To promote the transformation of hospital pharmacy in the field of rare diseases, the OrPhar-SEFH Strategic Plan 2024–2027 was developed. Twelve hospital pharmacists from the OrPhar-SEFH group participated in the project which was developed in 3 phases: Situation analysis, trends, and current challenges in rare diseases and their impact on the role of hospital pharmacist through literature review, interviews with hospital pharmacists and patients (FundAME and Fedhemo), and SWOT analysis; Definition of lines of action grouped into five strategic axes AEIOU (Alliances, Evidence, Research/Innovation, Optimization, and Union); and Prioritization of lines of action based on impact and feasibility. In order to achieve this, the existing trends and barriers in the field of rare diseases and hospital pharmacy were identified and classified around 4 levers for change: transformation of the care process, patient experience, evaluation and access to orphan drugs, and real-world evidence. The SWOT analysis identified key success factors, and interviews with patients identified their needs in four pillars: role of the hospital pharmacist, patient experience, patient education, and access to treatment. A total of 23 lines of action were agreed upon: Alliances (6), Evidence (3), Research/Innovation (4), Optimization (7), and Union (3). Based on their impact and feasibility, the following were prioritized: promoting hospital pharmacist collaboration in decision-making structures regarding the evaluation and positioning of orphan drugs, enhancing the role of hospital pharmacist in the critical analysis of scientific evidence in rare diseases and its translation to interdisciplinary clinical teams, encouraging the use of Multi-Criteria Decision Analysis methodology in orphan drugs, promoting the integration of hospital pharmacist into interdisciplinary teams managing patients with rare diseases, and creating a network of specialized hospital pharmacist services in rare diseases. Ultimately, the OrPhar-SEFH Strategic Plan 2024–2027 defines the roadmap to enhance the role of hospital pharmacist in addressing rare diseases, improving access and evaluation of orphan drugs, and optimizing the care process to improve health outcomes, quality of life, and patient experience.
Con el objetivo de impulsar la transformación de la farmacia hospitalaria en el ámbito de las enfermedades raras, se llevó a cabo el desarrollo del Plan Estratégico OrPhar-SEFH 2024–2027. Participaron en el proyecto los 12 farmacéuticos de hospital del grupo OrPhar-SEFH y se desarrolló en 3 fases: Análisis de situación, tendencias y retos actuales en enfermedades raras y su impacto en el rol del farmacéutico de hospital mediante búsqueda bibliográfica, entrevistas a farmacéuticos de hospital y pacientes (FundAME y Fedhemo) y realización de análisis DAFO; Definición de líneas de acción agrupadas en 5 ejes estratégicos AEIOU (Alianzas, Evidencia, Investigación/Innovación, Optimización y Unión); y Priorización de líneas de acción según impacto y factibilidad. En la consecución de este, se identificaron las tendencias y barreras existentes en el ámbito de las enfermedades raras y la farmacia hospitalaria y se clasificaron en torno a 4 palancas de cambio: trasformación del proceso asistencial, experiencia del paciente, evaluación y acceso de medicamentos huérfanos y evidencia en vida real. El análisis DAFO identificó los factores claves de éxito y las entrevistas con pacientes identificaron sus necesidades en 4 pilares: rol del farmacéutico de hospital, experiencia del paciente, educación del paciente y acceso al tratamiento. Se consensuaron un total de 23 líneas de acción: Alianzas (6), Evidencia (3), Investigación/Innovación (4), Optimización (7) y Unión (3). Según su impacto y factibilidad se priorizaron: fomentar la colaboración del farmacéutico de hospital en las estructuras de decisión sobre evaluación y posicionamiento de medicamentos huérfanos, potenciar el papel del farmacéutico de hospital en el análisis crítico de la evidencia científica en enfermedades raras y su traslación a los equipos clínicos interdisciplinares, fomentar el uso de la metodología de análisis de decisión multicriterio en medicamentos huérfanos, impulsar la integración del farmacéutico de hospital en los equipos interdisciplinares de manejo de pacientes con enfermedades raras y crear una red de servicios de farmacéuticos de hospital especializados en enfermedades raras. En definitiva, el Plan Estratégico de OrPhar-SEFH 2024–2027 define la hoja de ruta para potenciar el papel del farmacéutico de hospital en el abordaje de enfermedades raras, mejorando el acceso y evaluación de medicamentos huérfanos y optimizando el proceso asistencial para mejorar los resultados en salud, la calidad de vida y la experiencia de los pacientes.
