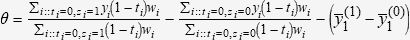Matching-Adjusted Indirect Comparison is a methodology that has been developed to assess new treatments vs alternatives when a direct comparison is not available through a randomized controlled trial. These comparisons are of particular interest in the areas of oncology and hematology where uncertainty in decision-making on the inclusion of new drugs is frequently accentuated by both the severity of the disease and the high cost of treatment. The objective of this study was to describe how Matching-Adjusted Indirect Comparison methodology has been used to date in the assessment of hematological cancer drugs by international agencies.
MethodBetween January 2015 and October 2019, an exhaustive search was conducted of the websites of European National Agencies that provided public information on the assessment process. The assessments provided by these agencies were reviewed to obtain a list of hematological cancer drugs for which the presentation of a Matched-Adjusted Indirect Comparison was recorded. For this list of drugs, the role of the comparison in the assessment process was analyzed for each selected agency.
ResultsThirteen hematological and oncological treatments were found in which the pharmaceutical marketing authorization holder had presented Matching-Adjusted Indirect Comparisons: most of this information referred to the first half of 2018. Acceptance of this methodology diverges among agencies, ranging from 50% in the case of the British National Institute for Health and Clinical Excellence, to 40% in the case of French National Authority for Health, to not having been taken into account in any of the 3 cases assessed by the German Institute for Quality and Efficiency in Health Care. The main cause of non-acceptance was matching-related problems.
ConclusionsMatching- Adjusted Indirect Comparison methodology is a tool that is being utilized in the decision-making process for assessing new hematological cancer treatments.
La comparación indirecta ajustada con emparejamiento es una metodología desarrollada para la evaluación de nuevos tratamientos frente a sus alternativas cuando no se dispone de comparación directa mediante un ensayo clínico aleatorizado y controlado. Estas comparaciones son de especial interés en el área de la hematooncología, en la que la incertidumbre en la toma de decisiones sobre la inclusión de nuevos fármacos se ve frecuentemente acentuada tanto por la gravedad de la enfermedad como por el elevado coste del tratamiento. El objetivo de este artículo es describir cómo la metodología de comparación indirecta ajustada con emparejamiento ha sido empleada hasta la fecha en la evaluación de fármacos hematooncológicos por parte de agencias internacionales.
MétodoPara la obtención de los datos del análisis se ha realizado una búsqueda exhaustiva en las páginas web de las agencias nacionales europeas entre enero de 2015 y octubre de 2019 que mostraran información pública del proceso evaluativo. Se revisaron las evaluaciones de estas agencias para obtener un listado de fármacos oncohematológicos para los que constara la presentación de documentación de una comparación indirecta ajustada con emparejamiento. Para este listado de fármacos se analizó para cada agencia seleccionada el papel que dicha comparación tuvo en la evaluación.
ResultadosSe han encontrado 13 tratamientos para patologías hematooncológicas en las que el laboratorio había presentado comparaciones con metodología de comparación indirecta ajustada con emparejamiento en su documentación, principalmente a partir del primer semestre de 2018. La aceptación de la metodología diverge entre agencias, pasando de un 50% en el caso del Instituto Nacional para la Salud y la Excelencia Clínica británico, a un 40% en el Alto Comisionado de Salud francés, a no haberse tenido en cuenta en ninguno de los tres casos evaluados por el Instituto para la Calidad y Eficiencia en los cuidados de salud alemán. La principal causa de no aceptación fue la existencia de problemas relacionados con el emparejamiento.
ConclusionesLa metodología de comparación indirecta ajustada con emparejamiento es una herramienta de comparación indirecta que está siendo considerada por las agencias analizadas en el proceso de toma de decisiones de evaluación de nuevos medicamentos.
Although randomized and controlled clinical trials are considered to be the most reliable source of evidence to assess the relative efficacy of two treatments, these trials offer limited support to those responsible for the decision-making process. This situation is due to the following factors: a) the lack of data: in general, there are no head-to-head clinical trials of all possible treatments for a given indication; and b) the uncertainty that arises due to the provision of efficacy data rather than effectiveness data, given that it cannot be determined if what is observed under ideal controlled conditions can be reproduced in real clinical practice1–3.
