The present work analyzed the stability of trastuzumab concentrations in blood and plasma samples stored at 4 °C and –;20 °C.
MethodBlood samples of patients treated with trastuzumab (Herceptin®) were analyzed. Stability of trastuzumab was analyzed under different conditions: whole blood stored at 4 °C during 72 hours, blood plasma stored at 4 °C during 120 hours and blood plasma stored at –;20 °C during 365 days.
ResultsIn whole blood trastuzumab concentration at 72 hours was 99.01 ± 0.02%, and in plasma samples at 120 hours it was 98.6 ± 2.0%. The concentration of trastuzumab was 95.22 ± 3.20% after 12 months’ storage at –;20 °C.
ConclusionsThe concentration of trastuzumab remains stable for at least 72 hours in whole blood stored at 4 °C, five days in plasma stored at 4 °C and one year in plasma stored at –;20 °C.
Analizar la estabilidad del trastuzumab en sangre y plasma a 4 °C y –;20 °C.
MétodoSe determinó la concentración plasmática de trastuzumab en las muestras de sangre y plasma de pacientes con cáncer de mama HER2 positivo tratados con trastuzumab (Herceptin®). Se analizó la estabilidad del trastuzumab en sangre a 4 °C durante 72 horas. En el caso de las muestras de plasma, se estudió la estabilidad a 4 °C durante 72 y 120 horas, así como durante 365 días congeladas a –;20 °C.
ResultadosEn sangre, la concentración de trastuzumab a las 72 horas fue de 99,01 ± 0,02%, y en las muestras de plasma a las 120 horas fue de 98,6 ± 2,0%. La concentración de trastuzumab fue de 95,22 ± 3,20% después de 12 meses de almacenamiento a –;20 °C.
ConclusionesEl trastuzumab permanece estable durante al menos 72 horas en sangre total almacenada a 4 °C, cinco días en plasma almacenado a 4 °C y un año en plasma almacenado a –;20 °C.
Trastuzumab is a monoclonal antibody used in the treatment of breast and gastric cancer where neoplastic tissue overexpresses the epidermal growth factor receptor HER21–4. Recently, a link has been established between trough levels (Cmin) of trastuzumab and effectiveness5–8, which has attracted interest in monitoring the pharmacokinetics of this drug.
One of the prerequisites for pharmacokinetic study is to determine the stability of the drug in blood samples, because sometimes they cannot be processed immediately after extraction and are stored. To date, there are no data on the long-term stability of trastuzumab in whole blood and/or plasma stored under different conditions. The present work analyzes the stability of trastuzumab concentrations in blood and plasma samples stored at 4 °C and –;20 °C, during differents points of time (24, 72, 120 hours and one year).
MethodsStudy design and sample collection
We performed a single-center, prospective, observational study of a year of duration, in which blood samples (5 mL) of HER2 positive breast cancer patients treated with trastuzumab (Herceptin®) were analyzed. The samples were collected in K2EDTA tubes (Becton Dickson Company®, USA) and centrifuged at 3,000 g for 5 minutes to obtain blood plasma.
We analyzed the stability of the samples stored under different conditions:
- •
Analysis 1: whole blood stored at 4 °C during time zero (C0), at 24 and 72 hours.
- •
Analysis 2: blood plasma stored at 4 °C during C0, 24, 72 and 120 hours.
- •
Analysis 3: blood plasma stored at –;20 °C, during C0, 60, 180 and 365 days.
Trastuzumab concentration measured at time zero (C0), i.e. immediately after extraction, was considered the reference value; all determinations were performed in duplicate. The results are expressed as mean ± standard deviation (SD) for both.
Statistical analysis was performed using IBM SPSS v22 (IBM Corporation, Armonk, NY).
Compliance with ethical standardsThe present analysis was part of a wider study (EPA-SP with code FER-TRA-2015–01), approved by the Clinical Research Ethics Committee of our hospital for implementation in February 2015. Written informed consent was obtained from all patients before inclusion in the study.
Determination of trastuzumab concentrationsPlasma concentrations of trastuzumab were determined using ELISA (enzyme-linked immunosorbent assay), following the manufacturer's specifications (Shikari® q-tras)9, employing ELISA triturus autoanalyzer (Grifols®). The samples were diluted 20 or 80 times (depending on the estimated concentration) with buffer solution (commercial kit) in duplicate to minimize pipetting errors and intrinsic variations of the method.
Stability of trastuzumabFor the analysis of the stability of trastuzumab in blood samples, the drug concentration calculated at time zero (C0) was taken as the reference value. Thus the stability of the drug in samples stored for different times was expressed as a percentage of the C0 value. A stability limit of 90% was established as acceptable.
For the analysis of stability at 4 ºC (whole blood and plasma) we used three samples (A, B and C) and for the study of stability at –;20 ºC (plasma) we used five samples (D, E, F, G and H).
ResultsA total of 82 determinations were made, with the following results:
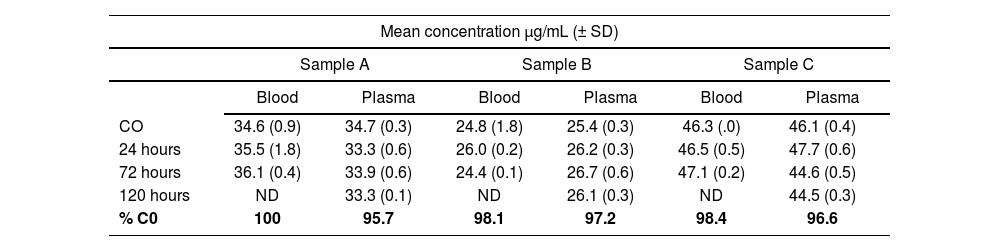
Stability of trastuzumab in samples stored at 4 °CThe mean concentrations of trastuzumab are shown in Table 1. In whole blood at 72 hours this was 99.01 ± 0.02, and in plasma samples at 120 hours it was 98.6 ± 2.0% (Table 1).
