To describe the effectiveness and safety of olaparib off-label indications in patients with impaired homologous recombination genes and solid tumors different than those authorized.
MethodsA single-center, observational and retrospective study including patients treated with olaparib for off-label use. The main variables were patient characteristics, prior therapies, response to therapy, progression-free survival, overall survival and adverse events.
ResultsA total of 6 patients were included. All patients had metastases and received 3 or more lines of prior treatment. The primary tumor locations and mutations were partner and localizer of BRCA2 (PALB2) intrahepatic cholangiocarcinoma, ataxia telangiectasia mutated (ATM) non-small cell lung adenocarcinoma, somatic breast cancer gene (sBRCA2) colorectal cancer, germinal breast cancer gene 2 (gBRCA2) breast neuroendocrine tumor, gBRCA2 ampullary cancer and gBRCA2 pancreatic neuroendocrine tumor. At the end of the study, one patient was still receiving olaparib showing more than 25 months of sustained stable disease response. No novel toxicities were observed besides those included in the product information.
ConclusionsThere is limited published evidence on the use of olaparib in patients harboring pathogenic variants other than breast cancer genes, like PALB2 and ATM and conditions different than those authorized such as digestive tract, neuroendocrine and lung tumors. Further research is to assess the efficacy of olaparib in these patients.
Describir la efectividad y seguridad de las indicaciones off-label de olaparib en pacientes con genes de recombinación homóloga alterados y tumores sólidos diferentes a los autorizados.
MétodoSe trata de un estudio unicéntrico, observacional y retrospectivo que incluye a pacientes tratados con olaparib para uso off-label. Las principales variables fueron las características de los pacientes, las terapias previas, la respuesta al tratamiento, la supervivencia libre de progresión, la supervivencia global y los efectos adversos.
ResultadosSe incluyeron un total de 6 pacientes. Todos los pacientes tenían metástasis y recibieron 3 o más líneas de tratamiento previo. Las localizaciones tumorales primarias y las mutaciones fueron colangiocarcinoma intrahepático mutado en partner and localizer of BRCA2 (PALB2), adenocarcinoma pulmonar de células no pequeñas mutado en ataxia telangiectasia (ATM), cáncer colorrectal con mutación somática en el breast cancer protein 2 (sBRCA2), tumor neuroendocrino de mama con mutación germinal en BRCA2 (gBRCA2), ampuloma con mutación gBRCA2 y tumor neuroendocrino pancreático gBRCA2. Al final del estudio, un paciente seguía recibiendo olaparib y presentaba más de 25 meses de respuesta estable sostenida a la enfermedad. No se observaron nuevas toxicidades además de las incluidas en la información del producto.
ConclusionesExisten pocos estudios publicados sobre el uso de olaparib en pacientes que albergan variantes patogénicas distintas del breast cancer gene, como PALB2 y ATM, y afecciones distintas de las autorizadas, como tracto digestivo, neuroendocrino y pulmón. Se necesitan más investigaciones para evaluar la eficacia de olaparib estos pacientes.
The deoxyribonucleic acid (DNA) damage repair (DDR) mechanisms include homologous recombination (HR), which repair double-strand damage. This process is regulated by genes which the most known are breast cancer gene 1 (BRCA1) and breast cancer gene 2 (BRCA2), but also there are other genes involved in HR-like ataxia telangiectasia mutated (ATM), partner and localizer of BRCA2 (PALB2), Fanconi anemia, complementation group A (FANCA), histone deacetylase 2 (HDAC2), checkpoint kinase 2 (CHEK2). If these HR-related genes (HRRg) are impaired, they can be detected and used as a predictor of response to certain therapies such as DNA cross-linking agents like cisplatin or carboplatin, which may kill cancer cells by damaging their DNA and stopping them from dividing. In addition, when HR is impaired, the Poly-adenosine diphosphate (ADP)-Ribose-polymerase (PARP) enzyme acts as an alternative repair mechanism. Therefore, when PARP is inhibited in vitro, it causes cell apoptosis conditioned to impaired HRRg.1 Olaparib is the first approved PARP inhibitor (PARPi) and currently has indication in somatic (s) and/or germline (g) BRCA1/2-mutated ovarian cancer, gBRCA1/2-mutated breast cancer, gBRCA1/2-mutated pancreatic cancer, somatic and/or gBRCA1/2-mutated prostate cancer and mismatch repair proficient endometrial cancer.2 Nevertheless, off-label indications were explored in different locations in a basket clinical trial (CT).3 Another study revealed the first use of olaparib in HR mutations other than BRCA such as PALB2 or ATM.4 Our objective is to describe the effectiveness and safety of olaparib off-label indications in patients with impaired HRRg and different solid tumors than those authorized.