Uncertainty in decision making is particularly evident regarding drugs for the treatment of severe disease and their high cost, as is the case in the settings of oncology and hematology. Such uncertainty is even more accentuated in relation to therapeutic indications for new treatments. Decisions on their inclusion must be made in the absence of comparing them with the standard treatments or with the most clinically relevant alternative treatments.
To help make decisions about the inclusion of new drugs, comparative effectiveness studies are conducted through pragmatic trials or are based on records and real-life data4. These studies are conducted once the new drug becomes available, which entails a lag between the time when the evidence becomes available and the decision-making process that should be based on it.
In this setting, indirect comparisons have been developed to address these limitations. In the absence of comparative clinical trials, indirect comparisons use data from different studies to assess the relative efficacy of two treatment alternatives1,5.
Naive (or unadjusted) indirect comparisons are those which directly compare the arms of each alternative in separate studies as if they were arms of the same study without taking into account the control arms.
In contrast, adjusted indirect comparisons are those which are based on two studies that separately assess the efficacy of 2 treatments vs a common control such that their efficacy can be indirectly compared6.
Indirect comparisons assume that there are clinical and methodological similarities between the studies assessed. Specifically, that the baseline characteristics of the patients, follow-ups, and the outcome variables measured were similar in the studies on which the comparison is based. The choice of different measures of relative effect (e.g. relative risk, odds ratio, or risk difference) can sometimes bias the results and lead to erroneous conclusions on compared effectiveness. These limitations can be avoided if individual patient data (IPD) are available from the clinical trials on which the comparison is based. In these cases, comparisons can be made by using regression methods or matching techniques that employ propensity scores, thus mitigating the biases derived from differences between trial populations6,7.
A common limitation of these approaches is the availability of IPD for all the treatments compared and all the trials of interest. Data privacy issues or commercial interests mean that researchers often have the IPD of one treatment but only the aggregated data obtained from the scientific literature on the other treatments. In recent years, the Matching-Adjusted Indirect Comparison (MAIC)8,9 method has been developed to address these issues. This method can be used to adjust differences in the baseline characteristics of trials when needed. It can also be used to reduce sensitivity to different effect measures and to resolve differences in the definition of study outcome variables as well as differences in the comparison of different doses with clinical relevance used in clinical trials.
The objective this article was to describe how MAIC methodology has been used in the assessment of hematological cancer drugs by several international agencies.
MethodsAssessment of MAIC evidence by international agenciesWe selected national agencies that provided public information on the drug assessment process on their web pages, thus making it possible to collect the data needed for the analysis. With this aim, we searched European agency websites between January 2015 and October 2019.
Once the agencies were selected, from among the drugs assessed we chose the hematological cancer drugs for which the drug company had presented information on a MAIC comparison in their documentation.
Information was collected on whether each of the chosen drugs had been assessed by any of the 3 selected agencies. If this was the case, we recorded the date of the assessment, whether the pharmaceutical manufacturer had presented MAIC information to the agency, whether the MAIC data had been analyzed by the agency, and whether the results of the indirect comparison had been taken into account or discarded during the assessment process.
Description of the MAIC methodologyMAIC methodology is based on using the available IPD from a clinical trial and weighting them such that the mean of their baseline characteristics and standard deviations are consistent with the aggregate data of those reported in the literature for the alternative treatment of interest. Thus, the results of both treatments can be compared between balanced populations. Matching is achieved as follows: patients in trials with IPD are re-weighted by their odds of being potential participants in trials for which we only have published aggregate data8,9.
Comparisons are considered to be anchored if a placebo or a common comparator treatment vs the alternatives assessed is used. They are considered to be unanchored if direct comparisons are made between two treatment arms.