Mean concentrations of trastuzumab in whole blood and plasma samples stored at 4 °C for periods ranging from 0 to 120 hours
| Mean concentration µg/mL (± SD) | ||||||
|---|---|---|---|---|---|---|
| Sample A | Sample B | Sample C | ||||
| Blood | Plasma | Blood | Plasma | Blood | Plasma | |
| CO | 34.6 (0.9) | 34.7 (0.3) | 24.8 (1.8) | 25.4 (0.3) | 46.3 (.0) | 46.1 (0.4) |
| 24 hours | 35.5 (1.8) | 33.3 (0.6) | 26.0 (0.2) | 26.2 (0.3) | 46.5 (0.5) | 47.7 (0.6) |
| 72 hours | 36.1 (0.4) | 33.9 (0.6) | 24.4 (0.1) | 26.7 (0.6) | 47.1 (0.2) | 44.6 (0.5) |
| 120 hours | ND | 33.3 (0.1) | ND | 26.1 (0.3) | ND | 44.5 (0.3) |
| % C0 | 100 | 95.7 | 98.1 | 97.2 | 98.4 | 96.6 |
CO: Concenlralion of Irasluzumab measured immedialely afler blood sample extraction (time zero); ND: not data.
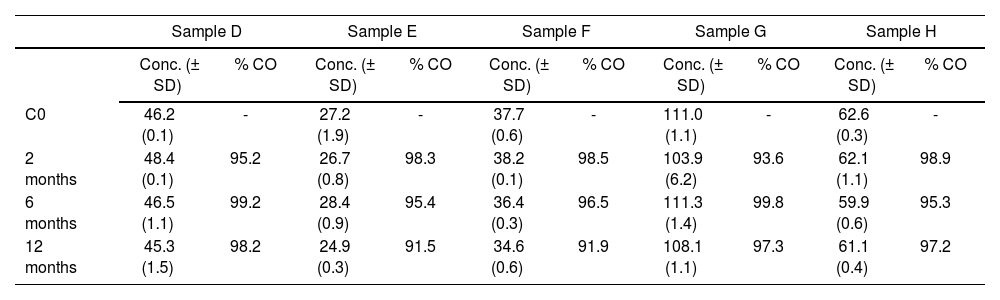
The mean concentration of trastuzumab was 96.9 ± 1.7%; 97.2 ± 0.9% and 95.22 ± 3.20%, after two, six and 12 months’ storage at –;20 °C respectively (Table 2).
Mean concentrations of trastuzumab in plasma samples stored at –;20 °C for periods ranging from 0 to 12 months
| Sample D | Sample E | Sample F | Sample G | Sample H | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Conc. (± SD) | % CO | Conc. (± SD) | % CO | Conc. (± SD) | % CO | Conc. (± SD) | % CO | Conc. (± SD) | % CO | |
| C0 | 46.2 (0.1) | - | 27.2 (1.9) | - | 37.7 (0.6) | - | 111.0 (1.1) | - | 62.6 (0.3) | - |
| 2 months | 48.4 (0.1) | 95.2 | 26.7 (0.8) | 98.3 | 38.2 (0.1) | 98.5 | 103.9 (6.2) | 93.6 | 62.1 (1.1) | 98.9 |
| 6 months | 46.5 (1.1) | 99.2 | 28.4 (0.9) | 95.4 | 36.4 (0.3) | 96.5 | 111.3 (1.4) | 99.8 | 59.9 (0.6) | 95.3 |
| 12 months | 45.3 (1.5) | 98.2 | 24.9 (0.3) | 91.5 | 34.6 (0.6) | 91.9 | 108.1 (1.1) | 97.3 | 61.1 (0.4) | 97.2 |
CO: plasma concentration of trastuzumab measured immediately after blood sample extraction (time zero). Cone: concentration (µg/mL).
Monitoring plasma levels of drugs has been shown to be an effective tool to improve clinical outcomes of treatments10. However, this is not always easy, as there are often limitations such as the processing and storage of samples11. Jamieson et al.9 have shown that trastuzumab is stable after 72 hours and 10 weeks in plasma samples stored at 4 °C and –;20 °C, respectively. With this work we wished to show that sample storage time can exceed 120 hours at 4 °C and that plasma samples can even be stored for one year at –;20 °C. The present study not only analyzed trastuzumab stability in blood plasma, but also confirmed its stability in whole blood samples stored for 72 hours at 4 °C. These data are of particular interest to health centers that lack the technical means for processing the samples which are then sent to external laboratories.
In conclusion, the concentration of trastuzumab remains stable for at least 72 hours in whole blood stored at 4 °C, five days in plasma stored at 4 °C and one year in plasma stored at –;20 °C.
FundingNo funding.
Conflict of interestsNo conflict of interests.
Contribution to the scientific literature
The stability of trastuzumab in blood or plasma has not been assessed. Such assessment is fundamental to conducting pharmacokinetic studies. The present study shows that the drug is stable in serum for 5 days when stored between 0°C and 5°C and up to 1 year when stored at –;20°C.