To compare radiographic progression-free survival in metastatic prostate cancer patients treated with low-dose abiraterone versus standard-dose abiraterone acetate (Abi-SD), and to evaluate prostate-specific antigen progression-free survival.
MethodsRetrospective cohort study of patients with metastatic prostate cancer, castration-sensitive or castration-resistant, treated with low or standard-dose abiraterone. All patients were followed until radiographic or prostate-specific antigen progression. Cox proportional hazards regression was used to assess radiographic progression-free survival and prostate-specific antigen progression-free survival according to abiraterone dose (low vs. standard-dose). The model was adjusted for Charlson Comorbidity Index, castration resistance status, disease volume based on CHAARTED criteria, and presence of de novo metastases.
ResultsA total of 144 patients with metastatic prostate cancer were included in the study, with 28.4% (n = 41) receiving low-dose abiraterone. The median age was 79 years (IQR: 75–85) in the low-dose group and 75 years (IQR: 70–81) in the standard-dose group. For radiographic progression-free survival, the crude hazard ratio for the low-dose group compared with the standard-dose group was 0.49 (95% CI: 0.23–1.07). After adjusting for clinical variables, the adjusted hazard ratio was 0.65 (95% CI: 0.29–1.45). For prostate-specific antigen progression-free survival, the crude hazard ratio was 0.48 (95% CI: 0.24–0.90), and the adjusted hazard ratio was 0.58 (95% CI: 0.29–1.14).
ConclusionThis study provides evidence supporting the use of low-dose abiraterone in patients with metastatic prostate cancer, showing survival and progression outcomes comparable to those of the standard-dose. This approach may improve access to treatment; however, larger studies are needed to validate these findings.
Comparar la supervivencia libre de progresión radiográfica en pacientes con cáncer de próstata metastásico tratados con abiraterona en dosis reducida versus dosis recomendada, y evaluar la supervivencia libre de progresión según antígeno prostático específico.
MétodosEstudio de cohorte retrospectiva de pacientes con cáncer de próstata metastásico, sensible o resistente a la castración, tratados con abiraterona en dosis reducida o dosis recomendada. Todos los pacientes fueron seguidos hasta la aparición de progresión radiográfica o progresión del antígeno prostático específico. Se utilizó la regresión de riesgos proporcionales de Cox para evaluar la supervivencia libre de progresión radiográfica y la supervivencia libre de progresión del antígeno prostático específico según la dosis de abiraterona (reducida versus recomendada). El modelo se ajustó según el índice de comorbilidad de Charlson, el estado de resistencia a la castración, el volumen de la enfermedad según los criterios CHAARTED y la presencia de metástasis de novo.
ResultadosSe incluyeron 144 pacientes con cáncer de próstata metastásico; el 28,4% (41) recibió abiraterona en dosis reducida. La mediana de edad fue de 79 años (RIQ: 75–85) en el grupo de dosis reducida y de 75 años (RIQ: 70–81) en el grupo de dosis recomendada. Luego de ajustar por variables clínicas, el grupo de dosis reducida presentó un hazard ratio de 0,65 (IC 95%: 0,29 a 1,45) para la supervivencia libre de progresión radiográfica y de 0,58 (IC 95%: 0,29 a 1,14) para la supervivencia libre de progresión basada en niveles de antígeno prostático específico, en comparación con el grupo de dosis recomendada. Para la supervivencia libre de progresión radiográfica, el hazard ratio ajustado fue de 0,65 (IC95%: 0,29 a 1,45) y para la supervivencia libre de progresión basada en niveles de antígeno prostático específico, el hazard ratio ajustado fue de 0,58 (IC95%: 0,29 a 1,14)2.
ConclusiónEste estudio aporta evidencia sobre el uso de abiraterona en dosis reducida en pacientes con cáncer de próstata metastásico, informando resultados de sobrevida y progresión comparables a los de la dosis recomendada. Este enfoque podría mejorar el acceso al tratamiento. Sin embargo, se necesitan estudios con mayor tamaño muestral para validar estos resultados.