The care of patients with rare diseases (RDs) or minority diseases is an ongoing challenge for health systems, due to their special characteristics (clinical complexity and care, social and financial costs). In Europe, RDs have been defined as those with a prevalence of less than 5 cases per 10,000 inhabitants.1 Currently, more than 7000 RDs have been identified. These are generally characterised as inherited diseases that often manifest in childhood, are chronic and degenerative, and cause high morbidity, mortality, and pronounced disability. They significantly affect the quality of life of patients and their families.2,3
It is estimated that RDs affect between 6% and 8% of the European population (i.e. between 24 and 36 million people, 3 million of whom live in Spain). A report by the Gaspar Casal Foundation entitled “Las Enfermedades Raras en España: estado de situación y oportunidades de transformación en el nuevo contexto digital” estimated that around 12 million people in Spain live with RDs, either because they or a family member has one.4
The approach to RDs is highly complex regarding aetiology, diagnosis, prognosis, and treatment. Patients usually wait an average of 4 years for a correct diagnosis, and in 20% of cases, diagnosis is delayed by 10 years or more.5 To address these diseases from a holistic and multidimensional perspective,5 patient follow-up generally requires multidisciplinary care, the use of supportive therapies, medical devices, support services (rehabilitation, mental health), and educational and social resources.5
Therapeutic innovation in the field of RDs is also a major challenge. Medicines developed to address unmet medical needs in RDs, known as orphan drugs (ODs), are often supported by limited scientific evidence of efficacy and safety at the time of marketing. The clinical trials conducted often have significant methodological limitations, including small and heterogeneous patient populations, a lack of valid therapeutic comparators, reliance on surrogate endpoints to assess clinical efficacy, and short follow-up periods. As a result, there are many uncertainties in decision-making that affect both access to and the funding of drugs and their use in clinical practice. In addition, the high cost of such medicines—due to the complexity of their development, often involving advanced therapies, and the small target population for which they are intended—is having a growing economic impact on healthcare systems that impacts their sustainability. In 2021, expenditure on ODs was approximately €1 billion, representing 5.5% of total expenditure on medicines in the Spanish health system.6
In this context, the OrPhar-SEFH group was established in recognition of the many challenges that the management of ODs and RDs poses to health professionals, the health system, patients, and society as a whole. This group is part of the Spanish Society of Hospital Pharmacy (SEFH) and comprises hospital pharmacists (HPs) specialising in RDs. Its mission is to help improve the management and treatment of RDs by promoting the active involvement of HPs, thereby adding value to the entire patient care process and aiming to improve both health outcomes and the overall experience of patients with RDs.
Since its creation, the OrPhar-SEFH group has led innovative projects with a patient-centred approach that have contributed significantly to progress in the field of RDs. These include the biannual dissemination of Horizon Scanning Reports on ODs (available on the group's website7), the application of multi-criteria decision analysis (MCDA) to the evaluation of ODs,8 the development of the Manual of Good Practices for the Humanisation of Pharmacy Services in the Care of Patients with RDs,9 and the publication of a Monograph on Hospital Pharmacy for Patients with RDs.10
The working group has recently developed its OrPhar-SEFH Strategic Plan 2024–2027 with the aim of continuing to drive the transformation of hospital pharmacy in the field of RDs. In line with the group's mission and vision, the plan aims to generate knowledge, strengthen the relationship between healthcare professionals and patients, and position itself as a leader in the treatment of RDs, all within a patient-centred approach.
MethodsThe OrPhar-SEFH 2024–2027 Strategic Plan was designed and drafted between March and September 2023 by the 12 HPs that formed the OrPhar-SEFH working group at that time, with the methodological support of a consultancy firm. A small coordinating group of 3 HPs was set up to organise the tasks through paired interviews, online group work sessions for deliberation and consensus, and individual exercises for reflection and prioritisation.
