The maximum expression of malnutrition in cancer patients is cancerous cachexia, always linked to an unfavorable prognosis. Given its evolutionary nature it is recommended to detect and act early in those patients with nutritional risk. The objective is to propose an action algorithm for the nutritional approach of patients with solid tumors.
MethodThrough the nominal group technique, specialists in hospital pharmacy, nutrition and oncology who established a prioritization of issues related to nutritional status and its approach in patients with solid tumors were brought together. Their discussion and analysis allowed us to design a performance algorithm.
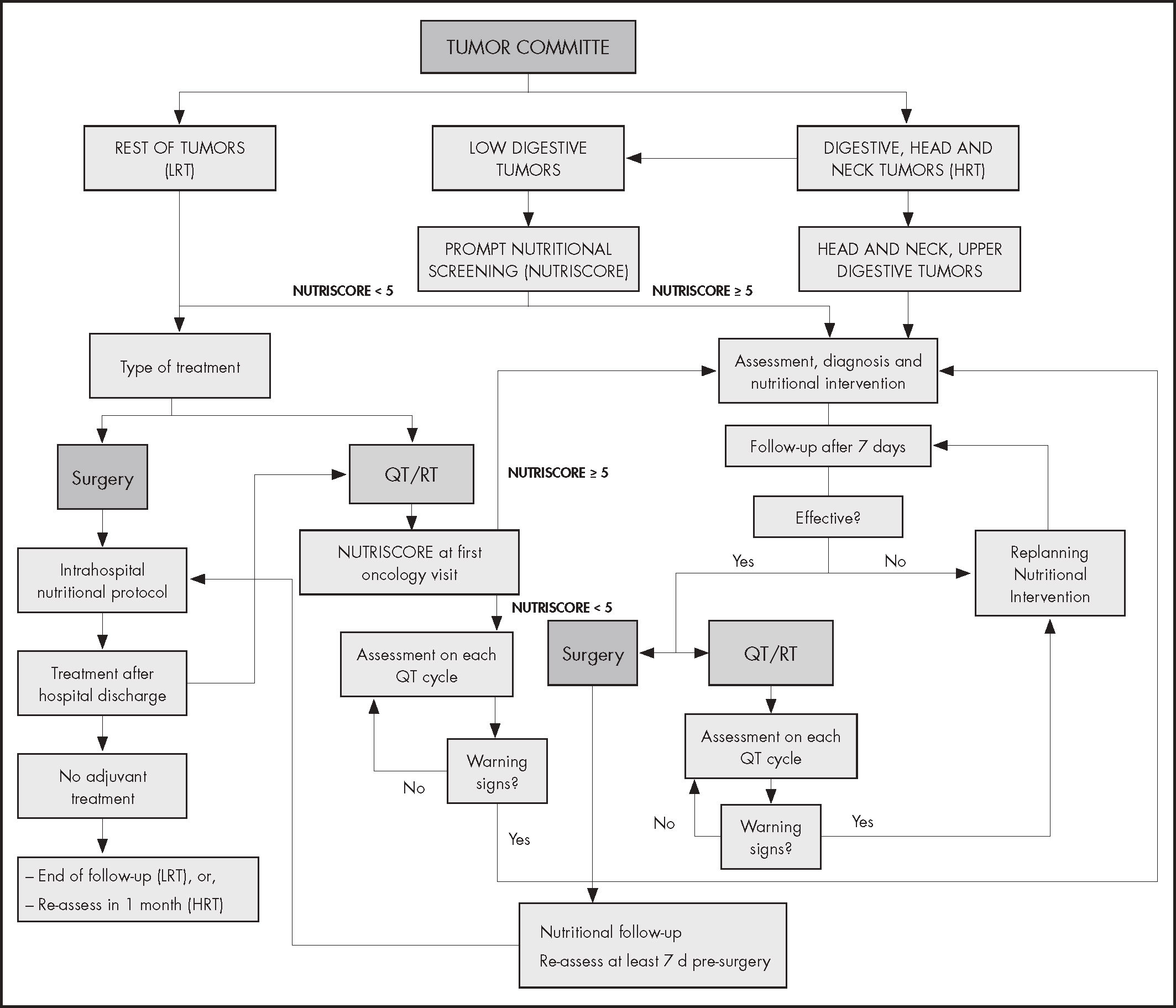
ResultsThe algorithm differentiates two groups of patients according to the location of the tumor and its impact on nutritional status: high-risk tumors (group 1) include cancers of the head and neck, upper digestive tract and colorectal and low-risk tumors (group 2) include the rest of the neoplasms. Group 1 patients (with the exception of those with colorectal cancer) are directly assessed nutritionally in the first 3-5 days after their presentation in the Tumor Committee, starting the nutritional support required at that time. Patients in group 2 and those diagnosed with colorectal cancer are screened (through NUTRISCORE) after their presentation in the Committee, those with positive risk being referred to nutritional consultation to perform a complete evaluation and propose treatment options. Patients without nutritional risk are periodically re-evaluated. Follow-up is planned according to cancer therapy, with continuous monitoring in each treatment cycle or during the perioperative period.
ConclusionsFrom the nominal group technique, agreements were reached to propose an algorithm of nutritional approach of the cancer patient. The adoption of the proposed algorithm could reduce variability in institutional clinical practice, promoting a timely and adequate nutritional approach in cancer patients.