Prostate cancer (PC) is a public health challenge worldwide, due to its high prevalence, substantial demand for healthcare resources, and the significant financial burden associated with its treatment.1,2 In Argentina, prostate cancer is the most frequently diagnosed cancer in men and represents the third leading cause of cancer-related deaths.3 This highlights the necessity of strategies that facilitate the accessibility of effective treatments while maintaining the financial sustainability of healthcare systems.
Abiraterone is approved for the treatment of patients with advanced PC, showing improvements in overall survival, radiographic progression-free survival (rPFS), and significant prostate-specific antigen (PSA) response.4–6
In 2019, the National Comprehensive Cancer Network (NCCN) included low-dose abiraterone (250 mg/day) as a treatment option for metastatic castration-resistant prostate cancer (mCRPC). This lower dose, now adopted in clinical settings worldwide,7 was supported by evidence from a phase II trial involving 72 patients, which demonstrated that administering 250 mg with a low-fat meal yields comparable clinical efficacy to the standard 1.000 mg dose taken while fasting.7
Using a low-dose of abiraterone can reduce the overall cost of cancer care, improve treatment affordability, and increase patient access, especially in resource-limited settings.8 Additionally, this strategy could enhance treatment safety and adherence by reducing the number of pills required per day. It may also lower treatment costs, allowing healthcare systems to reallocate resources toward managing other conditions and adopting innovative therapies.9–11
Our institution implements this strategy based on available preclinical evidence, observational studies, and clinical practice guideline recommendations.12 This study was designed to provide additional evidence on the use of low-dose abiraterone in real-world settings. We report survival and progression outcomes associated with the implementation of a low-dose strategy, which aims to improve treatment affordability. Specifically, the objectives are to describe rPFS in patients with metastatic prostate cancer receiving low-dose abiraterone (Abi-LD) versus the standard-dose (Abi-SD), and to evaluate PSA progression-free survival (PSA-PFS) and the PSA objective response rate (ORR-PSA) defined as a decline greater than 50%.
MethodsStudy designRetrospective observational cohort study.
SettingThe study was conducted at a university hospital, a high-complexity medical center located in Buenos Aires, Argentina. The hospital also manages its health insurance provider, the Plan de Salud.
ParticipantsThis study involved patients with metastatic prostate cancer who were treated at a university hospital between 2013 and 2023. The inclusion criteria were patients older than 18 years, enrolled in the university hospital's Plan de Salud, diagnosed with metastatic prostate cancer, and treated with Abiraterone.
Data sourcesThe study was approved by the local Ethics Committee for Research Protocols (Comité de Ética de Protocolos de Investigación, CEPI), approval number 7048. Clinical and administrative data were extracted from a centralized electronic health record (EHR) system. A manual review of EHRs was performed to collect demographic and clinical information, with all procedures ensuring patient confidentiality.
VariablesExposure variableUse of Abi-SD (abiraterone of 1.000 mg daily) or Abi-LD (abiraterone of 250 mg daily). All patients received prednisone at a dose of 5 mg orally twice daily.
Outcome variablesThe primary outcome was rPFS, defined as the time from the initiation of abiraterone to radiographic disease progression, assessed using the Response Evaluation Criteria in Solid Tumors (RECIST).13,14 Radiographic progression was determined by imaging studies showing either a ≥30% increase in the size of target lesions or the appearance of new lesions.13
Secondary outcomes included PSA-PFS, defined as the time from treatment initiation to PSA progression, as per the Prostate Cancer Clinical Trials Working Group 3 (PCWG3) criteria. PSA progression was characterized by a sustained rise in PSA of more than 25% and greater than 2 ng/mL above the nadir, confirmed at two consecutive time points at least 3 weeks apart.13
Another endpoint was the ORR-PSA, defined as the proportion of patients who achieved a reduction in PSA levels greater than 50% after treatment.
Given the likelihood of underreporting of less severe adverse events in EHR, only grade ≥3 events were analyzed using the available data.15
Descriptive variables included age, Charlson Comorbidity Index, Gleason score, presence of visceral metastases, castration sensitivity or resistance, de novo or recurrent metastatic status, disease volume according to the CHAARTED criteria (Randomized Trial of Chemohormonal Therapy Versus Androgen Ablation for Extensive Disease in Prostate Cancer), prior treatments, and PSA level.
