, dos y tres años.
ResultadosSe incluyeron un total de 88 pacientes con psoriasis de moderada a grave de los cuales 45 (51,1%) no habían recibido tratamiento biológico previo. La puntuación PASI inicial fue de 15,0 ± 2,9 y los pacientes recibieron 1,9 ± 0,9 líneas previas de tratamiento. Los tratamientos biológicos previos fueron anti-TNFα (60,5%) ustekinumab (20,9%). 34 (38,6%) pacientes suspendieron el tratamiento con secukinumab por los siguientes motivos.
19 (21,5%) por falta de efectividad, 8 (9,2%) por respuesta parcial e incompleta y 7 (7,9%) por efectos adversos. La persistencia de secukinumab fue de 61,5 ± [21,7] meses para todos los pacientes. Al realizar un análisis de subgrupos, los pacientes no naïve obtuvieron una persistencia de 63,5 ± [12,4] meses meses seguido de 54,1 ± [14,8] meses para los pacientes naïve (p = 0,0804). La tasa de retención de secukinumab al 1 año, 2 años y 3 años fue del 72,7%, 51,1% y 39,8% respectivamente.
ConclusiónSecukinumab demostró una persistencia de más del 70% en los pacientes con psoriasis de moderada a grave tras el primer año de tratamiento.
Psoriasis is a chronic immune inflammatory disease that affects 1%–3% of the world's population. It is characterized by the presence of well-demarcated erythematous and scaly plaques, preferentially located on the elbows, knees, and scalp, although it can also affect the palms, soles, nails, and joints.1 This clinical condition significantly limits the quality of life for some patients, thus numerous efforts and resources are continually focused on expanding the therapeutic arsenal. Currently, there is a wide variety of biological therapies targeting different molecular pathways with the aim of specifically addressing the mechanisms involved in the pathophysiology of the disease. Adalimumab, etanercept, certolizumab and infliximab (TNF-α inhibitors), ustekinumab (IL-12/IL-23 inhibitor), secukinumab, ixekizumab, bimekizumab, brodalumab (IL-17 inhibitors), and guselkumab, risankizumab, and tildrakizumab (IL-23 inhibitors)2 have label indication for psoriasis. This requires the correct prioritization of the different therapeutic alternatives, as well as the implementation of algorithms to ensure their cost-effective use. Having such a wide variety of mechanisms and molecules, even for the same mechanism, implies that a patient will likely receive several of these therapeutic alternatives over their lifetime. Therefore, it is also important to consider how the effectiveness profile of these drugs can be modified depending on whether the patient has previously been treated with biological drugs (non-naïve) or if it is their first time undergoing this type of therapy (naïve).3
The mechanism of action of secukinumab is based on the binding of the antibody to IL-17A. As a result, this inhibits the interaction of these interleukins with their receptor, thus preventing the activation of the inflammatory cascade that results in the release of tissue-damaging mediators and other pro-inflammatory cytokines such as TNF-α.4 Multiple clinical trials have demonstrated the efficacy and safety of secukinumab in the treatment of moderate-to-severe psoriasis.5Regarding safety, it has a favorable adverse effects (AEs) profile6 and shows no significant differences compared to other biological agents used in the treatment of psoriasis, with upper respiratory tract infections and injection site reactions being the most common AEs.7
Persistence is the length of time between initiation and the last dose, which immediately precedes discontinuation, that is, a definitive suspension of the treatment.8 Persistence could allow us to determine the value of secukinumab in psoriasis patients in real-world setting. It is important to highlight that the history of using other therapeutic options may impact the persistence of treatment with secukinumab. Understanding the factors that influence secukinumab persistence can help healthcare providers optimize treatment strategies and improve outcomes for patients with psoriasis.9 In Spain, previous studies of secukinumab persistence are limited for 1 year period.10 Long-term persistence real-world studies are needed to confirm these data. There is limited and conflicting evidence the long-term real-world persistence of secukinumab in patients with psoriasis. The objective of this real-life study is to determine the long-term persistence of secukinumab treatment in patients with moderate-to-severe psoriasis followed up for a period of 3 years. With this information, we can assess the long-term therapeutic benefit of secukinumab treatment, making the measurement of persistence useful as an indicator to evaluate the effectiveness and safety. The secondary objective is to compare persistence in secukinumab biologic-naïve patients with secukinumab patients who had previously been exposed to biologic agents.
