To establish a series of recommendations based on available evidence for monitoring surface contamination in the areas devoted to compounding hazardous drugs in pharmacy departments.
MethodBased on a literature search in the Medline and Embase databases (search period: January 2009 to July 2019), as well as on a review of standards and recommendations issued by different healthcare organines a committee of experts from the Spanish Society of Hospital Pharmacists defined a series of safe practices for handling hazardous drugs and monitoring compounding work surfaces. Recommendation decisions were adopted by consensus among the members of the expert group, considering the recommendations reviewed, the monitoring situation in Spanish hospital departments, and the associated costs.
ResultsTen recommendations were formulated, structured into eight sections. They include aspects related to the drugs to be monitored; the areas to be monitored; when samples should be taken; risk determination and preparation of a sampling protocol; analytical techniques; contamination thresholds; and design of an action plan based on the sampling and decontamination results obtained.
ConclusionsSurface monitoring allows hazardous drugs detection and evaluation of the effectiveness of current protocols for the safe handling of such drugs in hospital pharmacy departments. The evaluation should include an analysis of the efficacy of engineering controls, work practices and cleaning and decontamination processes.
Establecer unas recomendaciones, en base a la evidencia disponible, para la monitorización de la contaminación de superficies en las áreas de elaboración de medicamentos peligrosos de los Servicios de Farmacia.
MétodoA partir de una revisión bibliográfica en las bases de datos Medline y Embase desde enero de 2009 a julio de 2019, así como de la consulta de documentos de estándares y recomendaciones de organizaciozations, sanitarias, un comité de expertos de la Sociedad Española de Farmacia Hospitalaria ha definido una serie de prácticas seguras sobre manipulación de medicamentos peligrosos y monitorización de superficies de trabajo. Las decisiones de recomendación se tomaron por consenso entre el grupo de expertos teniendo en cuenta las recomendaciones encontradas, la situación en nuestro entorno y los costes asociados a la monitorización.
ResultadosSe han definido 10 recomendaciones estructuradas en ocho secciones. Se incluyen aspectos relacionados con los medicamentos a monitorizar; localizaciones a monitorizar; momento de la toma de muestras; determinación del riesgo y plan de muestreo; técnicas analíticas; umbrales de contaminación; plan de acción según los resultados del muestreo y descontaminación.
ConclusionesLa monitorización de superficies permite determinar la presencia de medicamentos peligrosos y evaluar la eficacia del programa de manejo seguro de los mismos en los Servicios de Farmacia. La evaluación debería incluir un estudio de la eficacia de los controles de ingeniería, de las prácticas laborales y de los procesos de limpieza y descontaminación.
Occupational exposure to hazardous drugs (HDs) is a matter of utmost concern given the potential risks posed to the health of workers.
The first publications on work surface monitoring date back to the 1990’s in the United States1,2, Canada2, and the Netherlands3–6. Since then, multiple articles have shown that surface contamination is present in the healthcare setting and that occupational exposure to HDs can result in acute and chronic adverse events such as skin rashes, reproductive problems, and chromosomal aberrations7–11. Although no cause-effect relationship has been certainly demonstrated between occupational exposure to HDs and the appearance of adverse events, it is generally believed that contamination levels should be kept as low as reasonably achievable (ALARA)12. Organizations such as the National Institute for Occupational Safety and Health (NIOSH) have issued recommendations regarding the safe handling of HDs and the regular updating of HDs lists so as to prevent occupational exposure13.
Professional organizations and governmental agencies have developed guidelines for the management of HDs, which include recommendations regarding surface monitoring12,14–19. Chapter <800> of the United States Pharmacopeia (USP), “Hazardous Drugs-Handling in Healthcare Settings”, recommends that such monitoring should be performed routinely, initially as a benchmark, and at least every 6 months, or more often if needed, there-after12. The recommendation has been applied for the last two decades in several hospitals2,20–27.
Studies have been published on the benefits of environmental monitoring28, and surface contamination has been evaluated in 338 hospital pharmacies29. The conclusion has been that continuous monitoring is beneficial to identify and correct suboptimal practices so as to prevent future exposure.
Nonetheless, the methodology and analytical tools employed vary substantially across studies30, and no risk exposure thresholds have been established (except for the USP standard, which suggested a threshold of exposure to cyclophosphamide of less than 1 ng/cm2, higher levels of exposure leading to a potential increase in the absorption risk)31. NIOSH, the Occupational Safety and Health Administration (OSHA) and the Conference of Governmental Industrial Hygienists (ACGIH) have all refrained from establishing maximum allowable contamination levels.
The purpose of this document is to review the available evidence and establish a set of recommendations for correctly surface contamination monitoring in the areas devoted to HDs compounding in hospital pharmacy departments (HPDs).
This document is an official position statement of the Spanish Society of Hospital Pharmacists (SEFH) and deals specifically with the surface monitoring in the areas devoted to HDs compounding in HPDs.
MethodsA working group was created whose members were selected by SEFH based on their experience, their publications on HDs and surface monitoring, and their membership to oncology, pharmaceutical compounding, and medical devices working groups.
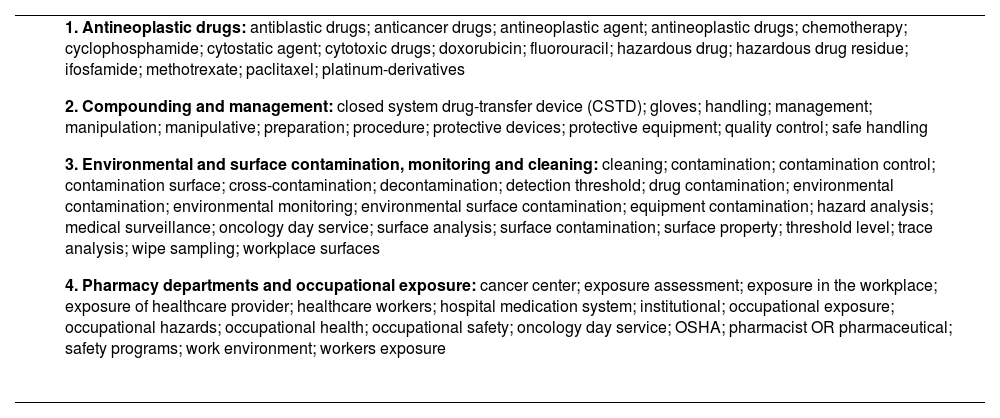
A literature searching was undertaken using the Medline and Embase data bases (search period: 1 January 2009 to 25 July 2019). Table 1 shows the MeSH search terms used, which were selected and agreed on by the co-authors. A total of 527 references were identified, which were manually supplemented by secondary references from the initially selected articles and documents containing standards and recommendations issued by healthcare organizations. A specific search was conducted of the legal and regulatory framework applicable to HPDs.
MeSH terms used in the literature search
|
OSHA: occupational safety and health administration.