Variables influencing the response (potential confounders)Charlson Comorbidity Index, CHAARTED criteria, castration sensitive state, and de novo or recurrent metastatic status. These variables were selected based on clinical criteria from oncology experts, which can be seen in the appendix through a directed acyclic graph (Supplementary material Fig. S1).
Study sizeA fixed sample size was used, as all available individuals who met the inclusion criteria were considered.
Statistical analysisDescriptive statistics were used to describe the study population, detailing central tendency and dispersion based on the distribution type. Normality was verified using Shapiro–Wilk or Kolmogorov–Smirnov tests as appropriate. Normally distributed data was presented as mean and standard deviation, while non-normal data was presented as median and interquartile range (IQR). Categorical and ordinal variables were expressed as absolute and relative frequencies. To assess the association between descriptive variables and Abi-SD or Abi-LD, bivariate analyses were performed based on the normality of the data and the nature of the variables (quantitative or categorical). For quantitative variables, the T-test or Mann–Whitney U test was utilized, while the chi-square test or Fisher's exact test was applied for categorical variables.
Radiographic progression-free survival and PSA-PFS were calculated using time-to-event analysis and were estimated graphically by the Kaplan–Meier (KM) method. Patients were censored at the end of follow-up, at the last data entry, at the last recorded visit, or due to administrative censoring on 19/06/2024. The log-rank test compared the PFS between abiraterone doses (1.000 mg/day or 250 mg/day) on rPFS and PSA-PFS. The Cox regression analysis was performed for adjustment by potential confounders. To identify potential confounders, oncology experts selected clinically relevant variables using a Directed Acyclic Graph (DAG) (Supplementary material Fig. S1). The variables chosen included the Charlson Comorbidity Index, CHAARTED criteria, castration-sensitive state, and de novo or recurrent metastatic status. The crude hazard ratio (cHR), adjusted (aHR), and 95% confidence interval (95% CI) were calculated. A significance level of less than 5% was considered.
The ORR-PSA was defined as the proportion of patients who achieved a ≥50% reduction in serum PSA levels from the start of treatment to study completion. Patients were categorized based on whether they achieved this PSA reduction, and the association with the dosing regimen (Abi-SD or AbiLD) was evaluated using a chi-square (χ2) test. A p-value of <0.05 was considered statistically significant. Statistical analyses were conducted using STATA version 16.
ResultsA total of 144 patients with prostate cancer were included in this study; 28.4% (41) were treated with Abi-LD. Baseline demographic and clinical characteristics are described in Table 1.
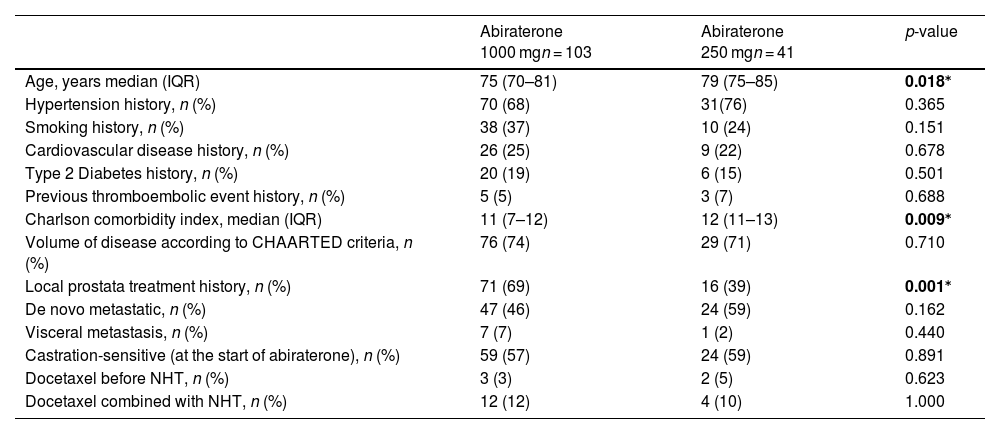
Baseline characteristics of patients receiving standard-dose and low-dose of Abiraterone.