This methodology is only valid if the studies to be included in the indirect comparison have been identified via a systematic review of the literature. Following this step, the baseline characteristics to be weighted are determined. Finally, these balanced averages are used to compare IPD studies to the aggregate data from the studies addressing the alternative treatments.
Regarding sample selection, there should be no protocol differences that could affect the results and that cannot be balanced by matching. Trials with available IPDs should have similar or more inclusive inclusion/exclusion criteria than the aggregate comparison studies. In addition, there should be consistency between the type of results reported and the way they were obtained in the different studies10.
The baseline characteristics can be matched if the data for each baseline characteristic in the aggregate study are available in the study from which the IPD are taken.
The baseline characteristics from trials are matched as follows: individual patients from trials with available IPD are re-weighted to make the means and standard deviations of their baseline characteristics match those obtained from studies with only aggregated data available.
Thus, each trial is characterized with 4 component vectors [X, T, Y, Z], where X is also another vector describing baseline characteristics (e.g. age, sex, ethnicity, or previous treatments), T is the treatment received (T = 0, treatment with IPD; T = 1, treatment with aggregated data); Y is the result of interest which can be binary (e.g. response to treatment: no = 0, yes = 1) or continuous (e.g. progression-free survival or overall survival); and Z is the placebo arm (Z = 0) or the treatment arm (Z = 1)10.
The IPD study will have a vector (xi, ti, yi, zi) for each individual patient, whereas the vectors corresponding to the patients in the aggregate data study will be (x¯, ti,y¯, zi) because all these patients will be described by the mean of their baseline characteristics (x¯) and mean results (y¯).
Differences in the treatment effect (T = 0 vs T = 1) can be estimated from the weight wi, which is obtained as the odds of patients in the IPD trial participating in the aggregate study vs the odds of them participating in the IPD study. These estimated weights (wi) are used to ensure that the mean baseline characteristics are balanced between the two trials.
Thus, the following formula is used to calculate differences between treatments:
Where the first term represents the effect on patients receiving treatment in the IPD study, the second term represents the effect on patients receiving placebo in this study, and the third term represents the difference between the average effects of the treatment and placebo arms in the aggregate data study.
This estimator is effective if the baseline characteristics vector contains all the confounding factors. In this sense, the inclusion of the placebo arms is useful in that missing confounders can be taken into account10.
The main limitations of MAIC methodology include its inability to adjust for differences regarding treatments (e.g. dosage, administration, or co-medications) between trials and the impossibility of ensuring that all prognostic and effect-modifying variables are known or available. Thus, adjusting the population in an indirect unanchored comparison requires that the absolute results can be reliably predicted such that the degree of bias due to the imbalance from potential covariates that have not been controlled is acceptable. Any estimates or conclusions obtained from unanchored comparisons may be strongly challenged because the magnitude of the bias in these estimates is unknown, likely to be substantial, and may even exceed the magnitude of the estimated treatment effects.
ResultsAssessment of MAIC evidence by international agenciesThe websites of the assessment agencies were reviewed and selected for analysis based on their fulfilling the inclusion and exclusion criteria. Three agencies were selected: The British National Institute for Health and Care Excellence (NICE), the French National Authority for Health (HAS), and the German Institute for Quality and Efficiency in Health Care (IQWIG).