The project was developed in 3 stages: assessment of the state of the art in the management of patients with RDs by HPs; definition of strategic axes and lines of action; and prioritisation of lines of action.
The first stage analysed the current situation, trends, challenges, and strategies in the field of RDs and their impact on the role of HPs, both nationally and internationally. To this end, a literature search was conducted in Pubmed and on the websites of organisations related to RDs and hospital pharmacy,2,3,11,12 along with benchmarking the role of HPs in similar international or national organisations. The information obtained was complemented by the experience of HPs of the OrPhar-SEFH group through a round of paired interviews, as well as by the inclusion of the patient perspective via interviews with representatives of 2 patient associations—the Fundación Atrofia Muscular Espinal and the Federación Española de Hemofilia—addressing aspects related to diagnosis, treatment, patient education, follow-up, and quality of life. The analysis and structuring of the data collected enabled the development of a SWOT matrix, the determination of key success factors in the areas of impact, needs, actions, and infrastructure and organisation, as well as the identification of barriers and trends, which were categorised into 4 strategic levers: transformation of the care process; patient experience; evaluation and access to drugs; and real-world data (RWD).
The second stage defined the lines of action of the Strategic Plan around 5 strategic axes—Alliances, Evidence, Innovation/Research, Optimisation, and Union (AEIOU)—in line with the SEFH Strategic Plan.12 The preliminary proposal drawn up by the coordinating group was discussed and agreed with the whole OrPhar-SEFH group during an online working session. For each line of action, an objective was defined along with indicators, professional profiles involved, required resources for implementation, main obstacles, and an action plan.