| Abiraterone 1000 mgn = 103 | Abiraterone 250 mgn = 41 | p-value | |
|---|---|---|---|
| Age, years median (IQR) | 75 (70–81) | 79 (75–85) | 0.018⁎ |
| Hypertension history, n (%) | 70 (68) | 31(76) | 0.365 |
| Smoking history, n (%) | 38 (37) | 10 (24) | 0.151 |
| Cardiovascular disease history, n (%) | 26 (25) | 9 (22) | 0.678 |
| Type 2 Diabetes history, n (%) | 20 (19) | 6 (15) | 0.501 |
| Previous thromboembolic event history, n (%) | 5 (5) | 3 (7) | 0.688 |
| Charlson comorbidity index, median (IQR) | 11 (7–12) | 12 (11–13) | 0.009⁎ |
| Volume of disease according to CHAARTED criteria, n (%) | 76 (74) | 29 (71) | 0.710 |
| Local prostata treatment history, n (%) | 71 (69) | 16 (39) | 0.001⁎ |
| De novo metastatic, n (%) | 47 (46) | 24 (59) | 0.162 |
| Visceral metastasis, n (%) | 7 (7) | 1 (2) | 0.440 |
| Castration-sensitive (at the start of abiraterone), n (%) | 59 (57) | 24 (59) | 0.891 |
| Docetaxel before NHT, n (%) | 3 (3) | 2 (5) | 0.623 |
| Docetaxel combined with NHT, n (%) | 12 (12) | 4 (10) | 1.000 |
Note: CHAARTED criteria: Chemohormonal Therapy versus Androgen Ablation Randomized Trial for Extensive Disease in Prostate Cancer, Charlson Comorbidity Index: Charlson Comorbidity Score predicts the 10-year mortality risk for a patient based on a range of concurrent conditions (comorbidities) and age. Each condition is assigned a score of 1, 2, 3, or 6, depending on the associated risk of death. Conditions and their scores include myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatologic disease, peptic ulcer disease, mild or severe liver disease, controlled or uncontrolled