The role of a Medication Safety Officer has emerged as a critical element in hospital pharmacy, addressing the persistent issue of medication errors. These errors, which can cause significant patient harm, have been documented for decades, prompting the establishment of formal roles dedicated to medication safety. Organizations such as the Institute for Safe Medication Practices (ISMP), the American Society of Health System Pharmacists (ASHP) as well as the United Kingdom's Medicines and Healthcare products Regulatory Agency (MHRA) and National Health Service (NHS) have been instrumental in supporting the Medication Safety Officer role.
Medication errors can result in severe consequences, including patient harm and death. Landmark publications like the Institute of Medicine's “To Err is Human” and “Crossing the Quality Chasm” have highlighted the prevalence and impact of these errors, advocating for system improvements and the necessity of dedicated safety roles.
Medication Safety Officers lead strategies and processes related to medication safety, develop strategic plans, and implement error prevention strategies. They analyze medication error reports, collaborate with healthcare staff, and optimize medication safety technologies. Medication Safety Officers play a key role in fostering a culture of safety within organizations, influencing practices to minimize harm and support second victim programs.
Studies have shown that employing a Medication Safety Officer can significantly improve hospital safety scores, demonstrating the effectiveness of this role in enhancing patient safety. The daily responsibilities of a Medication Safety Officer include reviewing medication errors, assessing harm, attending meetings, and collaborating with healthcare practitioners.
Overall, the role of a Medication Safety Officer is essential in identifying and mitigating medication risks, making hospitals safer, and ensuring the delivery of high-quality patient care.
La figura del responsable de seguridad de los medicamentos ha surgido como un elemento clave en la farmacia hospitalaria, para abordar el problema persistente de los errores de medicación. Estos errores, que pueden causar daños significativos a los pacientes, han sido documentados durante décadas, lo que ha llevado a la creación de profesionales especializados dedicados a la seguridad de los medicamentos. Organizaciones como el Institute for Safe Medication Practices (ISMP), la American Society of Health-System Pharmacists (ASHP), así como la Medicines and Healthcare products Regulatory Agency (MHRA) y el National Health Service (NHS) del Reino Unido, han desempeñado un papel fundamental en el apoyo a esta figura profesional.
Los errores de medicación pueden tener consecuencias graves, incluyendo daño e incluso la muerte del paciente. Publicaciones clave como To Err is Human y Crossing the Quality Chasm, del Institute of Medicine, han puesto de manifiesto la prevalencia e impacto de estos errores, y han abogado por mejoras en los sistemas y la necesidad de contar con profesionales dedicados a la seguridad.
Los responsables de seguridad de los medicamentos lideran estrategias y procesos relacionados con la seguridad en el uso de medicamentos, desarrollan planes estratégicos e implementan medidas para prevenir errores. Analizan notificaciones de errores de medicación, colaboran con los profesionales sanitarios y optimizan el uso de tecnologías que mejoran la seguridad de la medicación. Desempeñan una función clave en el fomento de una cultura de seguridad dentro de las organizaciones, promoviendo prácticas seguras para minimizar el daño y apoyando programas de atención a las segundas víctimas.
Diversos estudios han demostrado que contar con un responsable de seguridad de los medicamentos puede mejorar significativamente los indicadores de seguridad hospitalaria, lo que evidencia la eficacia de este puesto en la mejora de la seguridad del paciente. Sus responsabilidades diarias incluyen la revisión de errores de medicación, la evaluación de los daños asociados, la participación en reuniones y la colaboración con otros profesionales sanitarios.
En conjunto, la función del responsable de seguridad de los medicamentos es esencial para identificar y mitigar riesgos asociados a los medicamentos, hacer los hospitales más seguros y garantizar una atención sanitaria de alta calidad.