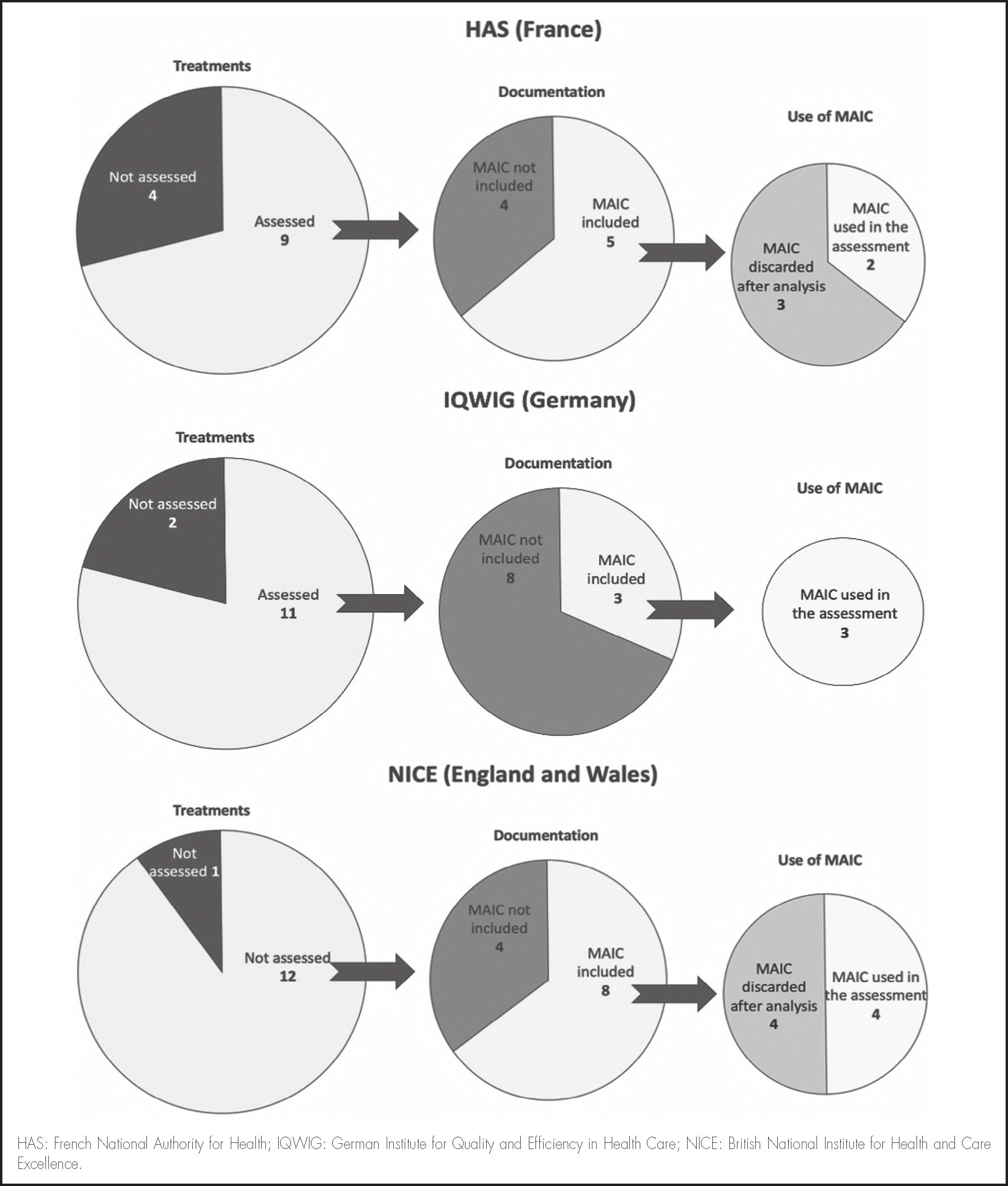
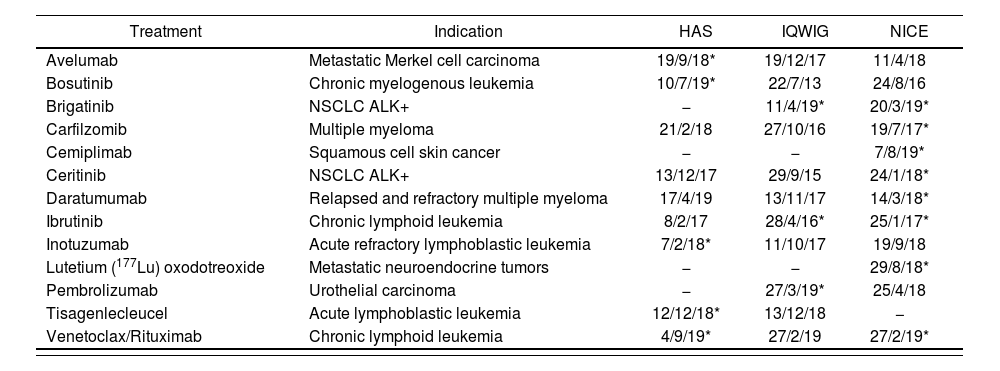
In total, 13 treatments for hematological cancer disease were found on the websites of the three agencies. All pharmaceutical manufacturers had included information on a MAIC comparison in their documentation. Table 1 shows these treatments, the agency which presented the documentation, and the date of the assessment.