Publications were reviewed by the authors to identify and compile safe hazardous drug handling and work surface monitoring practices. Their expected benefits as well as the feasibility of incorporating them to Spanish working environments were considered.
The different recommendations identified were discussed and analyzed by the working group members. Recommendation decisions were made by consensus among the members of the expert group on the basis of the literature review, considering the characteristics of the Spanish working environment and the costs associated to monitoring procedures.
Members worked online and had one face-to-face meeting. Once concluded, the document was submitted to SEFH's working groups for approval.
Regulatory frameworkThe risk derived from exposure to HDs has been recognized by the European Union, which has urged adoption of measures based on facilities engineering, closed-system drug transfer devices, personal protective equipment, and proper medical surveillance of workers32. In 2016, the Spanish Institute for Health and Safety at Work published a series of recommendations on the preventive measures required for compounding and administering hazardous drugs33, and developed the INFOMEP data base to record all HDs marketed in Spain34.
Royal Decree 374/200135 on the protection of workers’ health and safety against the risks posed by chemicals in work environments, which is applicable to HDs, establishes the measures to be undertaken to identify hazardous substances and mitigate their risks using safety data sheets (SDS) as a key instrument. However, there is no provision for compulsory risk mitigation in the case of HDs.
Protection of workers from exposure to carcinogenic agents is regulated by Royal Decree 665/199736, which rules that carcinogenic and/or mutagenic substances must be avoided. If this is unfeasible, the Royal Decree requires that production and use of such substances take place in a closed-system device. If that is not possible, the lowest technically possible exposure level should be ensured. The list of substances does not include drugs.
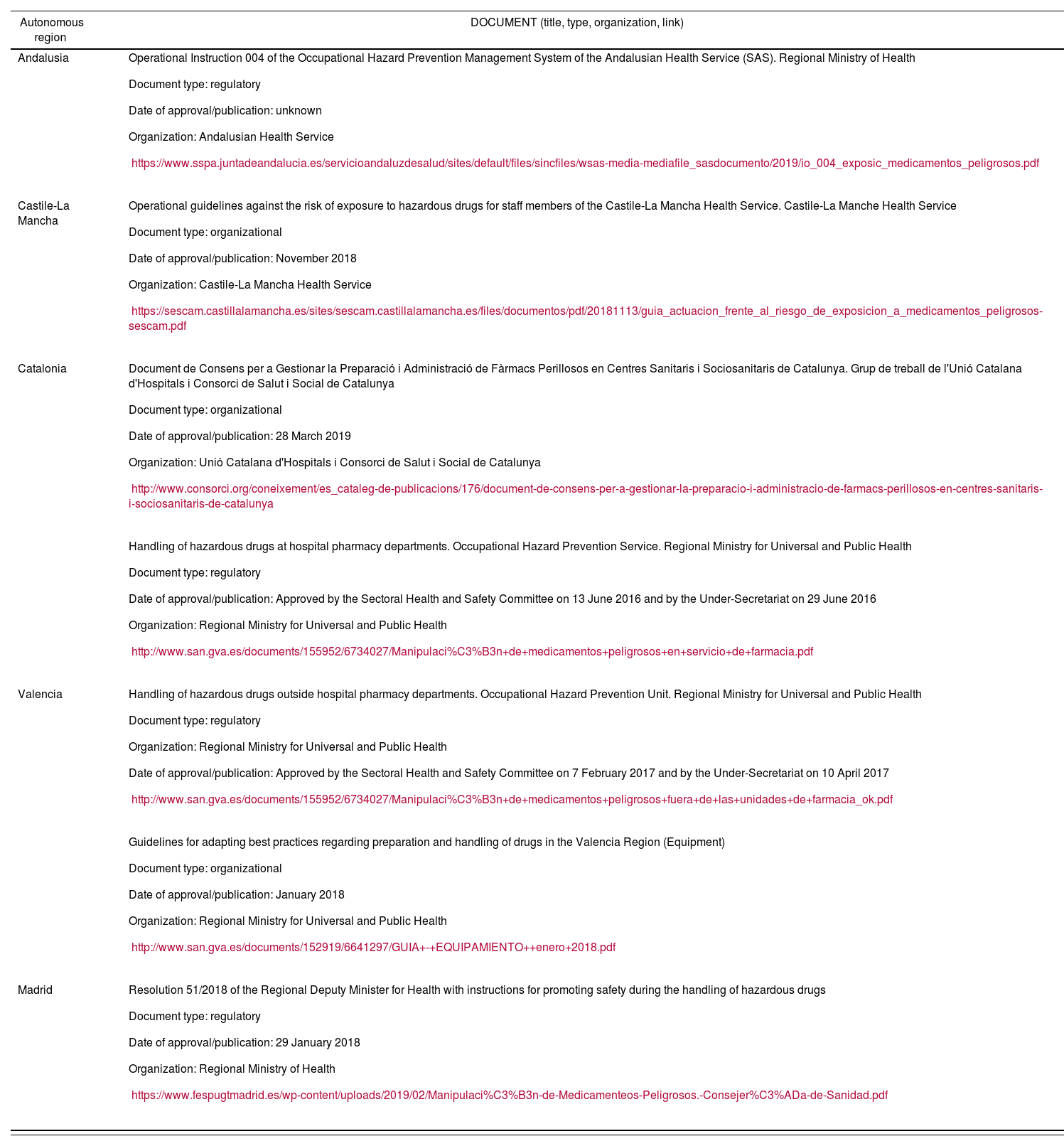
Different regions of Spain have undertaken initiatives in this area, publishing different types of regulations, which are presented in table 2.
Regulations in the different autonomous regions
| Autonomous region | DOCUMENT (title, type, organization, link) |
|---|---|
| Andalusia |
|
| Castile-La Mancha |
|
| Catalonia |
|
| |
| Valencia |
|
| |
| Madrid |
|
According to Act 31/1995, hospital pharmacists are responsible for ensuring implementation of the risk prevention protocol developed by their HPD with respect to the handling of HDs37. This Act establishes that every facility must ensure an optimal working environment, specifying that “the appropriate safety measures must be ensured by both employers and any senior staff reporting to them who may have authority over matters related to health and safety”. As regards the role of pharmacists and their responsibility in the realm of health and safety, Royal Decree 824/201038 states that the “technical director” (meaning the person in charge of the HPD) must ensure that “the staff receive initial and continuous training” and that “health and safety programs must be introduced and adapted to the activities to be carried out. Such programs must include procedures related to health, safety and protective equipment to be worn by the staff”.
RecommendationsDrugs to be monitoredMonitoring all HDs is unfeasible both from a methodological standpoint and because of the sheer cost of all the measurements that would be required. It is therefore necessary to carefully decide which drug(s) must be monitored as part of the surface contamination monitoring protocol applied to areas devoted to HD compounding in HPDs.
The first step should be an analysis of the most widely used drugs in the HPD. This will allow selecting the ones used most frequently and in the largest quantities as surrogate exposure markers. The selection process should also consider the number of times each drug is handled as well as their carcinogenic potential and physical-chemical properties (particularly their volatility and transdermal absorption potential).