La máxima expresión de la desnutrición en los pacientes oncológicos es la caquexia cancerosa, siempre vinculada a un pronóstico desfavorable. Dado su carácter evolutivo se recomienda detectar y actuar precozmente en aquellos pacientes con riesgo nutricional. El objetivo es definir un algoritmo de actuación para el abordaje nutricional de pacientes con tumores sólidos.
MétodoMediante la técnica de grupo nominal se reunió a especialistas en farmacia hospitalaria, nutrición y oncología que establecieron una priorización de temas relacionados con el estado nutricional y su abordaje en pacientes con tumores sólidos. Su discusión y análisis permitieron diseñar un algoritmo de actuación.
ResultadosEl algoritmo diferencia dos grupos de pacientes según la localización del tumor y su impacto en el estado nutricional: los tumores de alto riesgo (grupo 1) incluyen cánceres de cabeza y cuello, del tracto digestivo superior y colorrectal, y los tumores de bajo riesgo (grupo 2) engloban el resto de neoplasias. Los pacientes del grupo 1 (a excepción de aquellos con cáncer colorrectal) son directamente valorados nutricionalmente en los primeros 3-5 días tras su presentación en el comité de tumores, iniciando el soporte nutricional requerido en ese momento. Los pacientes del grupo 2 y los diagnosticados de cáncer colorrectal son cribados (mediante NUTRISCORE) tras su presentación en el comité, derivándose a consulta nutricional a aquellos con riesgo positivo para realizar una evaluación completa y proponer opciones de tratamiento, y reevaluándose periódicamente los pacientes sin riesgo nutricional. El seguimiento se planifica según la terapia oncológica, con una monitorización continua en cada ciclo de tratamiento o durante el periodo perioperatorio.
ConclusionesA partir de la técnica de grupo nominal, se alcanzaron acuerdos para proponer un algoritmo de abordaje nutricional precoz del paciente con cáncer. La adopción del algoritmo propuesto podría reducir la variabilidad en la práctica clínica institucional, promoviendo un enfoque nutricional oportuno y adecuado en pacientes con cáncer.