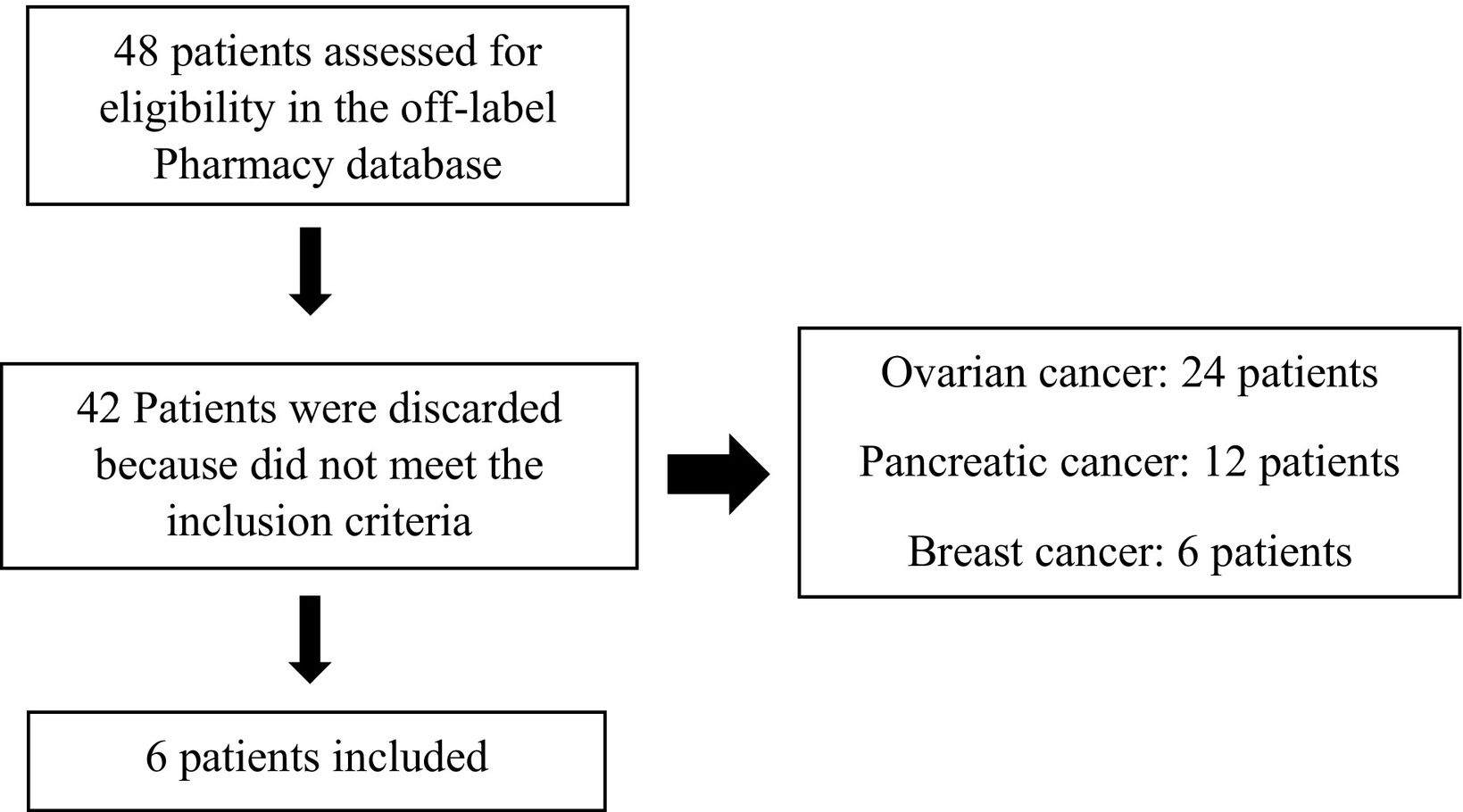
MethodsThe study was a single-center, observational and retrospective. The inclusion criteria were patients treated with olaparib as an off-label use for tumor sites different from ovarian, breast, prostate and pancreatic cancer. The exclusion criteria were patients evaluated for the treatment of ovarian, breast, prostate or pancreatic cancer, previously treated with PARPi in the context of CT, and pediatric patients. The usual process for the decision-making regarding off-label treatments consisted of a request sent by the doctor to the Pharmacy Department. The oncology pharmacists elaborated a report within the available scientific literature for the use of olaparib and the economic cost according to the expected duration of treatment for each patient. Afterward, each report was evaluated by a special drug use commission consisting of clinical pharmacologists, medical oncologists, internists, and pharmacists on behalf of the direction of the hospital. The treatment was approved according to the suitability of off-label olaparib therapy based on clinical factors such as tumor type, molecular profile, and prior treatment history. Even if the drug was indicated, the use of olaparib had to be assessed as an off-label by the special drug use committee when the indications did not have yet a positive opinion by the Catalan health authorities. This explains why so many patients did not meet the inclusion criteria in the Pharmacy database (Fig. 1).