Availability of a validated analytical method to quantify these HDs and their associated cost is another variable to be considered.
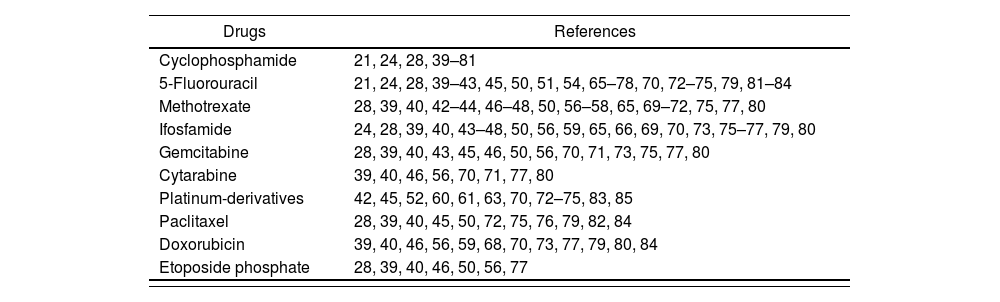
On the basis of the literature reviewed, the previously gathered experience, and the availability of validated analytical quantification methods, the drugs commonly selected as contamination markers are: cyclophosphamide, 5-fluorouracil, methotrexate, ifosfamide, gemcitabine, cytarabine, platinum derivatives, paclitaxel, doxorubicin and etoposide phosphate (Table 3).
Drugs used as contamination markers
| Drugs | References |
|---|---|
| Cyclophosphamide | 21, 24, 28, 39–81 |
| 5-Fluorouracil | 21, 24, 28, 39–43, 45, 50, 51, 54, 65–78, 70, 72–75, 79, 81–84 |
| Methotrexate | 28, 39, 40, 42–44, 46–48, 50, 56–58, 65, 69–72, 75, 77, 80 |
| Ifosfamide | 24, 28, 39, 40, 43–48, 50, 56, 59, 65, 66, 69, 70, 73, 75–77, 79, 80 |
| Gemcitabine | 28, 39, 40, 43, 45, 46, 50, 56, 70, 71, 73, 75, 77, 80 |
| Cytarabine | 39, 40, 46, 56, 70, 71, 77, 80 |
| Platinum-derivatives | 42, 45, 52, 60, 61, 63, 70, 72–75, 83, 85 |
| Paclitaxel | 28, 39, 40, 45, 50, 72, 75, 76, 79, 82, 84 |
| Doxorubicin | 39, 40, 46, 56, 59, 68, 70, 73, 77, 79, 80, 84 |
| Etoposide phosphate | 28, 39, 40, 46, 50, 56, 77 |
Cyclophosphamide is the most commonly monitored HD. Its recognized carcinogenic potential [it has been classified as a group 1 agent by the International Agency for Research on Cancer (IARC)] and its transdermal absorption potential make it an ideal candidate for our purpose. Moreover, it requires reconstitution prior to dilution in a vehicle that is acceptable for administration, which increases the number of times the drug must be handled. Also, cyclophosphamide is an active ingredient of which large quantities are handled with high frequency, and for which validated analytical quantification methods are available.
Areas to be monitoredBefore selecting which areas of the HDP should be sampled, an analysis must be made of the HDs handling circuits used in the facility.
It is recommended to select 1 to 5 common HDs contact points for each task that involves handling of such drugs in the HPD.
The areas where contamination with HDs is more likely to occur and which should therefore be evaluated are the following:
- •
Reception area, including the reception section in the preparation area.
- •
Storage area, which comprises the storage shelves, the floor and the reception bench or the work bench.
- •
Preparation and packaging area: this is where HDs are compounded and prepared for dispensation.
- •
HDs verification area: this is where HDs are inspected prior to dispensing.
Other published studies28,39,43,44,46,48,52,53,57,59,62,65,68,69,71–73,76,82,83 refer to the need to sample additional surfaces in the preparation sections and in the cleanrooms of HPDs:
- •
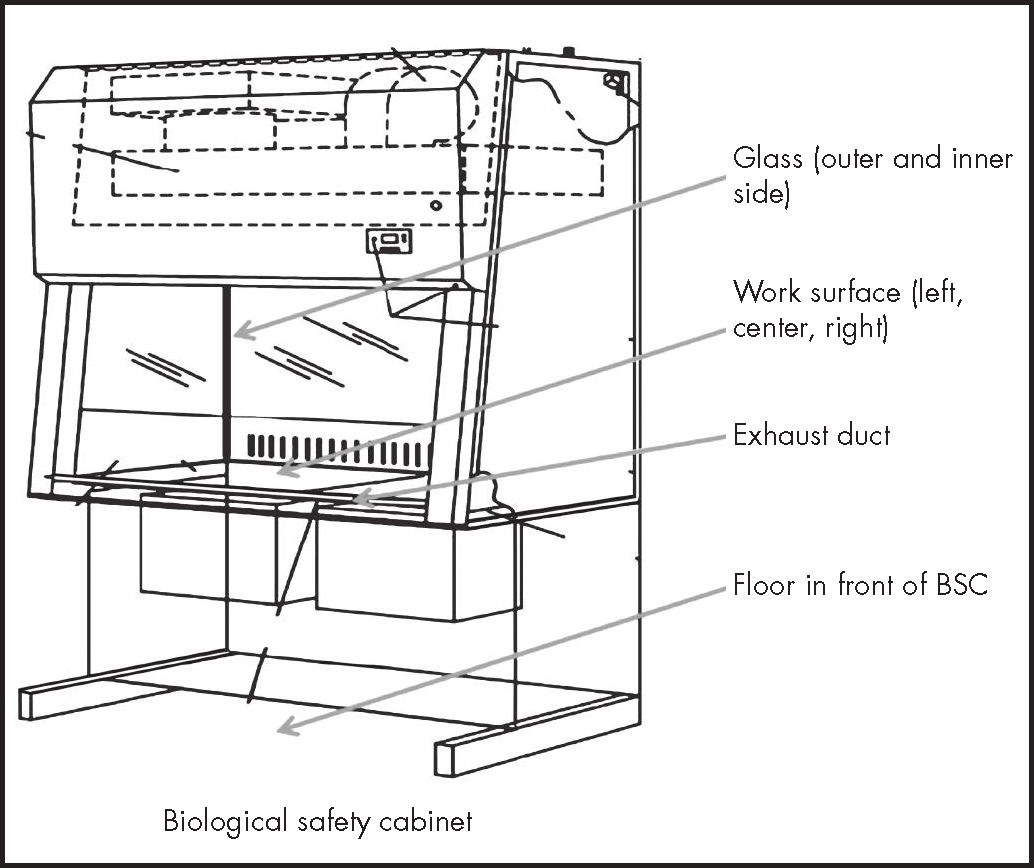
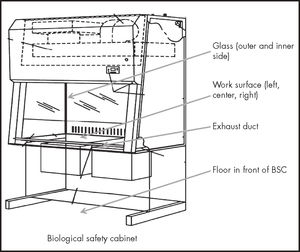
Biological safety cabinet or isolator and surrounding areas (Figure 1).