The third and final stage involved a prioritisation exercise that considered all the strategic lines within each axis to determine the most relevant and feasible actions to advance the identified strategic objectives. Prioritisation was based on 2 variables—the impact on managing RDs and ODs, and the feasibility of implementation—while taking into account the current situation of the OrPhar-SEFH group and the various professional profiles involved in the development of the lines of action. Thus, each member of the group assigned a score (1–10) to each line of action regarding its impact and feasibility. A score of 10 was reserved for actions that had the greatest impact and were the easiest to implement in terms of resources, time, and feasibility.
On the basis of all the information gathered, several documents were produced and are available on the OrPhar-SEFH group website: the final version of the OrPhar-SEFH Strategic Plan 2024–2027,13 an executive summary of the same,14 and an infographic highlighting the main data to facilitate its dissemination.15
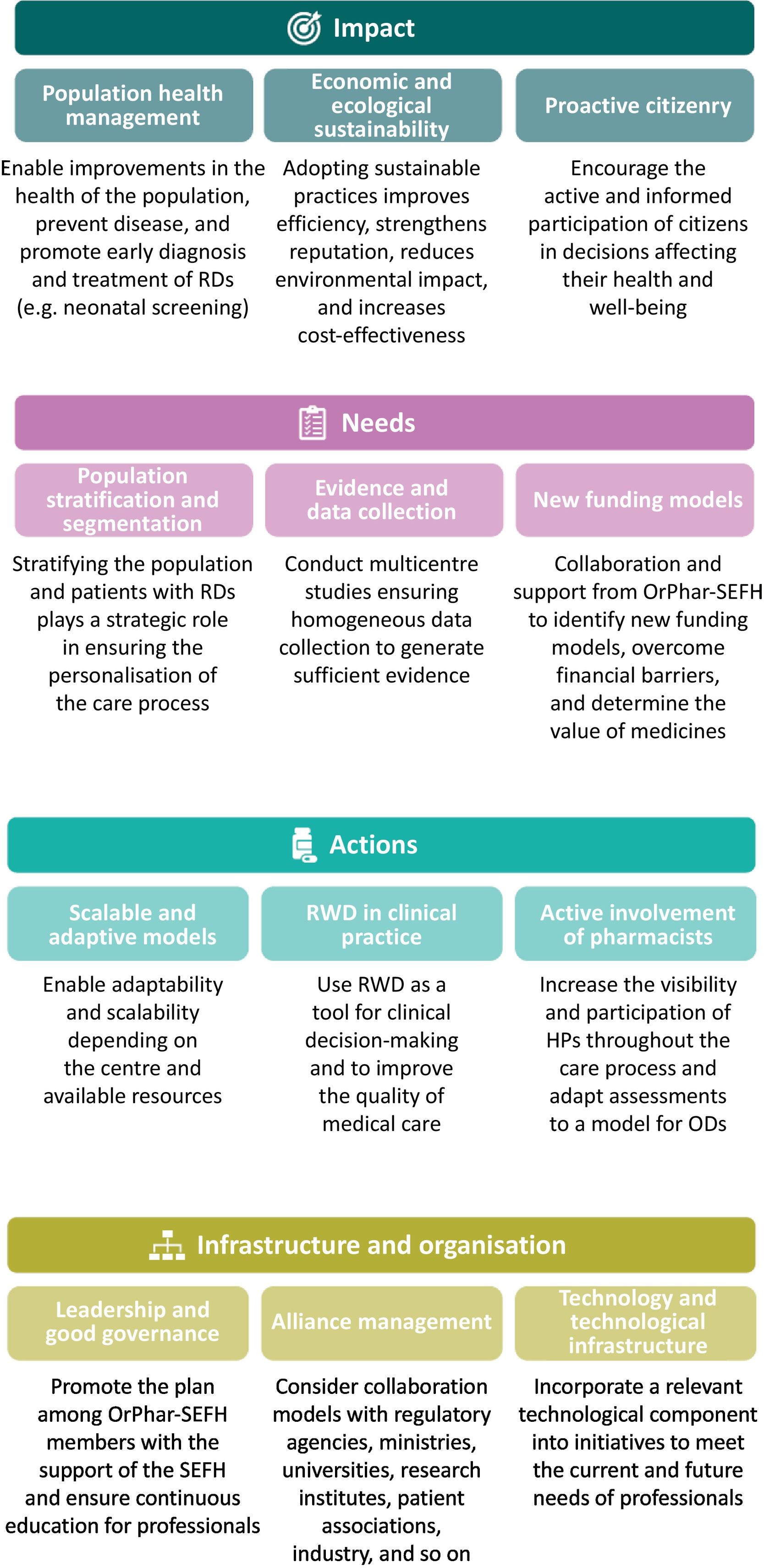
ResultsIn stage 1, the situation and context analysis at both national and international levels identified trends and barriers in the field of RDs and hospital pharmacy, which were categorised into 4 levers of change (Table 1). This stage included a SWOT analysis, thereby identifying strengths, weaknesses, opportunities, and threats (Table 2); the key success factors were divided into the areas of impact, needs, actions, and infrastructure and organisation (Fig. 1).
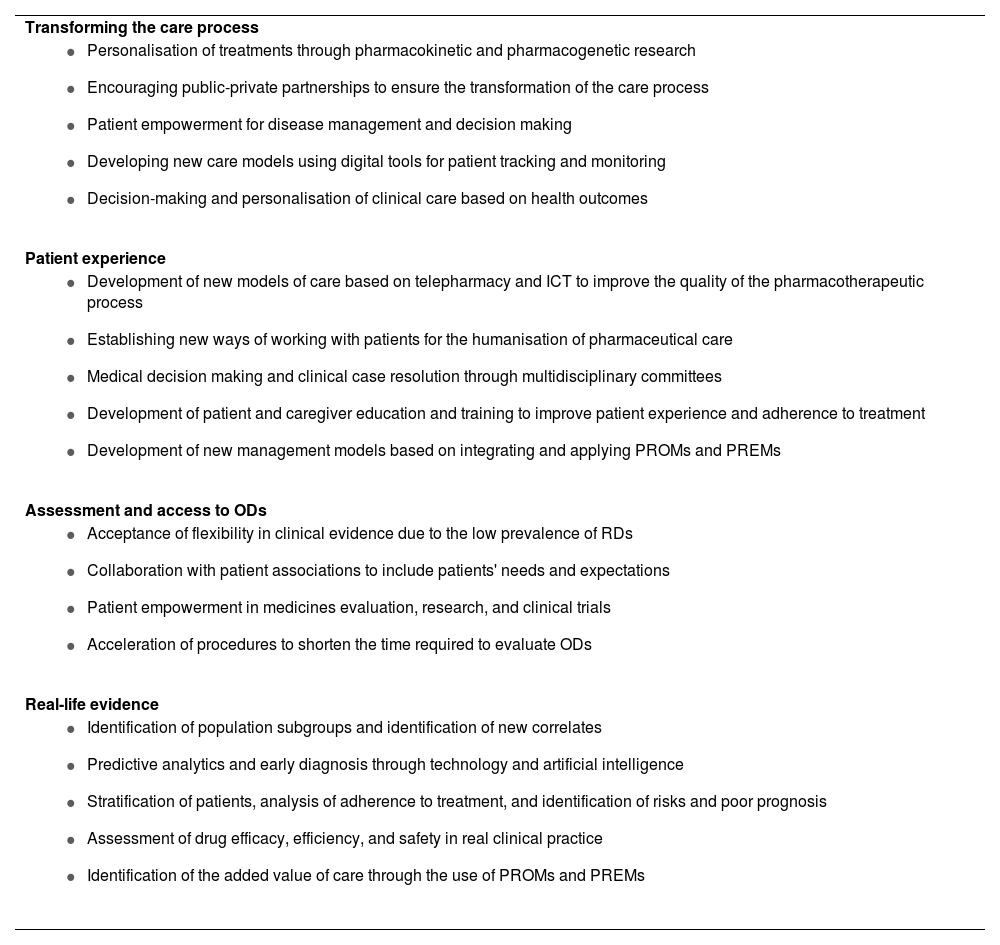
Trends identified in the field of rare diseases and hospital pharmacy.
| Transforming the care process |
|
| Patient experience |
|
| Assessment and access to ODs |