diabetes, hemiplegia or paraplegia, renal disease, localized or metastatic malignancy, leukemia, lymphoma, and AIDS. Additionally, patient age is incorporated, with one point added for each decade over 50 years. Median (IQR) = Median (Interquartile Range), NHT: New Hormone Therapy, n = number, (%) = percentage.
A total of 42 deaths were reported, corresponding to an overall mortality rate of 29.2% (42/144), with 33% (34/103) in the Abi-SD group and 19.5% (8/41) in the Abi-LD group.
Global progression was observed in 40% of patients (59/144), with a rate of 46.6% (48/103) in the Abi-SD group and 26.8% (11/41) in the Abi-LD group. Radiographic progression occurred in 32.6% of patients (47/144), including 37.8% (39/103) in the Abi-SD group and 19.5% (8/41) in the Abi-LD group. PSA progression was reported in 41.6% of patients (60/144), with 47.5% (49/103) in the Abi-SD group and 26.8% (11/41) in the Abi-LD.
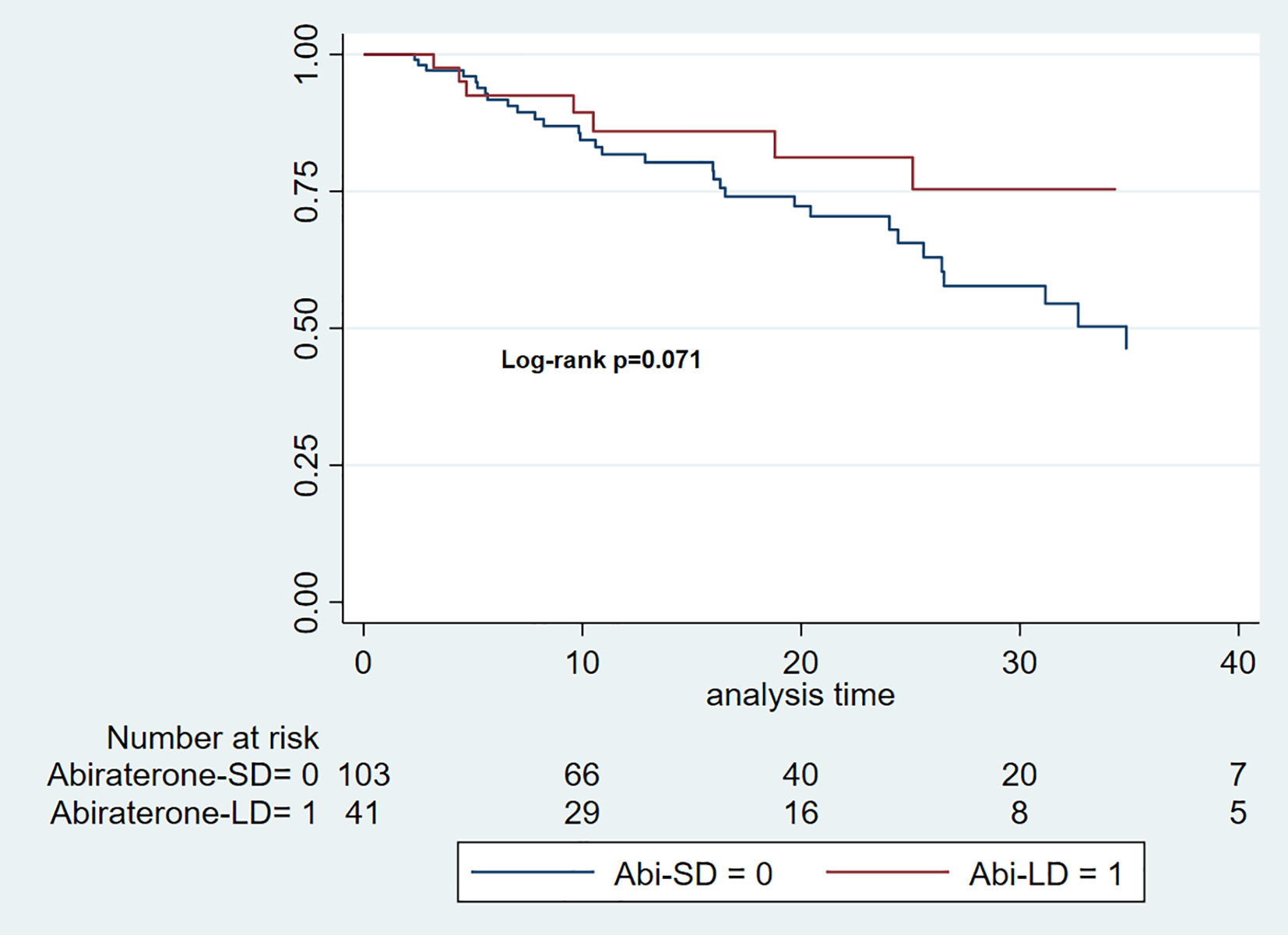
Radiographic progression-free survivalThe median follow-up time for the Abi-SD group was 16 months (IQR: 7–25), while for the Abi-LD group, it was 15 months (IQR: 9–27). The median rPFS was 34.87 months for the Abi-SD group (95% CI 25.6–42.4), while it could not be calculated for the Abi-LD group due to insufficient progression events. (Log-rank p 0.071). (Fig. 1).
The cHR for rPFS over a total at-risk time of 2634 months was 0.49 (95% CI: 0.23–1.07). After adjusting for key clinical variables (Charlson Comorbidity Index, CHAARTED criteria, castration sensitivity, and the presence of de novo metastatic disease), the aHR was 0.65 (95% CI: 0.29–1.45).