There is a Latin phrase, ‘primum non nocere’ which means ‘first, above all, do no harm.’ It is a phrase familiar to those in medical professions and is recognized as a principle for practice. It is a reminder when delivering care, we first want to ensure we do not cause harm to our patients. However, sometimes despite our best efforts in providing care, things do not always go as planned, and unfortunately, patients may suffer harm as a result. Medications can be life-changing and lifesaving when they are administered safely and appropriately to patients. Nevertheless, healthcare practitioners are human and therefore prone to error. Despite our expertise, knowledge and unwavering commitment to quality care, adverse medication can occur and may occasionally result in harm, suffering, and in rare instances, death.1
Medication errors have existed since the inception of medications, even if they were not always recognized. In the 1970s and 1980s, we began to see medication errors reported in newspapers and medical journals. In 1975, a critical incident was published where a patient received 40 units instead of 4 units of insulin due to a misinterpretation of the abbreviation “U” for units as a zero. This led to a regular column on documented medication errors in the US journal Hospital Pharmacy.2
In 1991, the Greensboro News and Record reported that a 5-year-old boy died after receiving vincristine instead of vinblastine.3 In 1997, we learned of the tragic death of a Canadian child who was accidentally injected intrathecally with vincristine.4 In 1998, the Journal of Hospital Pharmacy documented an overdose of penicillin G benzathine due to incorrect intravenous administration instead of the intended intramuscular injection.5 These are just a few examples of documented errors with harmful outcomes that prompted the initiation of conversations about medication errors.
In 2000, the Institute of Medicine (now the National Academy of Medicine [IOM/NAM]), published To Err is Human. This was the first publication of its type, which informed the public that humans make errors.6 In 2001, the IOM/NAM published a follow-up report, Crossing the Quality Chasm which informed the public that contributing factors are often related to system issues and not always human error. This represented a shift from the previous belief that human error was the primary cause of mistakes.7 In 2007, the IOM/NAM published Preventing Medication Errors which informed the public that medication errors cause harm. In this report, medication errors were estimated to account for over 7000 deaths annually and one medication error per day per patient.8 Recent publications indicate that medical errors, including medication errors, are the third leading cause of death in the United States.9
These landmark publications demonstrated to the healthcare community and the public the importance of identifying and discussing medication errors and associated error-reduction strategies. Since then, discussions about medication errors have become more common and open, leading to the establishment of the role of the Medication Safety Officer (MSO).
During the 1980s, patient safety emerged as a significant concern in the United States. The Institute for Safe Medication Practices (ISMP) emphasized the necessity of establishing formal roles dedicated to addressing medication safety issues. As a result, several hospitals instituted positions specifically aimed at enhancing medication safety.
Over the years, several articles have published varying statistics on the impact of medication errors. Despite the variability, medication errors are known to cause harm. According to the WHO, medication errors are the number one cause of harm globally. However, there is significant harm for one in 30 patients. Greater than 25% of medication errors are considered severe or life-threatening. In the United Kingdom, it is estimated that there are 237 million errors per year, costing 98.5 million pounds, 712 deaths, and over 181,000 lost bed days.10,11
Specific populations show varying error rates, for example, there are 4 errors per 1000 chemotherapy orders, pediatric patients are at a threefold risk for a medication error, and medication errors affect 4–14% of emergency department patients, with the pediatric population reaching as high as 39%. These data highlight the significance of medication errors, underscore the critical need for a practitioner dedicated to medication safety, and support the role of a MSO.12–14
Nowadays, the role of a MSO is common in the United States (US) and the United Kingdom (UK) but has had less uptake in other countries. It is up to each organization in the US to create and define an MSO role; however, in the UK, the MSO position has been required at the Trust level since 2014.15