|
| Real-life evidence |
|
RDs, rare diseases; ODs, orphan drugs; PROM, patient-reported outcome measures; PREM, patient-reported experience measures; ICT, information and communication technologies.
SWOT matrix.
| Weaknesses |
|
| Threats |
|
| Strengths |
|
| Opportunities |
|
ACs, autonomous communities; RDs, rare diseases; SWOT, strengths, weaknesses, opportunities, threats; ODs, orphan drugs; Orphar-SEFH, Rare Diseases and Orphan Drugs Group of the Spanish Society of Hospital Pharmacy; PROM, patient-reported outcome measure; PREM, patient-reported experience measure; RWD: real-world data; SEFH, Spanish Society of Hospital Pharmacists; ICT: information and communication technology.
Key success factors of the OrPhar-SEFH Strategic Plan 2024–2027.
RDs, rare diseases; HP, hospital pharmacist; ODs, orphan drugs; Orphar-SEFH, Rare Diseases and Orphan Drugs Group of the Spanish Society of Hospital Pharmacy; PROM, patient-reported outcome measure; PREM, patient-reported experience measure, RWD, real-world data.
From the outset, the Strategic Plan was designed to incorporate the voices of patients in relation to their current experiences, the difficulties they face in their daily lives, and their expectations of the role of HPs in the management of RDs. Patient needs were grouped into 4 main areas:
The role of HPs and pharmacy services, patient experience, patient education, and access to treatment (see Table 3).
Summary of patient needs in relation to hospital pharmacists, obtained through interviews with representatives of rare disease patient associations.
| Role of hospital pharmacists and pharmacy services |
|
| Patient experience |
|
| Patient education |
|
| Access to treatment |
|
ACs, autonomous communities; RD, rare disease; HP, hospital pharmacist; OD, orphan drug; PROM, patient-reported outcome measure; PREM, patient-reported experience measures.