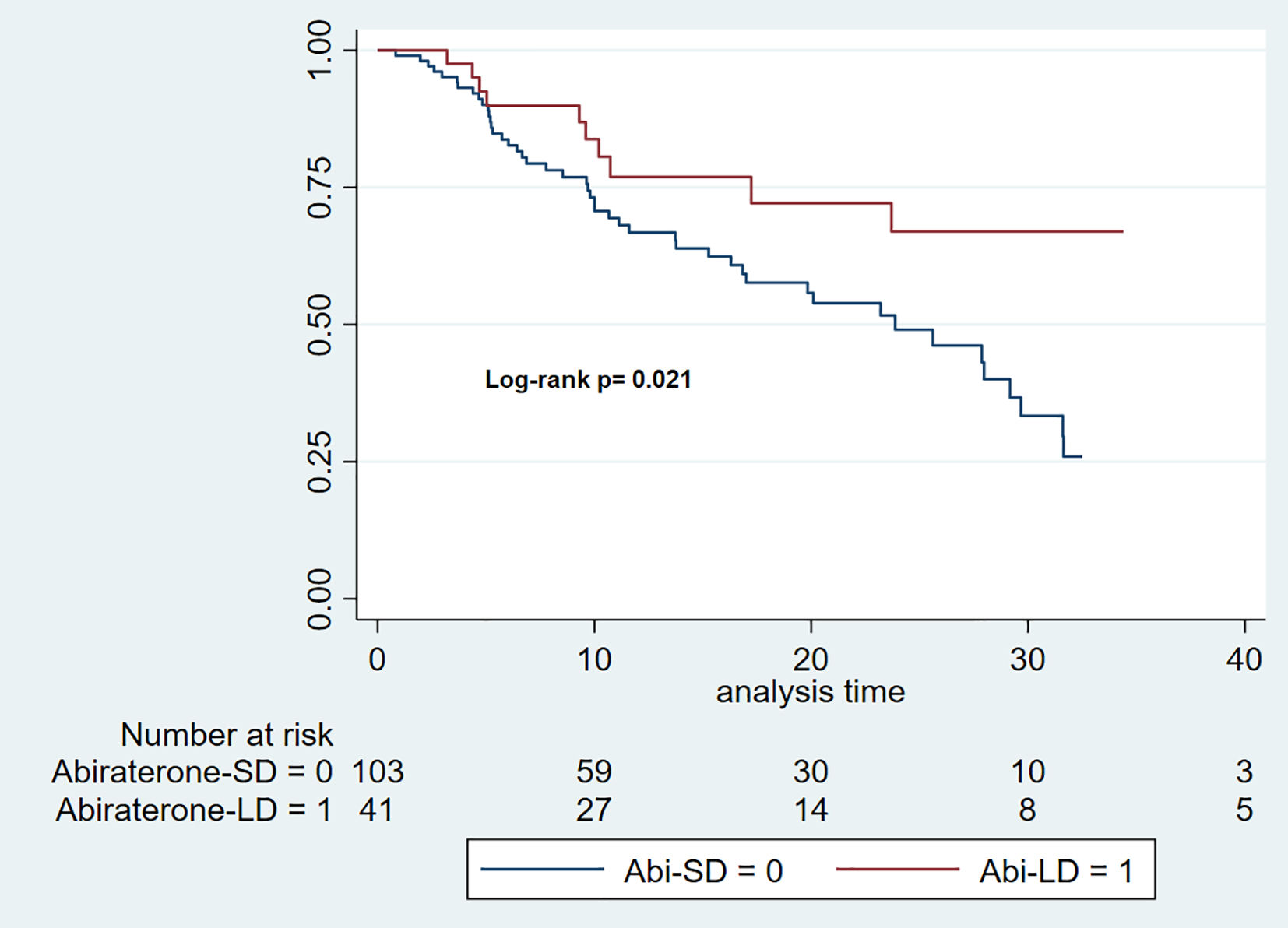
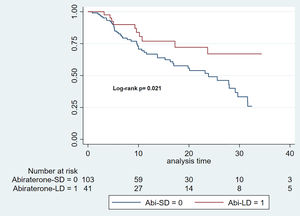
PSA progression-free survivalThe median follow-up time for the Abi-SD group was 11 months (IQR: 6–22), while for the Abi-LD group, it was 13 months (IQR: 8–27). The median PSA-PFS was 23.87 months for the Abi-SD group (95% CI: 16.3–29.6), while it could not be calculated for the Abi-LD group due to insufficient progression events. (Log-rank p 0.021). (Fig. 2).
The cHR for rPFS over a total at-risk time of 2267 months was 0.47 (95% CI: 0.24–0.90). After adjusting for key clinical variables (Charlson Comorbidity Index, CHAARTED criteria, castration sensitivity, and the presence of de novo metastatic disease), the aHR was 0.58 (95% CI: 0.29–1.14).
Objective response rate of PSA levels greater than 50%Among the 144 patients, 75.69% (109/144) achieved a PSA reduction greater than 50%, 5.56% (8/144) did not achieve such a reduction, and 18.75% (27/144) had missing PSA values. The association between the dosing regimen (Abi-SD or Abi-LD) and achieving a PSA reduction greater than 50% resulted in a p-value of 0.265.