Malnutrition is a common problem in patients with cancer. At the time of diagnosis of the tumor, the occurrence of malnutrition stands at between 15-20%, increasing as the illness progresses, so that it may affect up to 80% of patients at the advanced stage1. The malnutrition rate is higher both in patients with head and neck tumors and in those with digestive system tumors1,2. Segura et al. evaluated the prevalence, in Spain, of malnutrition in cancer patients and found that more than 50% present moderate or severe degrees of malnutrition1. Hebuterne et al.2 analyzed the use of nutrition support en cancer patients in 154 French hospitals wards and they found that only 28% of non-malnurished patients and 58% of malnourished patients received nutrition support.
The prevalence of malnutrition varies considerably depending on the neoplastic strain. Gastric and pancreatic cancers are associated with figures of malnutrition above 80%, whilst lymphomas and acute leukemias present prevalence below 30%. Regardless the nutritional impact that the cancer itself may have, the prevalence of malnutrition is influenced by the type of treatment applied, with nutritional complications being typical in patients with digestive carcinomas3.
It has been shown that nutritional deterioration has a negative impact on the evolution of cancer patients: reducing the tolerance and efficacy of their treatment4, increasing the risk for clinical and surgical complications5 and length of hospital stays with a concomitant increase in health care costs6. Malnutrition in these patients is also associated with poorer quality of life (QL)7,8.
The etiology of malnutrition in patients with cancer is multifactorial. In addition to the influence that the anatomical location of the tumor has on the intake and absorption of nutrients, we have to take into consideration both the nutritional and metabolic alterations associated with the inflammatory response and the impact of the side effects derived from the surgical and radio-chemotherapy treatments applied9. The maximum expression of malnutrition in the oncologic patient is the anorexia-cachexia syndrome, defined as a multifactorial syndrome characterized by a progressive loss of skeletal muscle, associated or not with a loss of adipose tissue. It is responsible either directly or indirectly for the death of a third of patients with cancer10.
The cancer cachexia syndrome can develop progressively through various stages: from pre-cachexia to cachexia and to refractory cachexia11. The focus by this definition is put on a minimum degree of body weight loss.
Even before diagnosis and treatment, weight loss is common. In one series of more than 3,000 cases, the frequency of weight loss ranged from 31% to 87% (patients with non-Hodgkin's lymphoma and those with gastric cancer respectively)12,13.
Tumor-related disease processes, host response and cancer-related treatments such as chemotherapy, radiotherapy, and surgery all culminate in the end point of under-nutrition14.
Pre-chemotherapy weight loss correlated with shorter overall survival and decreased response rate, QL and performance status13.
Given the incidence of nutritional risk in cancer and the fact that the management of cachexia remains a challenge in clinical practice15, a multidisciplinary approach is vital to define efficient strategies that can improve quality of care in cancer patients.
According to the reviewed data and guidelines, nutritional intervention should be complementary to any antineoplastic treatment and should be included in the multidisciplinary approach mandatory in oncology16.
Multidisciplinary follow-up, with early and regular nutritional intervention, is of major importance in oncology, thus being a key factor for successful treatment and recovery17,18.
A systematic review and meta-analysis from Baldwin et al.19, that evaluate the benefits of nutrition support, have been conducted in late stage patients with poor performance status. By this stage, both cachexia and cancer are refractory to treatment and a significant proportion of patients discontinuing trials because of toxicity, disease progression and death. As Aapro et al. say in his review on the early detection of cancer cachexia20: The earlier phase of active anti-cancer therapy, which frequently achieves good control of tumor, offers a window of opportunity for intervention against malnutrition and, by reducing catabolic drive, against cancer cachexia.
The clinical challenge is to identify those in the anorexia-cachexia spectrum who might gain demonstrable clinical benefit from current modes of nutritional support.
Therefore, the aim of this work was to address different issues related to the detection of patients with solids tumors and risk of malnutrition or malnourished and propose an algorithm based on prompt detection of the risk of malnutrition, periodic assessment and nutritional follow-up of these patients, and which envisages nutritional intervention at the early stages of the disease, prior to the appearance of refractory cachexia, in order to reduce the prevalence of malnutrition in this population.