Treatments assessed with MAIC documentation
| Treatment | Indication | HAS | IQWIG | NICE |
|---|---|---|---|---|
| Avelumab | Metastatic Merkel cell carcinoma | 19/9/18* | 19/12/17 | 11/4/18 |
| Bosutinib | Chronic myelogenous leukemia | 10/7/19* | 22/7/13 | 24/8/16 |
| Brigatinib | NSCLC ALK+ | − | 11/4/19* | 20/3/19* |
| Carfilzomib | Multiple myeloma | 21/2/18 | 27/10/16 | 19/7/17* |
| Cemiplimab | Squamous cell skin cancer | − | − | 7/8/19* |
| Ceritinib | NSCLC ALK+ | 13/12/17 | 29/9/15 | 24/1/18* |
| Daratumumab | Relapsed and refractory multiple myeloma | 17/4/19 | 13/11/17 | 14/3/18* |
| Ibrutinib | Chronic lymphoid leukemia | 8/2/17 | 28/4/16* | 25/1/17* |
| Inotuzumab | Acute refractory lymphoblastic leukemia | 7/2/18* | 11/10/17 | 19/9/18 |
| Lutetium (177Lu) oxodotreoxide | Metastatic neuroendocrine tumors | − | − | 29/8/18* |
| Pembrolizumab | Urothelial carcinoma | − | 27/3/19* | 25/4/18 |
| Tisagenlecleucel | Acute lymphoblastic leukemia | 12/12/18* | 13/12/18 | − |
| Venetoclax/Rituximab | Chronic lymphoid leukemia | 4/9/19* | 27/2/19 | 27/2/19* |
HAS: French National Authority for Health; IQWIG: German Institute for Quality and Efficiency in Health Care; NICE: British National Institute for Health and Care Excellence; NSCLC ALK+: Non Small-Cells Lung Cancer.
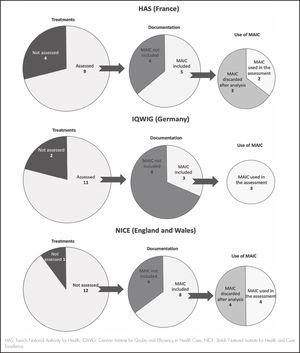
The HAS only received a MAIC for five treatments in their documentation: of these, only two were considered for the final assessment. The IQWIG only received a MAIC for three treatments, but none of these were used. Finally, the NICE received a MAIC for eight treatments, half of which were used in the assessment. Of these, 50% of those assessed by the NICE and 40% of those assessed by the HAS were considered by the agencies during their decision-making process on their inclusion and therapeutic positioning in the treatment sequence. None of the three MAIC comparisons received by the IQWIG were taken into account during decision making.
Figure 1 shows the assessment by the HAS, IQWIG, and NICE of these treatments with MAICs.
All the MAICs included as documentation for the assessment processes were conducted without anchoring. The only exceptions were the MAICs included with bosutinib for chronic myeloid leukemia and inotuzumab for refractory acute lymphoblastic leukemia, both of which were received by the HAS. Although inotuzumab was taken into account in the assessment, bosutinib was not because the comparator was considered inappropriate.