The case for Medication Safety OfficersSafety officers are prevalent in numerous industries, including manufacturing, pharmaceuticals, construction, transportation, aviation, and healthcare. The Institute for Safe Medication Practices (ISMP) was among the first organizations to endorse the establishment of the MSO role. It is important to note that other healthcare professions also have similar positions aimed at minimizing harm. For instance, infection preventionists have demonstrated efficacy in reducing infections, and antimicrobial stewards have proven successful in mitigating drug resistance. Although the task of ensuring medication safety involves multiple stakeholders, the coordination and strategic alignment of these efforts are most effective when directed by a single individual, namely, the MSO.16
Data have been collected indicating that an MSO can positively affect hospital safety scores in the United States. One study found that employing an MSO for at least 20 h per week resulted in a hospital's safety score improving from 12% to 40%, which is a 233% increase.17 In 2018, data indicated that 48% of US hospitals (n = 807) that responded to a self-assessment survey had created a MSO position to address ongoing medication safety challenges.18
Key characteristics of a Medication Safety OfficerAn MSO should lead the strategy, vision, and processes related to medication safety. They must develop strategic plans tailored to specific care areas within the organization. The MSO is recognized as an expert in medication safety practices, proactively identifying and addressing opportunities for improvement within the medication-use process. They play a critical role in facilitating and implementing error prevention strategies.16,19
Furthermore, an MSO regularly analyzes medication error reports and collaborates with pharmacy, nursing, and medical staff leaders to optimize the use of medication safety technologies such as automated dispensing machines, clinical decision support, and smart infusion pumps. In addition to these tangible tasks, the MSO is expected to significantly influence the organization's safety culture. They foster and build a culture of safety, where the organization's shared perceptions, beliefs, and values create a commitment to safety with the goal of minimizing harm. The MSO is a key influencer and drives implementing changes in practice. They establish systems to prevent avoidable medication-related harm. Equally important is their role in developing and supporting second-victim programs for those affected by errors or traumatic events.16,19
The MSO is an accountable expert on medication safety with in-depth clinical knowledge and experience. They understand the medication-use process, have strong analytic skills to identify trends, and are experienced in Failure Modes Effects Analysis (FMEA), Root Cause Analysis (RCA), Causal Analysis (CA), and process mapping. They possess leadership skills to guide staff and leaders and clear communication abilities. An MSO addresses medication errors system-wide, commits to fostering a Just Culture, and promotes transparency and sharing lessons learned. MSOs can link improvement efforts to the mission, patient safety, and care performance goals through advocacy and collaborative behavior.16,19
Responsibilities and role of the Medication Safety OfficerAn MSO can be a pharmacist or another healthcare practitioner. Pharmacists have the knowledge to manage safety throughout the medication-use process, from prescribing to monitoring medications, and understand the operational needs for delivering medications to patients. An MSO most often reports either to the pharmacy department or the quality department.
In the United States, many MSOs are positioned within the pharmacy department. They collaborate with nursing staff, physicians, and quality leadership to review data and implement systemic improvements. Additionally, the MSO engages with risk management professionals and those responsible for accreditation compliance. Being part of the pharmacy department enables the MSO to effectively manage the Medication Safety Committee, which is typically a subcommittee of the Pharmacy and Therapeutics Committee (P&T). In some organizations, the MSO is situated within the quality department, thereby bringing medication safety concerns closer to executive leadership. This role is often initially established within the pharmacy department.
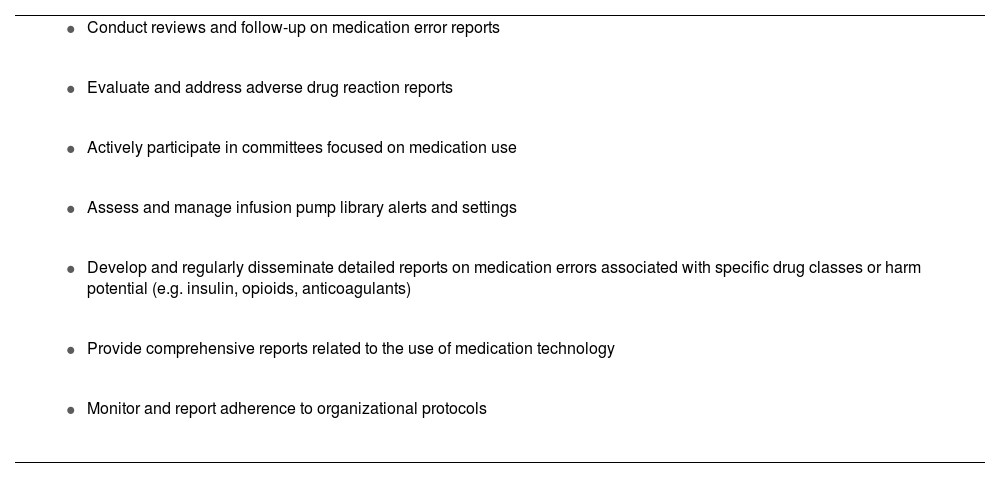
The MSO promotes medication safety throughout the organization. They actively participate in committees focused on medication use, collaborate with various healthcare disciplines, and engage with executive leadership. The MSO guides actions and decisions to enhance safety systems and provides education to staff (Table 1).
Common MSO responsibilities.
|
|
|
|
|
|
|
The MSO is responsible for monitoring and managing data related to medication errors, including assessing patient harm. They lead initiatives to identify the best practices and strategies aimed at reducing preventable harm resulting from such errors. Furthermore, they remain updated on regulatory and accreditation requirements pertaining to medication use.
To ensure system improvements, the MSO aligns efforts to bolster patient safety and quality strategic plans at both organizational and unit/ward levels. This involves integrating improvement principles, safety methods, and effective tactics to mitigate medication errors.