MethodsA multidisciplinary group formed by five specialists in Hospital Pharmacy, one nutritionist and one oncologist, working in collaboration, has developed an algorithm to fully integrate the prevention and nutritional treatment of cancer patients throughout their illness. The medical oncologist made a review to have her feedback and devise the algorithm with onward experience. A Hospital Pharmacyst with big experience in oncology patients was chose coordinator.
At the first meeting, held on March 13, 2018, by nominal group technique (NGT), each participant individually wrote the ideas that arise on the questions asked by the coordinator (Table 1), and then there is an exhaustive presentation of all the ideas generated.
Each idea or item was numbered and written on a panel after which a discussion about them is generated, explaining the logic that sustains it. The next step was the preliminary vote: Each group member individually selected nine items and wrote them down on a card that was ordered according to the importance given. They assigned 9 points to the most important item, one point less to the next and so on until the last item scored 1 point. After this, the moderator added the points of each response and listed all the items that resulted with scores greater than 6 points
Finally, the members of the group (through an anonymous and individual vote) scored on a scale of 0 to 100, the score given to each of the selected items.
After the obtaining the statistical results of the scores given to the questions of the questionnaire, the most conflicting points are submitted for further discussion. At the end of this meeting, the proposals are re-scored, and the most voted are defined as agreed by the panel. In each response the value of the median or other percentiles is used to measure the central tendency of the scores to each question and the arithmetic means of the agreement and disagreement, previously defined numerically.
Finally, the panel laid down a list of items and pathways the algorithm had to include.
At the second meeting, held on May 5th, 2018, a literature review was made and each item was discussed to reach a common site.
An initial document with the algorithm was drafted.
At the third and fourth meetings, the panel discussed the designed structure and wording algorithm written in the previous phases. After collecting the corresponding suggestions and modifications, it was finished with the elaboration of the final text of the algorithm (Figure 1).
ResultsList and agreement on the proposal and the reconstructed priorities obtained after analyzing the contributions of the experts who participated in the nominal group were:
- •
Different approaches according the risk of malnutrition of different types of cancer, considering different pathways to high and low risk tumors.
- •
Different pathways to manage the patients considering the impact on the nutritional state of the cancer therapies.
- •
When the screening tool should be implemented according the patient's nutritional risk?
- •
Surveillance that must be done patients to avoid nutritional deterioration depending on their nutritional risk.
- •
Timing to assess the effectiveness of nutritional interventions.
An algorithm is proposed starting with early evaluation of the patient (with solids tumors) within the Tumor Multidisciplinary Committee, where the situation of oncologic patients is exposed at the outset of their process.
The multidisciplinary approach algorithm differentiates two groups of patients in accordance with the location of the tumor and its impact on the nutritional state: Group 1) head and neck and gastrointestinal (GI) tumors, including colorectal cancer (CRC), named high risk tumors, and Group 2) the rest of tumors, named low risk tumors (Figure 1).
It is considered that high-risk cancer cohort (group 1) have to receive directly a complete nutritional assessment and early treatment if is necessary.
Although malnutrition is more common in CRC than in tumors from group 2, the prevalence rate is lower than patients with upper GI or head and neck cancer21. Even if a direct nutritional assessment is not carried out as in the case of upper GI and head and neck cancer, it is necessary to identify those patients with weight loss throw nutritional screening, immediately after its presentation in the committee.