Of the 16 assessments including MAICs, only three drugs were assessed by more than one agency. Of these three drugs, brigatinib and ibrutinib were assessed by the NICE and IQWIG, whereas venetoclax was assessed by the NICE and HAS. The remaining 10 assessments that included MAICs were assessed by only one agency; the HAS assessed avelumab, bosutinib, inotuzumab, and tisagenlecleucel; the IQWIG assessed pembrolizumab; and the NICE assessed carfilzomib, cemiplimab, ceritinib, daratu-mab, and lutetium (177Lu) oxodotreoxide.
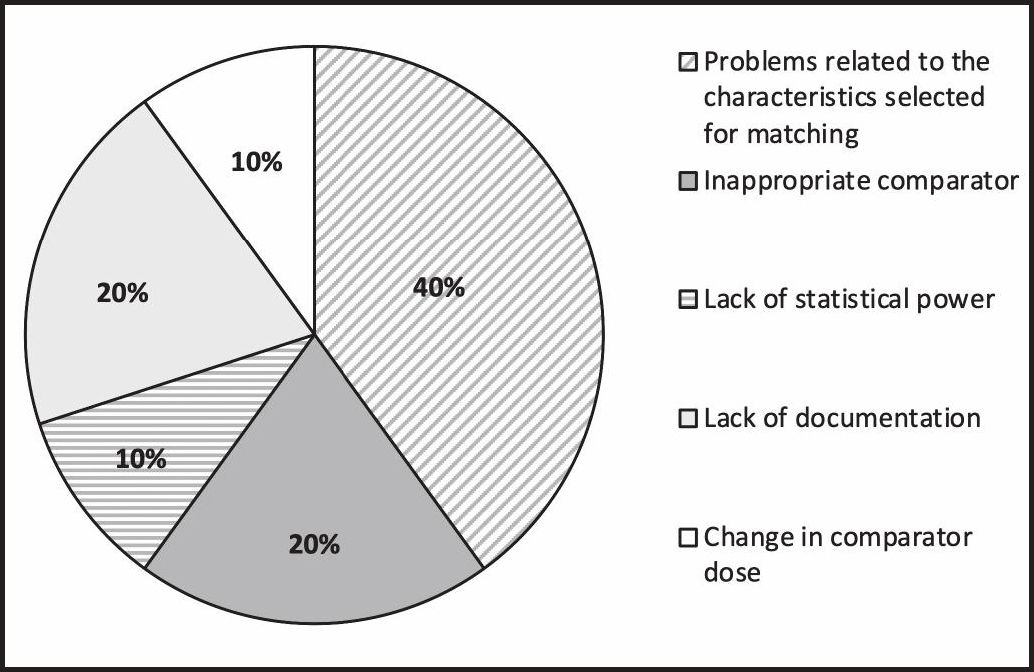
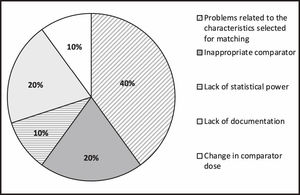
Of these drugs, avelumab (HAS), carfilzomib (NICE), and daratumumab (NICE) were not considered for assessment because of problems related to the characteristics selected for matching. In the case of pembrolizumab (IQWIG), the MAIC was not considered in the assessment because it did not have sufficient statistical power to determine if the observed differences were caused by bias. In the cases of bosutinib (HAS) and cemiplimab (NICE), the MAICs were not considered in the assessment because they used a comparator that was considered inappropriate for the assessment (i.e. the indication of the comparator drug was not approved in the country at the time of the assessment).
Of the treatments assessed by more than one agency, the NICE and the IQWIG made the same decision to not to use MAIC in their assessment of ibritinib because in both cases the pharmaceutical manufacturer did not include the data corresponding to the comparison made.
In the cases of brigatinib (NICE and IQWIG) and venetoclax (NICE and HAS), indirect comparisons were taken into account by the NICE, but not by the other agencies. The IQWIG assessment of brigatinib did not take the MAIC into account because of a decrease in the comparator dose (ceritinib) at the time of assessment in Germany. The HAS raised questions about the selection of the characteristics used for matching during the assessment of venetoclax.