- •
Floor in front of the cabinets or the corridors.
- •
Door handle, light switch, computer keyboards and mouses, calculator, label printer, pens and/or highlighter, scissors.
- •
Telephone or intercom.
- •
Tablet or touchpad devices used for gravimetrically controlling preparations, including scales.
- •
Shelves for the storage of vials and the devices used to prepare raw materials and inspect final products.
- •
Refrigerator.
- •
Trays or boxes used to transfer materials and/or finished products.
- •
Gloves.
- •
The surfaces of infusion bags, disinfectant bottles, and HD vials.
- •
Waste bins.
Regardless of what is sampled and at what locations, it is essential to precisely establish the size of the surface to be analyzed in each of them as results are usually expressed as nanograms or picograms of the HD per sq. cm. The size of the sampled surface varies across the different studies, but typically ranges between 10039,46,53,57,59,68,71–73,82 to 400-900 cm228,43,44,48,52,62,65,69,76,83.
Sampling protocols must be scrupulously followed to avoid falsifying the results. It is also important to specify what material the sampled surface is made of as the percentages of HD recovered may vary depending on the material type. If the sampled surface does not allow 100% recovery of the HD if may be necessary to apply a corrective factor to the result obtained in order to avoid underestimating HD levels. Tables are available which, depending on the quantitative technique used, apply one set of values or another.
Also, using photography or video could be an ideal way of unequivocally identifying the sampled areas and facilitate follow-up sampling.
It is essential for the responsible people for the sampling to be appropriately trained to do the job rigorously, avoiding cross contamination and protecting themselves from exposure to HDs. It is advisable for the same person to carry out all the samplings to avoid biases due to changes in the procedure.
Once the sampling process has been completed, the whole area must be cleaned and decontaminated as the sampling process itself could mobilize HDs on the work surfaces and increase the exposure risk. All the materials used should be handled as potentially chemically contaminated and discarded in a chemical waste container that meets local waste management regulations.
The number of samples to be taken will depend on how many preparations have been compounded; on the HD circuit established at the facility; on the previously defined goals of the surface monitoring protocol; and on the budget available, as analytical laboratories tend to establish costs as a function of the number of samples to be analyzed.
Sampling timeWhen to carry out the sample must be decided depending on the purpose of the sampling process.
For routine monitoring of surface contamination levels, USP <800>12 recommends sampling the surfaces at baseline and repeating the procedure at least every 6 months, or whenever confirmation of contamination levels is required. In this case, samples should be obtained in normal working conditions so that the data obtained is relevant and representative of actual work processes. It is advisable to carry out the sampling at the end of the working day, before any cleaning, inactivation or decontamination takes place, in order to obtain a clear insight into the maximum exposure that the staff could be liable to. Depending on the sampling protocol and the activity levels in the facility, monitoring could be performed at the end of a work cycle and at the end of the week.
It is recommended to carry out extraordinary sampling rounds in the event of spills, mishandling of HDs, following technical manipulation of a biological safety cabinet, or in the event any substantial change in the HD handling procedures to verify the impact of these occurrences on contamination levels.
If the sampling is aimed at verifying the effectiveness of new contamination prevention measures, new handling protocols or new cleaning and/or decontamination agents, the sampling should be performed before and after the change is introduced.
The cost of sampling is also a criterion to be considered when establishing the monitoring frequency and the number of samples to be taken. The sampling protocol should establish a prioritization of the surfaces to be sampled and a minimum number of samples to be taken.
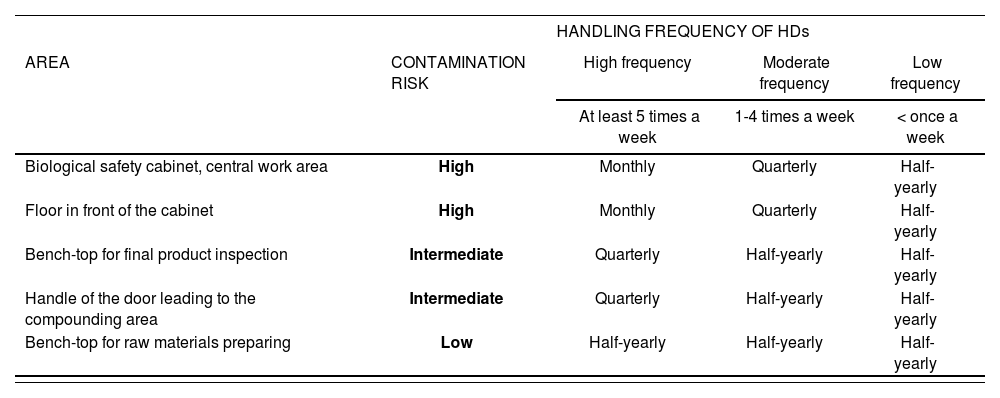
Risk determination as part of the sampling plan. Monitoring frequencyTo be efficient, any surface contamination monitoring plan should include an assessment of the contamination risk present in the different sections of the HPD's compounding area. This is essential to determine where to sample and establish a suitable monitoring frequency. These risk assessment must be carried out at least once a year but can be performed more frequently if any changes in the sampling procedures or the results of monitoring tests so warrant.
According to the litertature28,39,43,44,46,48,52,53,57,59,62,65,68,69,71–73,76,82,83, certain locations are associated with a higher contamination risk than others, which means that they should be classified as HIGH, INTERMEDIATE or LOW risk, depending on their liability to HDs contamination.
Another factor to be considered is the volume of HDs handled and the handling frequency on a given surface. The latter can also be classified into three categories: high frequency (at least 5 times a week), moderate frequency (1-4 times a week) and low frequency (less than once a week).
The sampling plan should also contemplate the effect that exposure to HDs may have on the staff's health, analyzing the severity of potential effects and considering them when determining overall risk. Effects will in most cases be considered as severe.
The combination of the contamination risk of the surface to be sampled with the frequency of HDs handling on that surface will determine the initial monitoring frequency in the sampling protocol (Table 4). Sampling frequencies are typically monthly, quarterly or half-yearly.