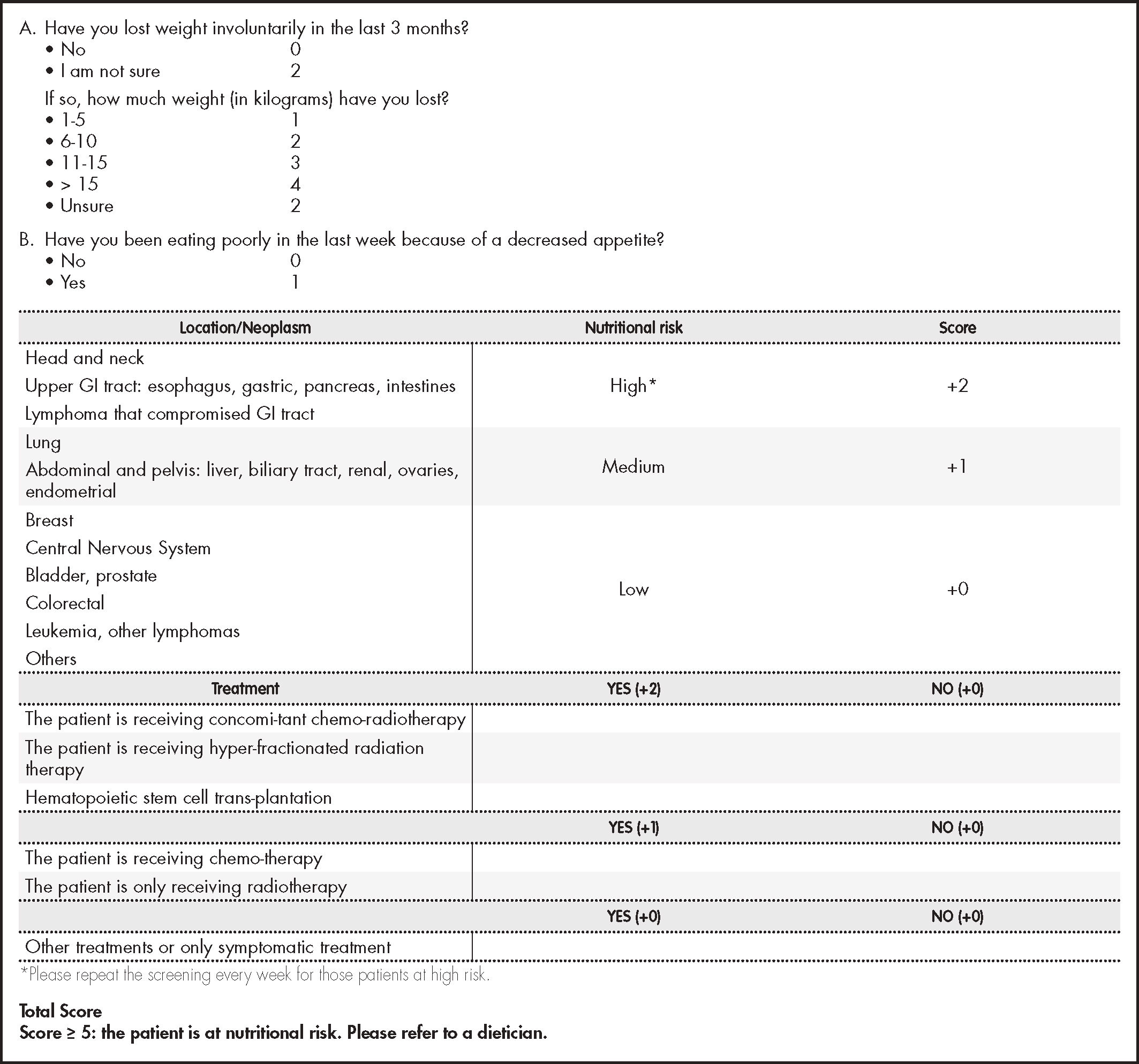
The nutritional screening tool (NST) proposed is NUTRISCORE (Figure 2) which takes into account weight loss over time, appetite in the last week, tumor location, and the treatment to be applied22. Because NUTRISCORE was designed to categorize oncology outpatients according to the presence of nutritional risk using a scoring system, patients who obtained ≥ 5 points were considered at risk, whereas those who scored < 5 were not.
As an additional precautionary measure, during the screening test, patients are informed of the importance of maintaining a suitable nutritional state and, at the same time, provided with training to identify and communicate warning signs and symptoms that might aggravate their nutritional situation.