The analysis period encompassed patients who initiated treatment with olaparib between June 2019 and April 2022, with follow-up data collected until October 2024. The variables reviewed for each patient were age, sex, primary tumor location, mutations, presence of metastases, tumor mutational burden (TMB), the Eastern Cooperative Oncology Group (ECOG) Performance Status, treatment history, time between last platinum and olaparib, best response to platinum, side effects, and laboratory abnormalities. The TMB was calculated as the number of detected and validated non-synonymous variants per megabase in the sample analyzed by next-generation sequencing targeted cancer gene panel. Internal validation of our Ilumina® test established a cutoff of ≥13 Muts/Mb as high TMB (equivalent to 10 Muts/Mb in the FoundationOne CDx® test). Imaging responses were assessed per response evaluation criteria in solid tumors (RECIST v.1.1) criteria.5 Progression-free survival (PFS) was defined as the time between the start of olaparib therapy and documented clinical and/or radiological evidence of disease progression. Overall survival (OS) was defined as the time between the start of olaparib therapy and the date of death. Adverse events (AEs) were assessed based on the Common Terminology Criteria for Adverse Events (CTCAE) v5.0.6 Data were obtained from the electronic medical record system (SAP®) and the outpatient-dispensing program (Silicon®). Qualitative variables were presented as proportions. Statistical analysis was performed using descriptive methods due to the small sample size and the exploratory nature of the study. The study adhered to the principles outlined in the Declaration of Helsinki. The study is approved by the Institution Research Ethics Committee from Hospital Universitari Vall d'Hebron by the code EOM (AG) 057/2024 (6338) with the exemption of informed consent due to the retrospective nature of the study.