Figure 2 shows the reasons for excluding the studies that were excluded discarded.
DiscussionMAIC methodology began to be used in different therapeutic settings following the publication of the first study applying MAIC methodology to psoriasis treatment in 201010. The first study applying MAIC in the setting of hematological cancer compared nilotinib and dasatinib as first-line treatment for chronic myeloid leukemia in 201111.
Since then, MAIC methodology has slowly become established. It was only in April 2016 that it was accepted as evidence in assessment processes, when it was presented as part of the documentation of ibrutinib in the treatment of chronic myeloid leukemia.
The results of the present study show that there has been an increasing trend in the use of MAICs in the assessment of new treatments.
However, only the NICE (2016) has published a technical support document on this type of assessment9. The IQWIG12 and HAS13 have not included MAICs as an option in their methodological documents. This aspect may be the reason for differences found between the NICE and the HAS regarding the inclusion of MAIC in their assessment of brigatinib and venetoclax.
Assessments conducted in Spain were not analyzed because in Spain this process is conducted using the therapeutic positioning report, which does not provide information on the assessment process but only reports the results of the final assessment. Thus, comparisons are only mentioned in the report if they were taken into account, but the report does not specify if the pharmaceutical manufacturer provided MAIC documentation or if the documentation provided was assessed in any way. Although other assessments such as the GENESIS reports produced by the Spanish Society of Hospital Pharmacy are more transparent in this sense, they were not taken into account as this body is not a national agency tasked with such assessments.
The increasing trend in the number of assessments using MAICs has not occurred in Spain. In the setting of hematological cancer, only one published therapeutic positioning report (August 2018) has used this methodology for positioning carfilzomib in the treatment of multiple myeloma14.
Unanchored indirect comparisons should only be considered for use where there is a disconnected treatment network or single-arm studies. Despite this, the results show that only two of the MAICs included in the assessments analyzed were anchored, which was mainly due to the use of single-arm studies. In no case was the absence of anchorage a reason for not considering the analysis.
Network-meta-regression and network-meta-analysis6,7,15,16 require a connected network of evidence and the fulfillment of the assumptions of transitivity (i.e. if drug B is superior to a drug A and drug A is superior to a drug C, drug B will also be superior to C) and consistency (i.e. the results of indirect evidence are congruent with direct evidence). Thus, they cannot be used in cases of unanchored indirect comparisons.
In these cases, other tools can be used in the decision-making process, such as multi-criteria decision analysis17–19, whose use has been proposed in the field of oncology20,21. However, this methodology has limitations, such as subjectivity, confusion between value items, or a lack of transparency that affects its external validity22. A recent systematic review showed that this methodology was only used on five occasions for a comparative risk-benefit assessment and none of them included treatments in the field of hematological cancer23.
In this sense, the 13 reports that included MAIC methodology in hematological cancer treatments versus no reports found with multi-criteria decision analysis suggest that MAIC has become more accepted for the comparative assessment of two treatment alternatives.
The main limitation of this study is that it is restricted to the 3 European agencies that, in a transparent manner, provide information on the assessment process of the different treatments. Future studies would benefit from having information available on the process of developing Spanish therapeutic positioning reports and the inclusion in these processes of documentation on these types of studies.
ConclusionTools that allow indirect comparisons are needed, given the difficulty of obtaining direct evidence on hematological cancer alternatives due to the high number of treatments developed.
MAIC methodology is used for indirect comparisons by the international agencies analyzed when IPD are available from 1 alternative and grouped data from another.
FundingThis study was funded by Takeda España, S.A.
Conflict of interestJM Martínez-Sesmero and J De Castro-Carpeño declare no conflicts of interest. J. Parrondo has received consultancy fees from Biogen, BMS, GSK, Lilly, Novartis, Pfizer, PierrreFabre, Roche, Servier, Takeda, and Vii-VHealthcare. A López-de las Heras and A Fernández-Nistal are employed by Takeda.