Monitoring plan
| HANDLING FREQUENCY OF HDs | ||||
|---|---|---|---|---|
| AREA | CONTAMINATION RISK | High frequency | Moderate frequency | Low frequency |
| At least 5 times a week | 1-4 times a week | < once a week | ||
| Biological safety cabinet, central work area | High | Monthly | Quarterly | Half-yearly |
| Floor in front of the cabinet | High | Monthly | Quarterly | Half-yearly |
| Bench-top for final product inspection | Intermediate | Quarterly | Half-yearly | Half-yearly |
| Handle of the door leading to the compounding area | Intermediate | Quarterly | Half-yearly | Half-yearly |
| Bench-top for raw materials preparing | Low | Half-yearly | Half-yearly | Half-yearly |
Certain studies59,64,69,86 determine monitorization frequency depending on the contamination results obtained. This means, for example, that if contamination is observed, the sampling frequency should be upped one level from half-yearly to quarterly, from quarterly to monthly, from monthly to fortnightly, etc. If, on the other hand, results are not indicative of contamination, and this finding persists over time, it may be decided to decrease sampling frequency by one level, ensuring in any event that monitoring frequency never falls below six months.
Analytical techniquesSelection of a proper analytical technique to ensure an accurate and validated sampling process is necessary for adequate contamination monitoring. If sampling is carried out by an outsourced laboratory, it is important to ensure they periodically evaluate their quality assurance processes and that they are duly accredited. If semiquantitative techniques are preferred, it must be taken into consideration that the information obtained will have limitations.
Several factors must be analyzed when selecting the analysis and sampling method as the interpretation and/or the quality of the results obtained may vary depending on the method chosen. If an external laboratory is recruited, candidates should be asked to provide information about the analysis and sampling methods they intend to use so that the one that most closely meets the needs of the contracting HPD can be selected:
- •
Degree of recovery of the HD or extraction efficiency, depending on the type of surface sampled: flat (stainless steel, glass), smooth (fabric or leather), rough (fitted carpets or wood), porous (vinyl), contaminated or otherwise. Extraction percentages between 75 and 90% are acceptable.
- •
Solubility of the HD in the solvent used in the sampling procedure.
- •
Type of material used for the sampling. Analytical laboratories usually make available all the materials required. The material should be the same one used to validate the analytical technique. For in situ semiquantitative techniques, the supplier must provide all the materials required for proper sampling of the drug.
- •
The solvent used for extraction should be compatible with the material of the surfaces to be sampled.
- •
The extraction solvent must be compatible with the one used for the analysis.
- •
Selectivity and sensitivity of the technique. These parameters include the threshold detection and quantification values, as well as the linearity of calibration curves.
- •
Sampling cost.
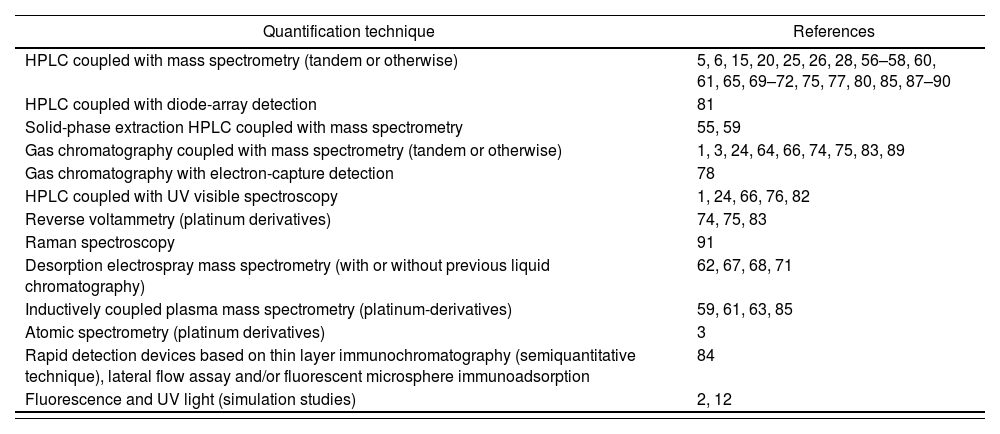
There are multiple validated analytical HD sampling techniques. Table 5 shows the different quantification methods used. The most widely used include: gas chromatography, high-performance liquid chromatography (HPLC) and ultra-performance liquid chromatography (UPLC), always combined with some high-sensitivity identification or quantification method such as mass spectrometry (MS) or tandem mass spectrometry (MS/MS).
Quantification techniques for hazardous drugs
| Quantification technique | References |
|---|---|
| HPLC coupled with mass spectrometry (tandem or otherwise) | 5, 6, 15, 20, 25, 26, 28, 56–58, 60, 61, 65, 69–72, 75, 77, 80, 85, 87–90 |
| HPLC coupled with diode-array detection | 81 |
| Solid-phase extraction HPLC coupled with mass spectrometry | 55, 59 |
| Gas chromatography coupled with mass spectrometry (tandem or otherwise) | 1, 3, 24, 64, 66, 74, 75, 83, 89 |
| Gas chromatography with electron-capture detection | 78 |
| HPLC coupled with UV visible spectroscopy | 1, 24, 66, 76, 82 |
| Reverse voltammetry (platinum derivatives) | 74, 75, 83 |
| Raman spectroscopy | 91 |
| Desorption electrospray mass spectrometry (with or without previous liquid chromatography) | 62, 67, 68, 71 |
| Inductively coupled plasma mass spectrometry (platinum-derivatives) | 59, 61, 63, 85 |
| Atomic spectrometry (platinum derivatives) | 3 |
| Rapid detection devices based on thin layer immunochromatography (semiquantitative technique), lateral flow assay and/or fluorescent microsphere immunoadsorption | 84 |
| Fluorescence and UV light (simulation studies) | 2, 12 |
HPLC chromatography with tandem mass spectrometry (LC-MS/MS) is the most commonly method used by laboratories for the quantitative analysis of most HDs as it employs a sensitive, specific, and accurate technique. However, the initial investment required is high and the processing of results usually takes a fairly long time, which could extend contamination exposure until the appropriate decontamination or mitigation measures are implemented54.
Although these techniques provide high sensitivity (of the order of pg/cm2), selectivity and accuracy levels, the initial investment in equipment required, the need of specifically trained staff and the delays in reporting test results increase their cost and make them unsuitable for routine high frequency monitoring. Having said this, any technique that has been validated may be appropriate if its detection and quantification thresholds are in line with the levels of HDs commonly present in a given facility.
There are also semi-quantitative sampling techniques84, based on rapid detection devices that use thin layer immunochromatography performed with lateral flow immunoassay (LFIA) and/or fluorescent microsphere immunoadsorption. NIOSH, for instance, has developed a new technology based on LFIA for detecting surface contamination with HDs. Although this technique does not seem ideal for baseline monitoring, it could prove useful for the establishment of routine or high-frequency monitoring programs given its low cost and lower response times as compared with currently used analytical techniques.
LFIA allows direct reporting of results through sensitive and easy-to-use portable field monitors that measure the levels of selected HDs on a given surface. In addition, results can be obtained in real time (within 10 minutes), which allows users to adopt immediate corrective measures and take further confirmatory samples. The disadvantage of LFIA is that currently commercially available readers provide semiquantitative results, whereas the LC-MS/MS technique provides quantitative ones.