ResultsAfter screening the requests for the off-label use of olaparib in our center, five females and one man were included. Forty-two patients were discarded due to their failure to meet the inclusion criteria, as they had tumors within the therapeutic indications, such as ovarian, breast, or pancreatic cancers, and therefore were not subjected to analysis. None of the patients were excluded. Patients were treated with olaparib based on evidence of at least one mutated HRRg and/or due to a lack of standard treatment options for heavily pretreated patients, with ≥3 lines of prior treatment.
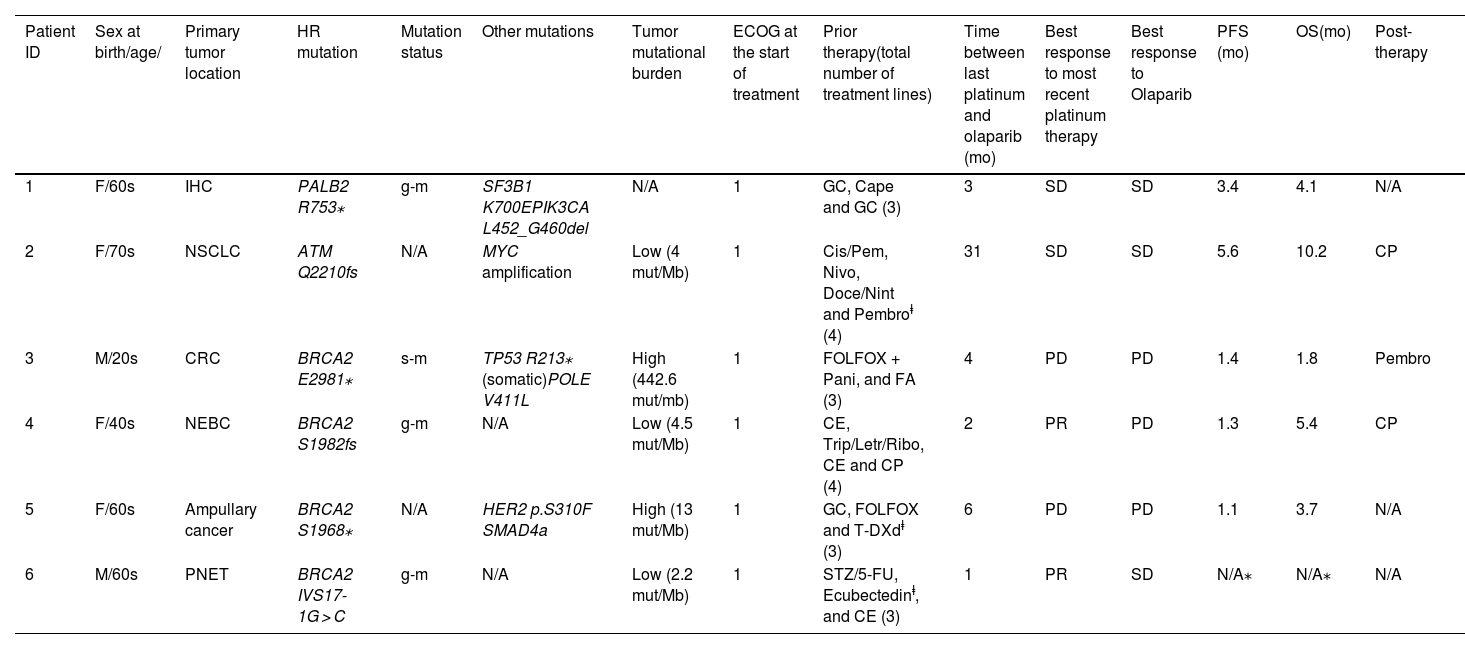
The primary tumor locations and mutations were intrahepatic cholangiocarcinoma with gPALB2 pathogenic variant (PV) (patient 1), non-small cell lung adenocarcinoma with ATM PV (patient 2), epidermal growth factor receptor (EGFR) wild-type v-raf murine sarcoma viral oncogene homolog B1 (BRAF) wild-type colorectal cancer with sBRCA2 PV (patient 3), breast neuroendocrine tumor with gBRCA2 PV (patient 4), BRCA2 PV ampullary cancer (patient 5) and pancreatic neuroendocrine tumor with gBRCA2 PV (patient 6). Most of the HRR genes were germline-mutated except for one patient being somatic-mutated and another that was not determined. Two of six patients were analyzed by the FoundationOne CDx® test while 3/6 patients were analyzed by Ilumina® assay. Two of them had high TMB while the other three had low. Homologous recombination deficiency (HRD) was not detected, except for patient 4 which was negative for radiation sensitive protein 51 (RAD51) foci test.
All patients had metastases and received ≥3 lines of prior treatment. Three patients received treatment in the context of clinical trials prior to olaparib. Most of the patients started treatment with an initial posology of tablets 300 mg/12 h (5/6) while one patient started with capsules 400 mg/12 h. Responses to the latest line of platin-based chemotherapy were as follows: partial response (PR) 2/6 stable disease (SD) 2/6 and progressive disease (PD) 2/6. Regarding olaparib therapy, 3 patients had SD as best overall response (BOR) while the rest 3 patients had PD. Among the patients who did not respond to olaparib treatment, 2/3 (patients 3 and 5) had previously progressed to platinum therapy while 1/3 (patient 4) had responded partially to prior platinum regimen. At data cutoff, one patient was still receiving olaparib with >25 months of sustained SD response. More detail is available in Table 1.
Patient characteristics.