Stage 2 defined the 23 lines of action of the Strategic Plan grouped around the 5 AEIOU strategic axes mentioned above. For each line of action, we defined an objective, indicators to evaluate implementation, the profiles of involved staff, necessary resources, obstacles to overcome, and an action plan. The goal was to implement the proposed lines of action, thereby laying the foundations for a new approach to RDs within the field of hospital pharmacy. Detailed information on the 23 lines of action can be consulted in the full Strategic Plan document available on the OrPhar-SEFH group website.13
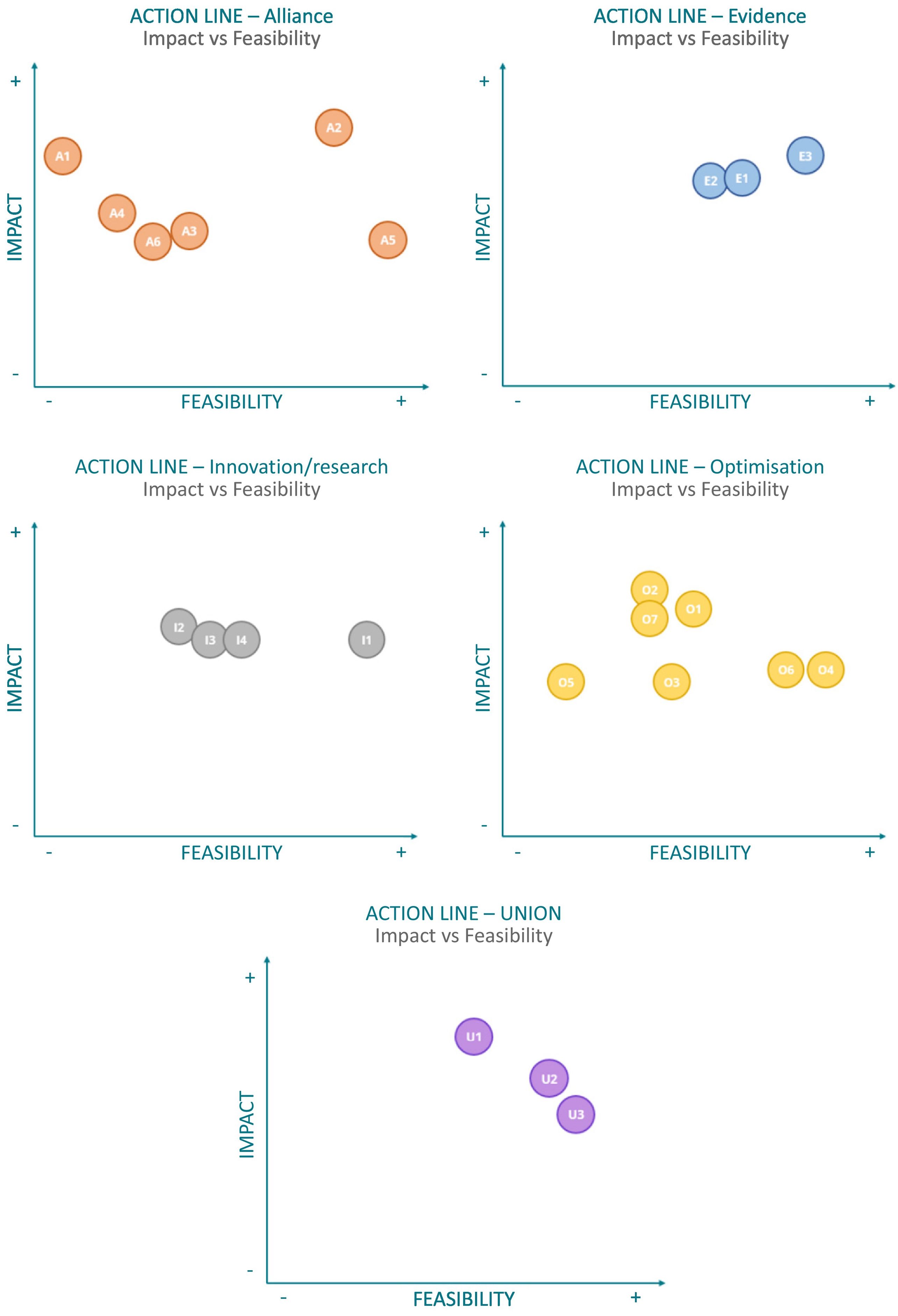
In Stage 3, the lines of action of the Strategic Plan were prioritised based on their impact on addressing RDs and their feasibility of implementation. Table 4 shows the prioritisation by each strategic axis, and Fig. 2 shows the scores for the 23 lines of action based on their impact and feasibility.