Before adoption of a monitoring method, it is essential to carefully weigh its pros and cons (agility, ease of use, cost, quantitative or semiquantitative nature of results). Combined use of LFIA and LC-MS/MS, the former for regular monitoring and adoption of immediate corrective action and the latter for periodic quantitative measurements, may be considered92.
It must be noted that HPDs that already have implemented these techniques could act as hubs where measurements could be made in a centralized way, as is the case for other hospital-based techniques and procedures.
Contamination thresholdsNo regulations or standards have been developed to date on surface contamination with HDs. Nor have any maximum allowable contamination levels been defined for the hospital setting. According to the literature, surface contamination higher than 1 ng/cm2 leads to absorption of HDs by exposed workers and to the presence of those substances in their urine12. No data has been published on the potential risk of environmental surface contamination with HDs for human health30.
Several authors3,5,6,15,20,28 have recommended establishing the maximum allowable exposure levels based on the historical results of controls carried out in HPDs. In this regard, they argue that contamination levels higher than the 75th, 90th and 95th percentile (depending on the different authors) of those historical controls should be considered unacceptable and therefore result in an overhaul of the HD handling circuits and techniques of the affected HPDs. These levels could be used to determine the staff's adherence to best HD handling practices, but not to determine the risk of developing long-term adverse events as the above-mentioned studies were not toxicologic trials evaluating health outcomes.
Another approach to establishing maximum exposure levels consists in correlating detected surface contamination levels with the drug's levels in the urine of exposed staff members. In this case, 0.1 ng/cm2 has been established as the maximum allowable quantity of HD on the surface (Table 6)93. It should be mentioned that this study only looked at the presence of cyclophosphamide, which means that the authors establish the same threshold for all HDs without having analyzed all of them and without having investigated their dermal permeation characteristics. USP has proposed a 1 ng/cm2 cyclophosphamide threshold to limit the risk of cyclophosphamide absorption by exposed workers12.
Maximum allowable exposure levels in the Netherlands, and actions recommended93
| Contamination (ng/cm2) | < 0.10 | 0.10-1.0 | 1.0-10 | > 10 |
|---|---|---|---|---|
| Actions | Monitoring once a year and evaluations every 4 years | Estimated risk. Monitoring every 3-6 months. Finally, application of corrective measures | Immediate application of corrective measures. Monitoring the efficacy of corrective measures | |
A Swedish study suggests establishing a practical and feasible threshold at the 90th percentile of the monitoring results available for a given (typical) working environment94. The notion of the 90th percentile is also proposed by the authors of the MEWIP study performed in German hospitals28.
It must be borne in mind that although surface contamination levels are not indicative of real-life exposure, they are helpful in determining whether there is a potential exposure source in the environment.
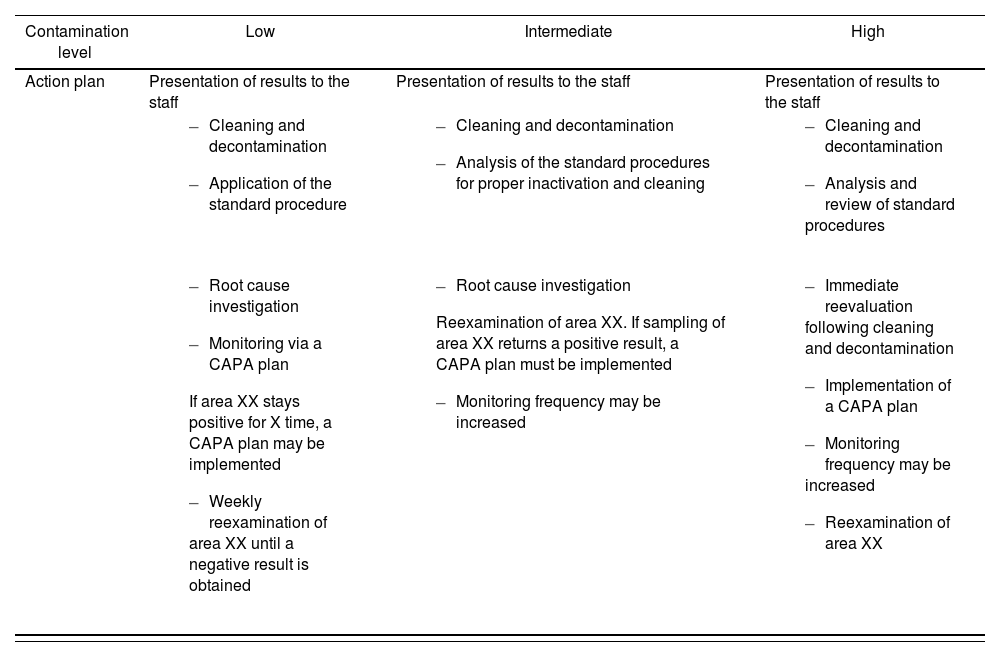
Action plan based on the results of the sampling procedureOnce the sampling parameters have been established, an action plan needs to be defined in case significant deviations or unexpected results are found. Table 7 shows an example of the measures that would be implemented in the presence of different surface contamination levels by application of the Corrective Action-Preventive Action (CAPA) continuous improvement plan. An HPD could adopt the CAPA model or create their own plan based on the ALARA model and the data they possess from their own surface monitoring analyses.
Example of an action plan based on the results of the sampling procedure.
| Contamination level | Low | Intermediate | High |
|---|---|---|---|
| Action plan | Presentation of results to the staff | Presentation of results to the staff | Presentation of results to the staff |
|
|
| |
|
|
|
CAPA: corrective action-preventive action.
Whichever model is selected to interpret the results, HPDs must establish an action plan to address significant deviations. It is recommended that all areas where residual contamination with HDs is identified be cleaned, decontaminated, and subsequently reanalyzed. Cleaning and decontamination procedures should be performed in accordance with each HPDs procedures.
DecontaminationMultiple factors must be considered when establishing an efficient decontamination protocol, not least because no clearly defined standard decontamination procedure exists for HDs. Factors to be considered include:
- •
Physical-chemical properties and number of contaminants89,95.
- •
Physical-chemical properties of the decontamination agent(s) to be used, specifically their hydrophilicity and lipophilicity89,95.
- •
Standardization of routine compounding processes96.
- •
Decontamination frequency89,95.
- •
Characteristics of the surfaces to be treated, both in terms of their ability to retain the contaminant95,97 (stainless steel and glass tend to be the most easily decontaminated surfaces), and to resist the effects of the decontamination agent used [degradation of stainless steel by sodium hypochlorite and of plastic surfaces by isopropyl alcohol (IPA) and other solvents, staining of surfaces with a potassium permanganate solution, etc.]88.
- •
Contact time between the decontamination agent and the surface to be treated.62,98,99
- •
Volume of decontamination agent used with respect to the surface area to be treated95,98.
- •
Likelihood that the inactivation process could generate equally hazardous products, such as KMnO4 or HCl100.
- •
Risk that the decontamination process could be poorly executed, leading to dissemination of the HD89,101.