| Patient ID | Sex at birth/age/ | Primary tumor location | HR mutation | Mutation status | Other mutations | Tumor mutational burden | ECOG at the start of treatment | Prior therapy(total number of treatment lines) | Time between last platinum and olaparib (mo) | Best response to most recent platinum therapy | Best response to Olaparib | PFS (mo) | OS(mo) | Post-therapy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F/60s | IHC | PALB2 R753⁎ | g-m | SF3B1 K700EPIK3CA L452_G460del | N/A | 1 | GC, Cape and GC (3) | 3 | SD | SD | 3.4 | 4.1 | N/A |
| 2 | F/70s | NSCLC | ATM Q2210fs | N/A | MYC amplification | Low (4 mut/Mb) | 1 | Cis/Pem, Nivo, Doce/Nint and Pembroⱡ (4) | 31 | SD | SD | 5.6 | 10.2 | CP |
| 3 | M/20s | CRC | BRCA2 E2981⁎ | s-m | TP53 R213⁎ (somatic)POLE V411L | High (442.6 mut/mb) | 1 | FOLFOX + Pani, and FA (3) | 4 | PD | PD | 1.4 | 1.8 | Pembro |
| 4 | F/40s | NEBC | BRCA2 S1982fs | g-m | N/A | Low (4.5 mut/Mb) | 1 | CE, Trip/Letr/Ribo, CE and CP (4) | 2 | PR | PD | 1.3 | 5.4 | CP |
| 5 | F/60s | Ampullary cancer | BRCA2 S1968⁎ | N/A | HER2 p.S310F SMAD4a | High (13 mut/Mb) | 1 | GC, FOLFOX and T-DXdⱡ (3) | 6 | PD | PD | 1.1 | 3.7 | N/A |
| 6 | M/60s | PNET | BRCA2 IVS17-1G > C | g-m | N/A | Low (2.2 mut/Mb) | 1 | STZ/5-FU, Ecubectedinⱡ, and CE (3) | 1 | PR | SD | N/A⁎ | N/A⁎ | N/A |
Abreviations: ID: identification; mo: months; ECOG: eastern cooperative oncology group; PFS: progression-free survival; OS: overall survival; F: female; M: male; IHC: intrahepatic cholangiocarcinoma; NSCLC: non-small cell lung cancer; CRC: colorectal cancer; NEBC: neuroendocrine cancer of the breast; PNET: pancreatic neuroendocrine tumor; HR: homologous recombination; PALB2: partner and localizer of BRCA2; ATM: ataxia telangiectasia mutated; BRCA2: breast cancer protein 2; g-m: germline-mutated; s-m: somatic-mutated; N/A: not aplicable; SF3B1: splicing factor 3b subunit 1; PIK3CA: phosphatidylinositol-3-kinase catalytic subunit p110α gene; MYC: myelocytomatosis oncogene; TP53: tumor protein p53; POLE: DNA polymerase epsilon catalytic subunit; HER2: human epidermal growth factor receptor 2; SMAD4a: sterile alpha motif domain containing 4A; GC: gemcitabine/cisplatin; Cape: capecitabine; Cis/Pem: cisplatin/pemetrexed; Nivo: nivolumab; Doce/nint: docetaxel/nintedanib; FOLFOX: fluorouracil/calcium folinate/oxaliplatin; Pani: panitumumab; FA: FOLFIRI/aflibercept; Trip/Letr/Ribo: triptorelin/letrozole/ribociclib; CP: carboplatin/paclitaxel; T-DXd: trastuzumab-deruxtecan; CE: carboplatin/etoposide; SD: stable disease; PD: progressive disease; PR: partial response; pembro: pembrolizumab; STZ/5-FU: streptozocin/5-fluoruracil.
AEs of any grade were reported in 4/6 patients being none of them G3 or more. The AEs reported were asthenia G1 (2/6) and G2 (1/6); anemia G1 (1/6) and G2 (1/6), nausea and vomiting G1 (1/6) and G2 (1/6), mucositis G2 (1/6); a dose interruption was required due to adverse effects and a temporary dose reduction to 300 mg/12 h for 6 days and 1 day off per week.
DiscussionWe report the outcomes of the use of olaparib in uncommon tumor locations and/or mutations. In general, the lack of response to previous platinum therapy led to worse results in patients 3 and 5.
Tumors with HR deficiencies, such as BRCA mutations, often show sensitivity to both platinum-based therapies and PARPi, but secondary mutations restoring BRCA1/2 can lead to resistance.7,8 Despite this, some platinum-resistant patients still respond to PARPi, highlighting the complexity of the relationship between platinum sensitivity and PARPi efficacy.9,10
In 2015, the Kauffman et al. basket study assessed the use of olaparib in patients with germline BRCA1/2 mutation in different locations. Most of the patients were part of the ovarian, breast, pancreatic or prostate cancer group but there was also an “others group” of 12 patients which had tumors of the biliary tract (4), lung (3), bladder (2), colorectum (1), esophagus (1) and uterus (1). This group presented SD in 58.3% of patients that persisted >8 weeks.3 In the same year, Mateo et al. evaluated the use of olaparib in metastatic prostate cancer with DDR defects analyzing additional mutations than BRCA. Of 49 patients evaluated, 16 had a response having 88% of them HRRg mutations in BRCA, ATM, PALB2, FANCA and HDAC2.4
PARP inhibition might be active in cancers with other HRRg mutations or cancer types than currently documented or approved. Thanks to our large CT program, we were able to do gene screening panel in patients with a lack of treatment options so that we can detect PV in HR-related genes that may be targetable both in the context of CT or off-label use. The European Medicines Agency (EMA) approval for olaparib includes ovarian, breast, prostate, pancreatic and endometrial cancer.2 No novel safety signals were observed besides those included in the product information.