MethodsA retrospective observational study was carried out between February 2015 to March 2024 of a population of moderate-to-severe psoriasis patients treated with secukinumab at 2 centers of the public network of hospitals in Valencia Region. The following inclusion criteria were used: all adult patients with moderate-to-severe psoriasis who failed treatment with glucocorticoids, immunosuppressants, or both, and who completed the secukinumab label induction and maintenance dosage (administered as a 300 mg subcutaneous injection monthly, after starter doses at weeks 0,1,2,3, and 4) were selected. Exclusion criteria: patients with a current diagnosis of psoriatic arthritis. Patients were defined as either biologic naïve (no evidence of biologic therapy use at any time) or biologic experienced (at least one dispensing record for any biologic). The variables collected were: sex, age, diagnosis, previous biological treatments, start date with secukinumab, dosage regimen, psoriasis area severity index (PASI), adverse reactions, and cause for discontinuation. The PASI was used to evaluate the severity of psoriasis.11 Major adverse events leading to discontinuation of the drug were documented. Dermatology Life Quality Index (DLQI) was utilized to evaluate the broader implications of psoriasis on the patients' health-related quality of life12 across 6 domains: symptoms and feelings, daily activities, leisure, work or school performance, personal relationships, and treatments. The DLQI scores range from 0, indicating no effect on the patient's life, to 30, signifying an extremely large effect, thus offering a broad perspective on the disease's impact.
Persistence on secukinumab was calculated based on the dates of initiation and end of treatment. The end-of-treatment date was considered to be the date at which the attending physician decided to discontinue the treatment as reflected in the patient's medical record. If the patient was still on the treatment, persistence was calculated based on the date when the follow-up ended (March 2024). Losses to follow-up, understood as failure by patients to visit their dermatology doctor or the pharmacist over the course of 1 year, were considered to be censored data in the persistence analysis.13 Data were obtained from the Pharmacy Department's and validation software (Oncofarm® IMF) and the patients' electronic medical record (Integrador® and Abucasis®). The statistical analysis was conducted using the SPSS Statistics® v23 software. Persistence for biologic treatment was calculated using Kaplan–Meier estimates. The results of categorical variables were described by means of frequencies (%) and compared through Pearson's chi squared test. The results of the quantitative variables were described using means and standard deviation (SD) in cases where they followed a normal distribution, which was previously determined by Shapiro–Wilk's normality test, and by medians and interquartile ranges in cases where the distribution was not normal.
Adherence was measured using medication possession ratio (MPR). It estimates the percentage of time a patient has access to their medication. Treatment adherence was obtained from the dispensing records of the Hospital Pharmacy Department. Individualized secukinumab dispensings and correlated dates during the study period were collected using Outpatient Clinic Hospital Pharmacy software DISPENSA, [Oncopharm® Health Information Technology, Valencia, Spain] which allows dispensing and follow-up of outpatient treatment. The MPR rate was calculated using data from the pharmacy dispensing records corresponding to the overall secukinumab treatment.14
The study was approved by the hospital's Clinical Research Ethics Committee, and was conducted according to the principles of the Declaration of Helsinki.
ResultsThe study population included 88 adult patients diagnosed with moderate-to-severe psoriasis who received treatment with secukinumab. Table 1 shows the baseline characteristics of patients included in the study. Forty-five (51.1%) patients were naïve to biologic treatment, 36 (83.7% of non naïve) patients received 1 biologic treatment, and 7 (16.3%) patients received at least 2 biologic treatments. No differences in baseline characteristics were found between naïve to biologic treatment and non-naïve to biologic treatment. Baseline PASI scores were comparable between patients from the 2 hospitals, with a median value of 15.0 ± 6.5. Patients had received an average of 1.4 ± 0.8 prior biological treatments. There was no significant difference between the 2 groups in baseline PASI and previous lines of treatment. A total of 43 (48.9%) patients were grouped in the subgroup of non-naïve patients and therefore received some biological treatment for psoriasis prior to the use of secukinumab. The most common drugs being used by biological experienced patients were anti-TNFα (60.5%) and ustekinumab (20.9%).