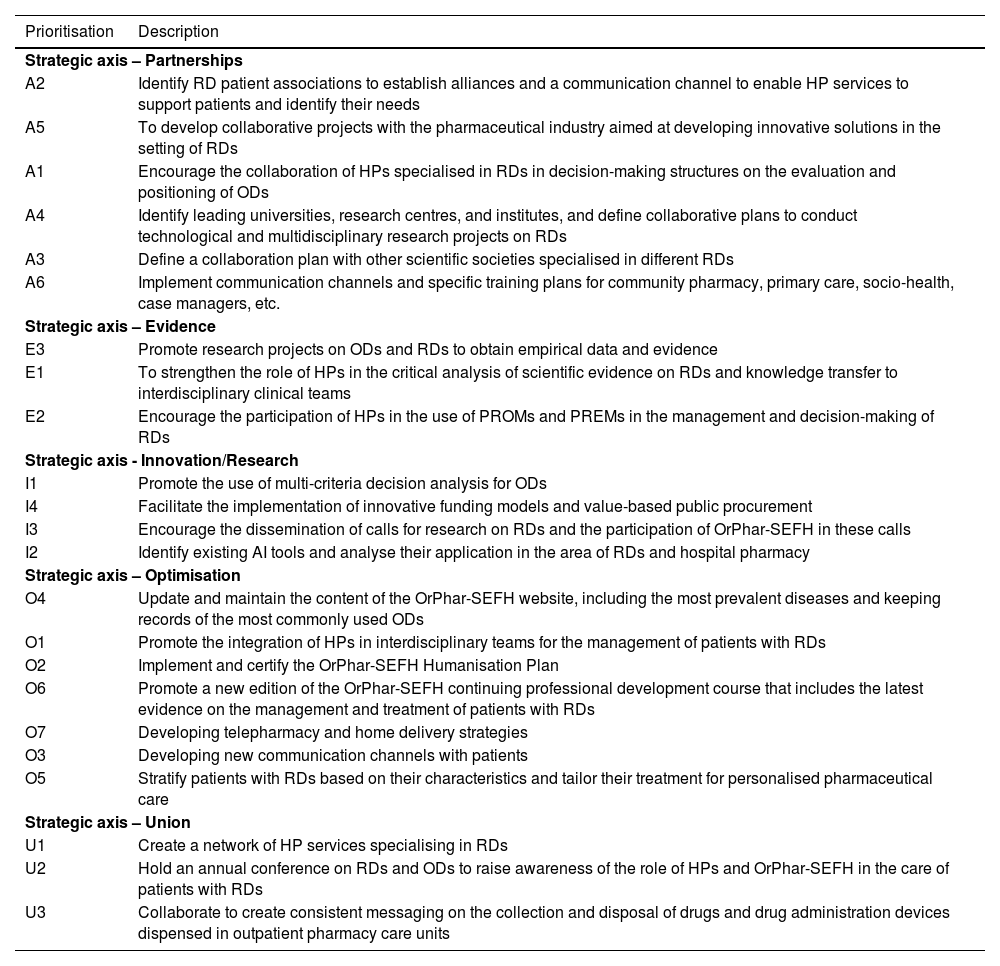
Prioritisation of the 23 lines of action by strategic axis.