In a study of 13,599 patients, cholangiocarcinoma was the most common tumor with PALB2 alterations,11 and several cases with BRCA mutations showed responses to PARPi. These include partial responses and long-term remissions, suggesting that patients with BRCA2 mutations may benefit from PARPi therapy, particularly in combination with treatments like pembrolizumab.12–16 To date, this is the first report about olaparib treatment in PALB2 mutated intrahepatic cholangiocarcinoma.
PARPi have shown mixed clinical results in ATM-mutated tumors.17 While studies like Profound18 demonstrated the benefits of olaparib in prostate cancer with ATM, BRCA1, or BRCA2 mutations, other trials, such as JAVELIN BRCA/ATM,19 reported poor outcomes in ATM-mutated tumors, leading to early termination due to low response rates.20,21
The poor response of patient 3 to treatment may be due to a somatic tumor protein p53 (TP53) mutation, which is associated with resistance to PARPi, and a DNA polymerase epsilon catalytic subunit (POLE) alteration leading to high tumor burden.22TP53 mutations result in RAD51 overexpression, reducing PARPi sensitivity by enabling alternative DNA repair pathways.23 Although POLE alterations typically enhance sensitivity to immunotherapy,24 the patient did not respond to pembrolizumab, likely due to the advanced disease stage.
Regarding ampullary cancer cohort, 18% of patients harbor pathogenic mutations in BRCA2, ATM, RAD50, and mutY DNA glycosylase.25 There is no insight into PARPi treatment outcome in these tumors. A CT which evaluated rucaparib in tumors with HRD including ampullary cancer was terminated due to a change in development priorities.26
Consisting with our outstanding response in patient 6, there is a recent case in the literature of 17-month response to olaparib maintenance therapy in a PNET gBRCA2 (Ki67 62%) patient after receiving immunotherapy combined with platin-based chemotherapy.27 Another patient exhibited partial response for 5 months in a cyclin-dependent kinase 12 mutated metastatic lung neuroendocrine tumor after treatment with olaparib in combination with paclitaxel as third-line therapy.
The limitations of the study are those of an individual, observational and retrospective study. On the other hand, it is a small sample of 6 patients with heterogeneous characteristics both in terms of tumor location and the mutation involved. All patients had metastatic disease and had received ≥3 lines of prior treatment. This could affect the olaparib response, as heavily pretreated patients often have more resistant diseases. Furthermore, only 2/6 patients had partial response to prior platinum therapy, although, in the scientific literature there are cases in which the response to PARPi treatment is independent of prior response to platinum, this is not the general trend.
There is a lack of evidence on the use of olaparib and PARPi in mutations different than BRCA like PALB2 and ATM and in conditions different than those authorized such as digestive tract, neuroendocrine and lung tumors. Finally, we have described the off-label use of olaparib in 6 patients with HR-related genes in uncommon tumor locations. Safety was adequate and similar to previous studies. Further studies are needed to assess the efficacy and safety of olaparib in patients with mutated HRRg in new tumor sites.
FundingThere was no funding for this work. Treatment and tests for the patients were covered by the Catalan Health Service.
Previous presentationsPart of this work has been previously presented as an oral communication in the International Symposium of Oncology Pharmacy Practitioners in March 2023, Seville, Spain.
Ethical approval and informed consentThe investigation was conducted in accordance with the principles of the Declaration of Helsinki. The study is approved by the Institution Research Ethics Committee from HUVH by the code EOM (AG)057/2024(6338) with the exemption of informed consent due to the retrospective nature of the study.
Contribution to the scientific literatureThis study explores olaparib use in tumors with HRRg mutations beyond BRCA variants and authorized tumor sites.
The results may help medical oncologists and clinical pharmacists to assess olaparib use in these patients, considering platinum response, mutations, or prior treatments.
CRediT authorship contribution statementHéctor Carlos García-Díaz: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Conceptualization. María Larrosa-Garcia: Validation, Investigation, Conceptualization. Javier Gómez-Alonso: Validation, Investigation. Mara Cruellas: Validation, Supervision. Enriqueta Felip: Validation, Supervision. Teresa Macarulla: Validation, Supervision. Anna Farriols: Validation, Supervision. Maria J. Carreras: Validation, Supervision, Investigation, Conceptualization.