Demographic and disease characteristics at baseline.
| NAÏVE | NON-NAÏVE | |
|---|---|---|
| N patients | 45 (88) | 43 (88) |
| Male sex (%) | 26 (58%) | 14 (33%) |
| Age, mean ± SD | 50 ± 15 | 56 ± 15 |
| Weight, mean ± SD | 75 ± 12 | 73 ± 15 |
| Previous lines of treatment, mean ± SD | – | 1.4 ± 0.8 |
| Persistence, median ± [IQR] | 54.1 ± [14.8] | 63.5 ± [12.4] |
| Overall persistence, median ± [IQR] | 61.5 ± [21.7] | |
| Baseline PASI, median ± [IQR] | 15.0 ± [2.9] | |
SD: standard deviation; IQR: inter-quartile range.
The 1-year probability of persistence was 72.7%. The cumulative persistence for patients who have been treated at least 2 and 3 years with secukinumab was 51.1% and 39.8%, respectively. Persistence to treatment showed a median ± [IQR] of 61.5 ± [21.7] months (minimum 25.2 months and maximum 85.9 months) as represented in Fig. 1. When performing a subgroup analysis, non-naïve patients obtained persistence of 63.5 ± [12.4] months (minimum 37.2 months and maximum 85.9 months) followed by 54.1 ± [14.8] months (minimum 25.2 months and maximum 83.1 months) for naïve patients, without finding statistically significant differences (p = .804) (Fig. 1).
Regarding of therapeutic response, patients were categorized based on the percentage of relative improvement in comparison to the baseline PASI value. The findings indicate that 19.4% of patients achieved a PASI 100, followed by 38.9% with a PASI 90, and 59.7% with a PASI 75. DLQI was observed from the initial stage from 12.9 ± 4.3 to 7.7 ± 4.2 after treatment. A mean adherence rate of 82.4% ± 8.4 was obtained for the 88 patients included in the study.
Throughout the study, 34 (38.6%) patients discontinued secukinumab treatment due to the following reasons: 19 (21.5%) due to a lack of effectiveness, 8 (9.2%), due to achieve only a partial response, and 7 (7.9%) due to AEs. One experienced an elevated of transaminases that requiring discontinuation of treatment. The others AEs that led into discontinuation were upper respiratory infection, hemiparesis, peripheral facial paralysis, arthralgia, asthma, and gastrointestinal discomfort. At the end of the study period, 54 (61.3%) patients were still receiving treatment with secukinumab.
DiscussionLong-term control of moderate-to-severe psoriasis has been extremely important in the management of psoriasis, permitting the achievement of long-term remission of disease signs and symptoms in an increased proportion of patients. Persistence is becoming a useful measure to evaluate the long-term effectiveness of therapies in real clinical setting in chronic diseases. It may be considered a surrogate of treatment success, dependent on the effectiveness and safety, comfort of administration, patient satisfaction, and other factors.15 Our study analyzed the persistence of secukinumab in a cohort of 88 patients treated according to routine clinical practice. The included patients had a median PASI score of 15.0, being similar to others real-world studies.16,17 Patients naïve to biologic therapy accounted for 51% of all patients included. Our study has shown secukinumab persistence at 1 year, 2 years, and 3 years was 72.7%, 51.1%, and 39.8%, respectively. The observed 1-year persistence is consistent with previous reports in real-word studies. Conesa-Nicolás et al.10 conducted a real-world study in Spain to evaluate the persistence and effectiveness of treatment with secukinumab in psoriasis. The persistence obtained at 12 months was 56.3%, lower than our study. 43.7% of patients discontinued their treatment, 59.3% of them because of secondary failure. However, in a 2-year real-world, the persistence rates of secukinumab in patients with psoriasis at 12, 18, and 24 months were 89.0%, 81.1%, and 74.3%, respectively.18 In a multicenter European study in psoriasis patients with 65.9% naive patients to biologic treatment, the secukinumab treatment retention rates after 1, 2, and 3years in the study were 88.0%, 76.4%, and 60.5%, respectively. The 36.9% of patients discontinued secukinumab, the most common reasons were lack of efficacy (42.6%), adverse event (17.4%), physician decision (12.2%), and subject decision (11.6%).17 Regarding therapeutic response, PASI 100 was obtained only in 19.4% of patients similar to other real-world studies.19 Similar results were obtained regarding the DLQI reduction after secukinumab 1 year therapy.20 Secukinumab had a manageable adverse event discontinuation rate and only 7.9% of patients had to discontinue treatment for safety reasons, similar to other studies.10,17