The MSO also supports a robust error reporting culture where staff are encouraged to report errors and near misses/close calls. They advocate for the understanding that transparent communication of errors and hazards can lead to system enhancements and a reduction in errors and harm.
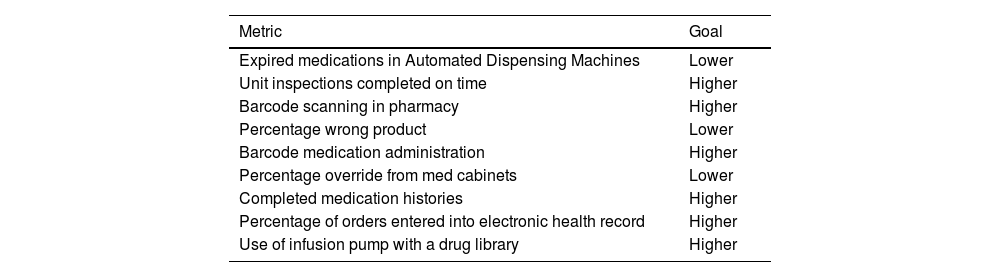
The MSO is responsible for tracking event reporting as it pertains to the culture of safety. In addition to this, they also monitor other data such as serious safety incidents, adherence to routine safety checks, data from medication use technologies, and specific organizational targets. Specific metrics that can be tracked include the utilization of smart infusion pump libraries, compliance with medication use protocols, application of barcode verification prior to medication administration or during medication preparation, and the safe use of drugs stored in automated dispensing machines (Table 2).20
Medication safety metrics.20
| Metric | Goal |
|---|---|
| Expired medications in Automated Dispensing Machines | Lower |
| Unit inspections completed on time | Higher |
| Barcode scanning in pharmacy | Higher |
| Percentage wrong product | Lower |
| Barcode medication administration | Higher |
| Percentage override from med cabinets | Lower |
| Completed medication histories | Higher |
| Percentage of orders entered into electronic health record | Higher |
| Use of infusion pump with a drug library | Higher |
Although medication errors cannot be benchmarked between institutions, an institution can conduct internal comparisons. Calculating the internal harm rate enables a hospital to monitor the impact on patients. This is achieved by determining the number of harm events relative to patient days or doses dispensed, standardized per 1000 units. By understanding the internal rate of harm, hospitals and MSOs can implement measures to mitigate and prevent patient harm.
This dashboard allows quick data analysis on the high-level items to focus system fixes on.
Institutions can create an interactive dashboard to filter data by care location, drug, or high-alert medication to identify actions to prevent harm. This allows quick data analysis of key items for focused system fixes. Reports can also focus on specific medication classes like insulin, opioids, or anticoagulants. However, dashboards may not always be effective; for example, a near miss with IV vincristine mistaken for an intrathecal medication should prompt a root cause analysis and system review. For targeted focus, MSOs should consider monitoring compliance with the IMSN Global Targeted Medication Safety Best Practices or the ISMP Targeted Medication Safety Best Practices for Hospitals or Community Pharmacies.21–23
A typical day in the life of an MSOThe daily responsibilities of a MSO typically encompass a range of tasks. These include reviewing medication errors, assessing harm and contributing factors, and following up with staff to identify events requiring further evaluation. MSOs attend meetings to offer insights on medication safety and engage in broader initiatives such as data collection, literature reviews, and preparing presentations. Additionally, analyzing trends from error reports related to specific drugs or issues forms an integral part of their role. Collaboration with nurses, physicians, technicians, and other healthcare practitioners is essential to ensure a comprehensive approach.
ConclusionMedication errors continue to occur across all areas of healthcare, with preventable harm from these errors being extensively documented. The role of a MSO is well-established in both the United States and the United Kingdom and is gradually being adopted by other countries. Given the complexity of healthcare systems, it is crucial to have a dedicated individual focused on identifying and mitigating risks before they result in medication errors. MSOs have proven to be an effective strategy for convening leaders to pinpoint system improvements that enhance patient safety and contribute to making hospitals safer.
Declaration competing interestThe authors declare to have no conflict of interest in relation to this work.
CRediT authorship contribution statementElizabeth Hess Ford: Conceptualization, Writing – original draft, Writing – review & editing. Christina Michalek: Conceptualization, Writing – review & editing.
FundingNone declared.
This article has not been submitted to another journal or conference previously or simultaneously.