The main highlight in procedure lies in the fact that an early nutritional approach is carried out in the 3-5 days subsequent to the presentation of the cases in the Tumor Committee.
Patients with colorectal cancer and positive screening (NUTRISCORE ≥ 5 points)If a positive result is obtained in the NST, a nutritional evaluation is performed to define the degree of malnutrition. A positive result always entails a nutritional intervention in line with the recommendations recently published by the ESPEN on nutritional care for cancer patients16.
Adherence to recommendations and effectiveness (correct intake according to prescription, absence of gastrointestinal symptoms derived, maintenance or weight gain) of the early nutritional intervention carried out is assessed in the first week. This may be done through a telephone questionnaire if in-person consultation is not possible, in line with the questionnaire propose for Wanden-Berghe et al.23, which will assist in detecting any problem early and in re-planning the nutritional intervention in the case of any evidence of lack of effectiveness and adherence is found. If the intervention is considered effective the patient's nutritional management is planned in accordance with the cancer therapy:
- •
If the patient is going to receive chemotherapy and/or radiotherapy, the nutritional follow-up continues on the first day of each chemotherapy cycle, watching out for the appearance of warning signs (loss of weight or appetite). The weight and variations are evaluated with respect to the initial weight and, in case of weight loss (≥ 5%) it is sent to the nutrition unit for a complete evaluation (in this case, the patient must be assessed during the next five days of detecting problems and, if possible, it should be done in the same act on the same day).
- •
When the patient is going to undergo a surgical intervention, the nutritional follow-up and re-assessment of his/her nutritional state is carried out at least seven days prior to surgery. During hospital admission, the nutritional care procedures are carried out in accordance with the protocols of each center in line with the recommendations recently published by the ESPEN on clinical nutrition in surgery24.
We recommended that the surgical management of choice follows the strategy of an enhanced recovery after surgery (ERAS) program. Within this program, every patient should be screened for malnutrition, and if deemed at risk, given nutritional therapy25.
If the patient does not require adjuvant treatment after surgery and discharge from hospital, a new assessment is made within a period of one month. If adjuvant chemotherapy and/or radiotherapy are required, the nutritional assessment follow-up is made on the first day of each chemotherapy cycle, as described previously.
If a negative result in the initial NST in patients with CRC and, in general, in the rest of patients with tumors of another type, the course of action differs depending on the treatment applied:
- •
If the patient is going to receive treatment with chemotherapy and/or radiotherapy, the screening test is performed again at the first visit to the Oncology/Day Hospital Unit, by any professional trained in clinical nutrition (nurse, nutritionist, dietitian, hospital pharmacist, oncologist) that has contact with the patient on the day of the treatment. In the event of obtaining a positive result, the patient should be sent to the nutrition unit. In this point the nutritional evaluation process starts, with subsequent nutritional intervention and close follow-up of the effectiveness of the intervention, as described above. When the patient is not in a nutritional risk situation, the appearance of warning signs (weight loss, lack of appetite) is monitored the first day of each chemotherapy cycle, to act immediately at the time of detection.
- •
When the patient requires surgical treatment or chemotherapy and/or radiotherapy after hospital discharge, the procedure is as described previously.
Even though the health professional considers nutritional support as a key element in the treatment of cancer patients, clinical malnutrition prevention and treatment practices vary considerably and, as a result, we continue to find a high prevalence of malnutrition in this population19,26.
The most recent recommendations on the nutritional care of cancer patients coincide in underlining the importance of detecting and acting promptly on those at nutritional risk, as one of the most effective measures to prevent the appearance of malnutrition and minimize the devastating impact this may have on the evolution of these patients17,19. In accordance with these recommendations, the proposed algorithm aims to address the nutritional care at the moment these patients are evaluated by the Tumor Committee, as a key starting point. The presence and participation of a health professional specialized in nutrition on this multidisciplinary committee is of vital importance for taking early action in those patients with a high nutritional risk, either by the type of tumor or by the associated cancer treatments.