Establishment of a decontamination procedure is complex given the number of requirements that must be met. Indeed, the procedure must:
- •
Efficiently remove HDs.
- •
Avoid generation of products that may be hazardous in themselves or when combined with other products used in the working environment.
- •
Be easy to use and safe for the operator.
- •
Avoid alterations of the working environment.
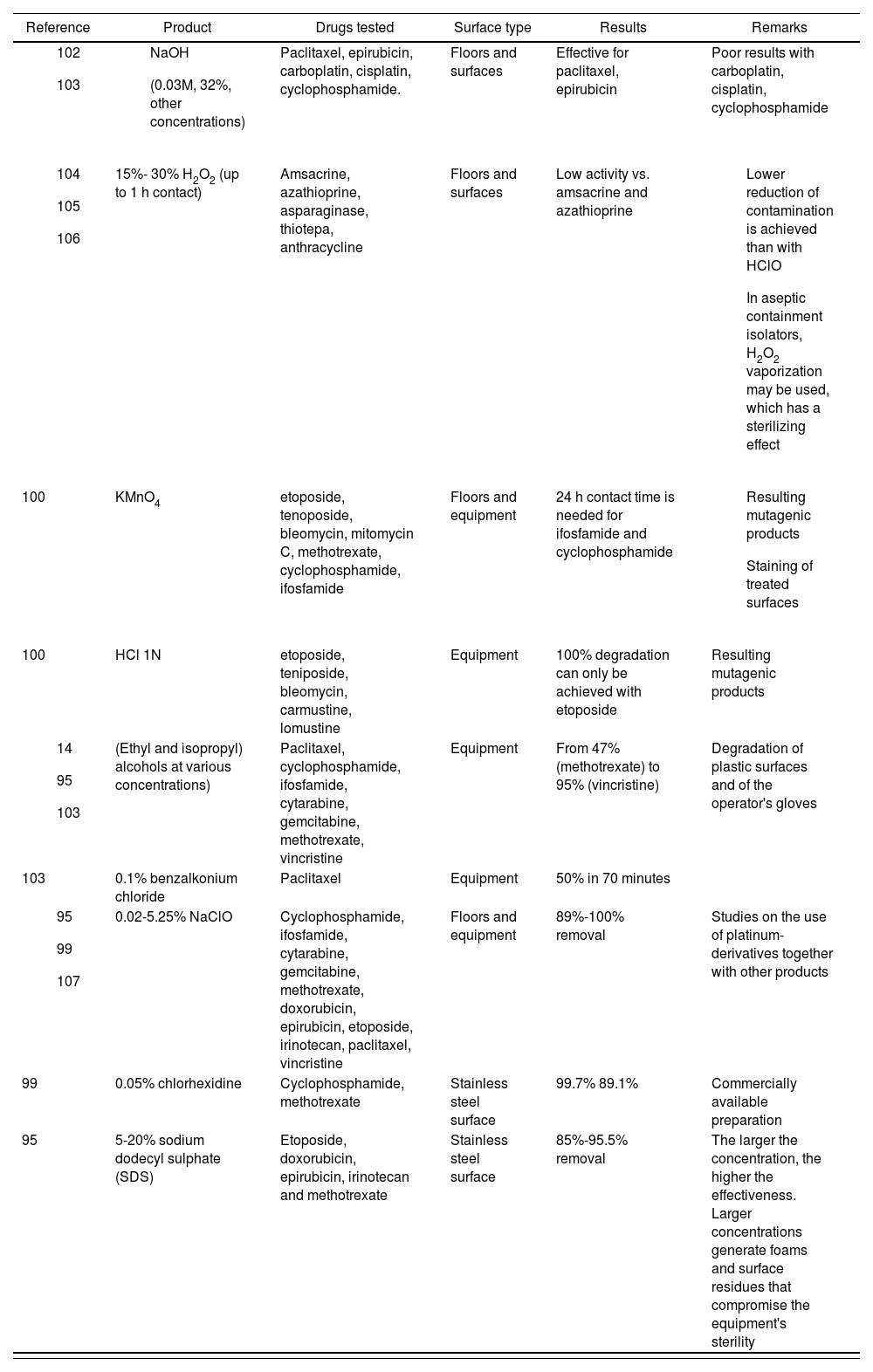
It must be noted that no single agent has shown itself to be able to decontaminate or inactivate all HDs. A review of different publications101 found that sodium hypochlorite concentrates are the most effective decontamination agents in that context, but this could be because that agent is the most frequently used one for that purpose101.
Table 8 contains all the data available on the different decontamination agents.
Usefulness of different decontamination agents against hazardous drugs
| Reference | Product | Drugs tested | Surface type | Results | Remarks |
|---|---|---|---|---|---|
| Paclitaxel, epirubicin, carboplatin, cisplatin, cyclophosphamide. | Floors and surfaces | Effective for paclitaxel, epirubicin | Poor results with carboplatin, cisplatin, cyclophosphamide | |
| 15%- 30% H2O2 (up to 1 h contact) | Amsacrine, azathioprine, asparaginase, thiotepa, anthracycline | Floors and surfaces | Low activity vs. amsacrine and azathioprine |
| |
| 100 | KMnO4 | etoposide, tenoposide, bleomycin, mitomycin C, methotrexate, cyclophosphamide, ifosfamide | Floors and equipment | 24 h contact time is needed for ifosfamide and cyclophosphamide |
|
| 100 | HCl 1N | etoposide, teniposide, bleomycin, carmustine, lomustine | Equipment | 100% degradation can only be achieved with etoposide | Resulting mutagenic products |
| (Ethyl and isopropyl) alcohols at various concentrations) | Paclitaxel, cyclophosphamide, ifosfamide, cytarabine, gemcitabine, methotrexate, vincristine | Equipment | From 47% (methotrexate) to 95% (vincristine) | Degradation of plastic surfaces and of the operator's gloves | |
| 103 | 0.1% benzalkonium chloride | Paclitaxel | Equipment | 50% in 70 minutes | |
| 0.02-5.25% NaClO | Cyclophosphamide, ifosfamide, cytarabine, gemcitabine, methotrexate, doxorubicin, epirubicin, etoposide, irinotecan, paclitaxel, vincristine | Floors and equipment | 89%-100% removal | Studies on the use of platinum-derivatives together with other products | |
| 99 | 0.05% chlorhexidine | Cyclophosphamide, methotrexate | Stainless steel surface | 99.7% 89.1% | Commercially available preparation |
| 95 | 5-20% sodium dodecyl sulphate (SDS) | Etoposide, doxorubicin, epirubicin, irinotecan and methotrexate | Stainless steel surface | 85%-95.5% removal | The larger the concentration, the higher the effectiveness. Larger concentrations generate foams and surface residues that compromise the equipment's sterility |
Surface monitoring must be applied to determine the presence of HDs and establish the efficacy of the HD management protocol used by a given HPD, as no standard exists that can guarantee the safety of drug compounding processes across all HPDs. The evaluation must include an analysis of the efficacy of engineering controls, of work practices and of the cleaning and decontamination procedures used.