Adverse eventsNo significant differences were observed in the incidence of adverse events (grade ≥3) between the low-dose and Standard-Dose groups.
DiscussionThe purpose of our study was to present an institutional strategy to improve the affordability of metastatic prostate cancer treatment. To achieve this, we compared the survival and progression outcomes between patients receiving Abi-SD and those treated with Abi-LD.
Our analysis demonstrated comparable survival and progression outcomes between the two groups. After adjusting for disease volume (CHAARTED criteria), castration sensitivity, Charlson comorbidity index, and presence of de novo metastatic disease, no statistically significant differences in rPFS were observed. These findings suggested that Abi-LD was non-inferior to Abi-SD, consistent with previous pharmacological evidence indicating that low-dose of abiraterone could achieve adequate therapeutic levels for efficacy under specific conditions.7,11
To minimize confounding, we adjusted the Cox proportional hazards models using variables selected a priori based on clinical relevance and review of the literature. These included the Charlson Comorbidity Index, castration resistance status, disease volume as defined by the CHAARTED criteria, and the presence of de novo metastases.16,17 Variable selection was guided by a directed DAG (Supplementary material Fig. S1) and supported by the clinical expertise of our oncology team, with an emphasis on clinically relevant variants rather than relying exclusively on statistical significance. This methodological approach aimed to improve the validity of our effect estimates regarding low-dose abiraterone and progression-free outcomes.
Although we did not find studies with a comparable design, previous research on low-dose abiraterone administered with meals supported the notion that lower doses of abiraterone could provide similar efficacy to standard-dose. However, patient populations in these studies differed in baseline characteristics such as age, disease burden, and comorbidities.18–20 Our study, conducted at a university hospital in Buenos Aires, Argentina, presents results comparable to those of other studies conducted in other single-center settings.20
A survey of 118 medical oncologists in India found that nearly 62% reported using low-dose abiraterone, with 6.8% using it routinely and 55.1% using it in resource-limited settings.12 These findings suggested that, in the absence of randomized clinical trials, real-world evidence, supported by clinical practice guidelines, could contribute to the widespread adoption of this approach in routine practice.
In contrast to our findings, the study by Yamada et al. (2022), which retrospectively analyzed a Japanese cohort of patients with castration-resistant prostate cancer (CRPC), reported that abiraterone dose reduction was associated with shorter progression-free survival, although no differences in overall survival were observed. However, differences in patient population, healthcare system, and dose reduction strategies limit the direct comparability between the studies.21
Our analysis of adverse events focused exclusively on grade ≥3 toxicities, with no significant differences observed between the treatment groups. This limitation reflects the retrospective design of the study and the potential for underreporting in medical record-based documentation. Since the phase II trial comparing low-dose and standard-dose abiraterone did not show significant differences in toxicity, we did not expect such differences in our cohort.
While assessing adverse events was not the primary objective of our study, low-dose of abiraterone may offer benefits in terms of reducing dose-dependent toxicities, including a lower incidence of mild to moderate adverse events, which were not evaluated in our analysis. Future prospective studies are needed to explore the full toxicity and safety profile of low-dose abiraterone.
In our study, all patients received 10 mg of prednisone daily, independent of the abiraterone dose or hormone sensitivity. Following current clinical guidelines, low-dose prednisone was coadministered to prevent mineralocorticoid excess, which can result from CYP17A1 inhibition induced by abiraterone treatment. This mechanism of action could cause adverse effects, regardless of whether the standard or low-dose of abiraterone is used. Consequently, a fixed prednisone dose is recommended as part of the regimen, regardless of the abiraterone dose administered.
Although we did not perform a direct cost evaluation, the 75% dose reduction of abiraterone constitutes a strategy to improve treatment accessibility and facilitate resource redistribution.9
The limitations of this study include concerns regarding external validity and generalizability. As the study was conducted at specific centers, the findings may not apply to other institutions, even within Argentina, due to differences in patient populations. Additionally, the decision to administer low-dose was left to the discretion of the clinician, which could introduce variability based on physician judgment. While this approach reflects real-world practice, it also carries the risk of indication bias, which we attempted to mitigate by adjusting for potential confounders. It is important to acknowledge that baseline differences between treatment groups may have influenced the observed outcomes. Despite adjustments for key clinical variables, the observational design inherently carries the risk of residual confounding. Therefore, these findings should be interpreted with caution, and further studies are needed to confirm our results.