The model establishes a difference right from the outset between the two groups of patients in accordance with their nutritional risk, maintaining a closer monitoring in those at greater risk in order to optimize the resources available, as Arends et al. suggest, and in line with the recommendations recently published by the ESPEN on nutritional care for cancer patients16,17. In the group of digestive tumors, CRC has not been excluded, even though by its location is considered as a low nutritional risk tumor. The decision is based on the fact that these patients tend to lose weight because of complications associated with cancer (stenosis, etc) and the nutritional impact of certain treatments (restrictive diets, extensive intestinal resection, adverse effects of chemotherapy on the gastrointestinal tract, etc), as well as the prevalence of sarcopenia in colorectal cancer (25-60%)27.
There are various nutritional screening tools validated. In the model we propose the use of NUTRISCORE as the method of nutritional screening. It is a quick, straightforward tool which offers a high level of sensitivity and specificity (97.3% and 95.9%, respectively). It has been validated in the Spanish population for the detection of risk of malnutrition in oncologic outpatients, using as a reference test the Patient-Generated Subjective Global Assessment (PG-SGA), showing sensitivity and specificity levels higher than the Malnutrition Screening Tool (MST)22.
The NUTRISCORE screening test detects the risk of malnutrition more specifically than other tests used in cancer patients, taking in consideration the nutritional impact of the treatment received and the location of the neoplasia16,26.
Nutritional interventions are often carried out not accompanied by the necessary re-assessments that allow the detection of inefficient therapies, problems of tolerance and even situations of low adherence to the treatment, which determine the efficacy of such intervention28. In patients with malnutrition or at nutritional risk, the time elapsed between the cycles of chemotherapy or consultations with the oncologist may result too long to assess the effectiveness of the nutritional intervention performed, since in the case of failure, the nutritional deterioration continues without we are aware of it until several weeks have passed. This is why the most important variable in this model is time. It has been seen that he early detection of malnutrition or the risk of having it and its correction, that may improve survival outcomes in esophageal cancer patients treated with chemoradiotherapy29.
Cancer cachexia gets worse with time and the longer we wait to address it, the harder will be to treat.
We propose closer follow-up intervals after performing a nutritional intervention, which allows early detection of treatment failure and, therefore, enables the nutritional care schedule to be modified in time to prevent nutritional deterioration. Silvers et al. shown the potential of a novel telephone-based early and intensive dietetic model of care for newly diagnosed upper gastrointestinal cancer patients30.
The close follow-up allows both the patient and the team of healthcare professionals to receive information periodically on his/her nutritional state, an aspect consider a key to any model of nutritional care in this population.
One of the highlight limitations was the lack of the validation. It would be desirable to pilot the operation of the algorithm before starting it in a generalized way.
From the NGT, agreements were reached to propose an algorithm of nutritional approach of the cancer patient. The adoption of this proposed algorithm will reduce the variability in institutional clinical practice, promoting common criteria for action to focus on the nutritional problems of these patients and that are one of the causes of deterioration of their quality of life, which could improve health outcomes.
FundingFresenius Kabi España, S. A. U.
Conflict of interestNo conflict of interests.
Contribution to the scientific literature
Hebuterne et al.2 underscored the need for systematic detection and treatment of malnutrition. Taking into account the evolutional nature of tumor-induced cachexia and the risk that the disease may become refractory, it is essential to act preemptively to detect patients eligible for nutritional therapy and get them treated as soon as possible. This study proposes a therapeutic model based on a proactive approach that follows an intervention protocol comprising early identification of patients at malnutrition risk, thorough patient evaluation, implementation of a nutritional therapy that is suited to their needs and in line with the recommendations established in the relevant clinical guidelines, and close monitoring of the efficacy of the nutritional program instituted.