Based on the above, the following recommendations are put forward with the caveat that they are founded on expert opinion level of evidence in table 9:
Recommendations for monitoring contamination of hazardous drug compounding surfaces at hospital pharmacy departments
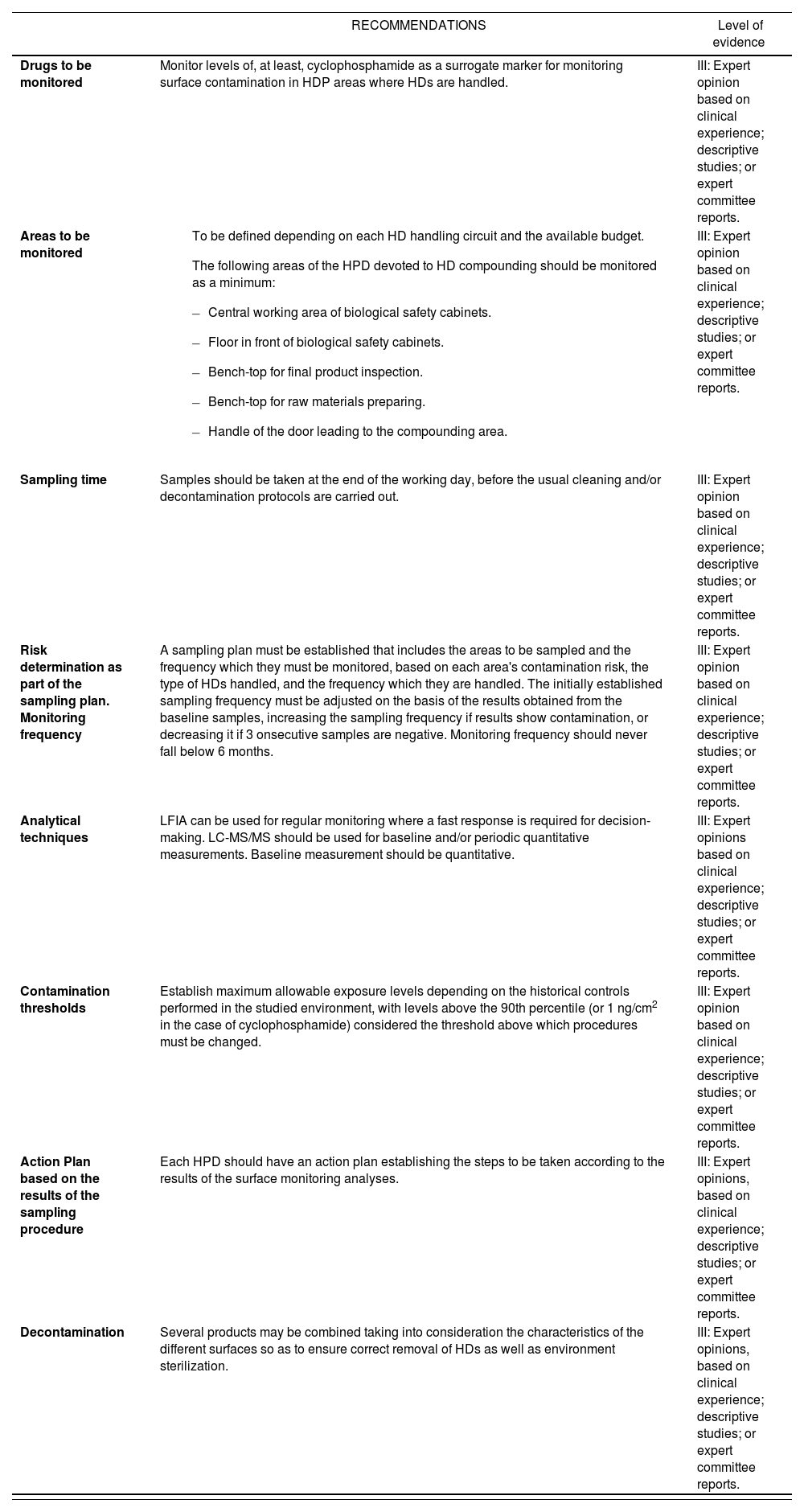
| RECOMMENDATIONS | Level of evidence | |
|---|---|---|
| Drugs to be monitored | Monitor levels of, at least, cyclophosphamide as a surrogate marker for monitoring surface contamination in HDP areas where HDs are handled. | III: Expert opinion based on clinical experience; descriptive studies; or expert committee reports. |
| Areas to be monitored |
| III: Expert opinion based on clinical experience; descriptive studies; or expert committee reports. |
| Sampling time | Samples should be taken at the end of the working day, before the usual cleaning and/or decontamination protocols are carried out. | III: Expert opinion based on clinical experience; descriptive studies; or expert committee reports. |
| Risk determination as part of the sampling plan. Monitoring frequency | A sampling plan must be established that includes the areas to be sampled and the frequency which they must be monitored, based on each area's contamination risk, the type of HDs handled, and the frequency which they are handled. The initially established sampling frequency must be adjusted on the basis of the results obtained from the baseline samples, increasing the sampling frequency if results show contamination, or decreasing it if 3 onsecutive samples are negative. Monitoring frequency should never fall below 6 months. | III: Expert opinion based on clinical experience; descriptive studies; or expert committee reports. |
| Analytical techniques | LFIA can be used for regular monitoring where a fast response is required for decision-making. LC-MS/MS should be used for baseline and/or periodic quantitative measurements. Baseline measurement should be quantitative. | III: Expert opinions based on clinical experience; descriptive studies; or expert committee reports. |
| Contamination thresholds | Establish maximum allowable exposure levels depending on the historical controls performed in the studied environment, with levels above the 90th percentile (or 1 ng/cm2 in the case of cyclophosphamide) considered the threshold above which procedures must be changed. | III: Expert opinion based on clinical experience; descriptive studies; or expert committee reports. |
| Action Plan based on the results of the sampling procedure | Each HPD should have an action plan establishing the steps to be taken according to the results of the surface monitoring analyses. | III: Expert opinions, based on clinical experience; descriptive studies; or expert committee reports. |
| Decontamination | Several products may be combined taking into consideration the characteristics of the different surfaces so as to ensure correct removal of HDs as well as environment sterilization. | III: Expert opinions, based on clinical experience; descriptive studies; or expert committee reports. |
HD: hazardous drug; HPD: hospital pharmacy department; LC-MS/MS: tandem mass spectrometry; LFIA: lateral flow immunoassay.
SEFH was awarded a Becton Dickinson grant to cover the meeting and rapporteur's expenses involved in the review and publication of this manuscript.
All authors were selected by SEFH. None of them received any funding for the work done.
Conflict of interestSponsor: Spanish Society of Hospital Pharmacists (SEFH).
Sanctioned by the Spanish Association of Occupational Medicine in the Healthcare Sector (ANMTAS)