Although our EHR system provided comprehensive clinical data, the retrospective design of the study introduced the potential for information bias. Nonetheless, although data collection was retrospective, the generation and documentation of data in the EHR occurred prospectively, before the outcomes were assessed. This approach helped reduce the risk of information bias typically associated with retrospective studies, where reliance on participants' recollection of past behaviors or events could result in errors.
Finally, pharmacokinetic parameters and potential drug–drug or drug-food interactions were not assessed, as such information is not routinely or systematically collected in standard clinical practice. We recognize that these factors are crucial in the context of abiraterone treatment, and their omission represents a limitation in the safety evaluation.22
A key strength of our study is the inclusion of real-world data derived from routine clinical practice, with complete follow-up of a closed cohort. Moreover, our study included a larger number of patients compared to other published studies to date.19,20,23
In conclusion, this study provides additional evidence on the use of Abi-LD in a real-world setting, suggesting its survival and progression outcomes are comparable to those of Abi-SD.
This approach reduces treatment burden while maintaining therapeutic efficacy and may improve adherence and patient satisfaction by lowering the number of daily tablets required. However, further prospective studies with larger sample sizes are necessary to conclusively assess the efficacy and safety of low-dose abiraterone in real-world settings.
Contribution to the scientific literatureThis study provides real-world evidence on the use of low-dose of abiraterone in patients with metastatic prostate cancer.
Implications of the resultsThe use of low-dose abiraterone may help reduce the financial burden of treatment and promote more efficient distribution of healthcare resources.
CRediT authorship contribution statementLuis Angel Di-Giuseppe: Writing – review & editing, Writing – original draft, Visualization, Supervision, Project administration, Investigation, Conceptualization. Rodney Alexander Ramirez-Murillo: Writing – review & editing, Writing – original draft, Visualization, Validation, Investigation, Data curation. Mariano Daniel Aymar: Writing – review & editing, Writing – original draft, Validation, Supervision, Software, Project administration, Investigation, Data curation. Luis Roberto Basbus: Writing – original draft, Validation, Supervision, Methodology, Investigation, Formal analysis, Conceptualization. Milagros Cornec: Writing – original draft, Visualization, Validation, Methodology, Investigation. Sergio Adrián Terrasa: Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Methodology, Investigation, Formal analysis. Maria Lourdes Posadas-Martínez: Writing – original draft, Visualization, Validation, Supervision, Resources, Methodology, Investigation, Formal analysis. Mariana Andrea Burgos: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Methodology, Formal analysis, Data curation. Gabriela Buela: Writing – original draft, Supervision, Methodology, Investigation, Conceptualization. Federico Cayol: Writing – original draft, Visualization, Validation, Supervision, Project administration, Methodology, Investigation, Formal analysis, Conceptualization.
Ethical considerationsThe study was approved by the local Ethics Committee for Research Protocols (Comité de Ética de Protocolos de Investigación, CEPI), approval number 7048.
FundingThe authors did not receive financial support for this research.
Presentation at congressesPresented at the XLIV Post Chicago 2024 Update Meeting, July 4–5, Mendoza, Argentina. Scientific Work – Poster Number 0008 – Effectiveness comparison between 1 g and 250 mg doses of abiraterone in the treatment of metastatic prostate cancer (ABECS).
Presented at the XXXIII National Congress of Medicine, November 12-14, 2024, Buenos Aires, Argentina. Oral Presentation Number 21663 – Institutional experience in the treatment of patients with metastatic prostate cancer with standard and low-doses of abiraterone.
We have attached the required pro forma documents, completed and signed by each author, as per the submission guidelines.
The authors would like to thank the staff of the Oncology section, the Health Insurance Provider of the Hospital Italiano de Buenos Aires, and the Department of Research, Non-Sponsored Area, Hospital Italiano de Buenos Aires, Buenos Aires, Argentina.