Bio-experienced patients might have a higher burden of disease severity, requiring more aggressive treatment strategies.21 Bio-experienced patients were more likely to discontinue their treatment than bio-naïve patients.22 However, there are few real-world evidence series that evaluate differences in the persistence between bio-naïve and bio-experienced patients.23 Although biologic survival rate tends to be higher in bio-naïve patients, statistically significant differences between them and bio-experienced patients for any of the approved biologics are challenging to find. Wang et al. conducted a real-world study of patients diagnosed with plaque psoriasis to assess the persistence of treatment with interleukin-17 inhibitors. Subgroup analysis by experience with biologics showed similar persistence rates among bio-naïve and bio-experienced patients with PsO at 12 (73.1% vs 75.6%), 24 (64.2% vs 66.6%), and 36months (59.5% vs 58.3%).24 In our cohort of patients, persistence was slightly higher in the non-naïve group vs naïve group without showing statistically significant differences (p = .804). These differences may be due to the small sample size and immaturity of the data in the naïve group, as these were patients who started treatment later. Therefore, it is foreseeable that in the future, the persistence of naïve patients will increase and be higher than non-naïve patients in a similar way to what occurs in the literature described.
One of the strengths of this study is that the patients included were considered adherent to secukinumab based on the MPR data presented. A high MPR indicates that a patient is consistently refilling their prescriptions and is therefore likely to be taking their medication as prescribed. This makes the persistence findings of the study more generalizable and reliable to real-world clinical practice.25
Interpretation of the study results requires consideration of the limitations of this study, such as our limited sample size, and the observational and retrospective nature of the study. Furthermore, only one method, the MPR, was used to estimate adherence. It is well-known that this method tends to overestimate adherence. Therefore, further studies are needed to confirm the real-world effectiveness of secukinumab by assessing outcomes in the clinical setting with the aim of improving psoriasis treatment.
In this real-world study, the patients with psoriasis treated with secukinumab had moderate to severe disease activity and more than 70% of patients with a 1-year follow-up persisted on secukinumab similar to previous real-world studies presented in Spain. However, 2- and 3-year secukinumab persistence rates are lower than those reported in other studies.16,17 These results add to existing data by providing evidence generated from real-world data of secukinumab long-term persistence in patients with moderate-to-severe psoriasis.
Contribution to the scientific literatureThis study contributes to the existing literature by presenting real-world data on the long-term persistence of secukinumab in patients with psoriasis. The findings provide valuable insights into factors associated with treatment persistence, including prior exposure to biologic therapies. The article highlights the importance of long-term persistence data in evaluating the cost-effectiveness of biologic treatments for psoriasis. Healthcare providers can utilize this information to optimize secukinumab therapy and improve patient outcomes.
FundingThe authors received no financial support for the research, authorship, and/or publication of this article.
CRediT authorship contribution statementJoaquín Borrás-Blasco: Writing – review & editing, Writing – original draft, Visualization, Validation, Methodology, Investigation, Formal analysis, Conceptualization. Silvia Cornejo: Writing – review & editing, Validation, Methodology, Investigation, Formal analysis, Data curation. Alejandro Valcuende-Rosique: Writing – review & editing, Visualization, Methodology, Formal analysis, Data curation. Rebeca Alcala: Writing – review & editing, Validation, Supervision, Formal analysis. Ana Navalon Bono: Data curation, Formal analysis, Investigation, Methodology, Visualization.
The authors have no affiliations with or financial interest in any company or organization that could conflict with the views expressed in this manuscript.
The work has not been previously published, nor is it under review by any other journal.
-The instructions for manuscript submission and ethical responsibilities have been taken into account, including ensuring that all signing authors meet the authorship requirements and have declared no conflicts of interest.”