| Prioritisation | Description |
|---|---|
| Strategic axis – Partnerships | |
| A2 | Identify RD patient associations to establish alliances and a communication channel to enable HP services to support patients and identify their needs |
| A5 | To develop collaborative projects with the pharmaceutical industry aimed at developing innovative solutions in the setting of RDs |
| A1 | Encourage the collaboration of HPs specialised in RDs in decision-making structures on the evaluation and positioning of ODs |
| A4 | Identify leading universities, research centres, and institutes, and define collaborative plans to conduct technological and multidisciplinary research projects on RDs |
| A3 | Define a collaboration plan with other scientific societies specialised in different RDs |
| A6 | Implement communication channels and specific training plans for community pharmacy, primary care, socio-health, case managers, etc. |
| Strategic axis – Evidence | |
| E3 | Promote research projects on ODs and RDs to obtain empirical data and evidence |
| E1 | To strengthen the role of HPs in the critical analysis of scientific evidence on RDs and knowledge transfer to interdisciplinary clinical teams |
| E2 | Encourage the participation of HPs in the use of PROMs and PREMs in the management and decision-making of RDs |
| Strategic axis - Innovation/Research | |
| I1 | Promote the use of multi-criteria decision analysis for ODs |
| I4 | Facilitate the implementation of innovative funding models and value-based public procurement |
| I3 | Encourage the dissemination of calls for research on RDs and the participation of OrPhar-SEFH in these calls |
| I2 | Identify existing AI tools and analyse their application in the area of RDs and hospital pharmacy |
| Strategic axis – Optimisation | |
| O4 | Update and maintain the content of the OrPhar-SEFH website, including the most prevalent diseases and keeping records of the most commonly used ODs |
| O1 | Promote the integration of HPs in interdisciplinary teams for the management of patients with RDs |
| O2 | Implement and certify the OrPhar-SEFH Humanisation Plan |
| O6 | Promote a new edition of the OrPhar-SEFH continuing professional development course that includes the latest evidence on the management and treatment of patients with RDs |
| O7 | Developing telepharmacy and home delivery strategies |
| O3 | Developing new communication channels with patients |
| O5 | Stratify patients with RDs based on their characteristics and tailor their treatment for personalised pharmaceutical care |
| Strategic axis – Union | |
| U1 | Create a network of HP services specialising in RDs |
| U2 | Hold an annual conference on RDs and ODs to raise awareness of the role of HPs and OrPhar-SEFH in the care of patients with RDs |
| U3 | Collaborate to create consistent messaging on the collection and disposal of drugs and drug administration devices dispensed in outpatient pharmacy care units |
RD, rare disease; HP, hospital pharmacist; OD, orphan drug; Orphar-SEFH, Rare Diseases and Orphan Drugs Group of the Spanish Society of Hospital Pharmacy; PROM, patient-reported outcome measure; PREM, patient-reported experience measure.
The OrPhar-SEFH Strategic Plan 2024–2027 provides a comprehensive and innovative vision for the approach to RDs. It has been developed with a scientific and strategic focus, applying a participatory working methodology that includes the perspective of professionals and patients. Thus, this plan defines and prioritises a total of 23 lines of action to be implemented by the working group over the period 2024–2027. It will strengthen the role of HPs in the care of patients with RDs, with the aim of addressing the challenges posed by ODs and RDs for the Spanish health system.
The analysis of the current situation, trends, challenges, and existing strategies in the management of RDs, both nationally and internationally, reveals a healthcare model showing signs of exhaustion and inefficiency, as well as a healthcare ecosystem that is constantly facing new challenges and including more stakeholders involved in the approach to RDs.16 We are clearly in a key transitional period driven by many influences, including the post-COVID setting, regulatory pressures, economic instability, the search for system sustainability and efficiency, digital transformation, the need to listen to patients, and the development of innovative and transformative models of engagement and collaboration.17
For these reasons, the European Union (EU) has established a strategic aim to improve access to diagnosis, information, and healthcare for the approximately 36 million patients with RDs in Europe. It will promote the development of national RD plans and strategies, the establishment of European Reference Networks (ERNs), facilitate the definition, coding, and inventory of RDs (European Rare Disease Registry Platform, Orphanet), promote the designation and authorisation of ODs, support research and development projects (Horizon Europe 2020 Project, ERICA: European Rare disease research Coordination and support Action), and empower patient organisations.18
In line with EU efforts, there are also several international initiatives to improve access to ODs, such as the Australian Life Saving Drugs Programme,19 which reimburses non-cost-effective medicines outside of the standard drug funding scheme. Similarly, the UK NHS has established innovative pricing agreements with the pharmaceutical industry to facilitate access to non-cost-effective treatments—40% of which are medicines for RDs. These agreements include simple discounts, caps on cumulative cost or quantity used per patient, and outcome-based payment models.20 Other innovative experiences worth highlighting include the international social network PatientsLikeMe,21 an open online community designed to transform patient stories into data, which already tracks more than 2800 diseases, or the RareConnect platform,22 created by EURORDIS (Rare Disease Europe and Care4Rare Canada) to connect and empower patients with RDs and their caregivers worldwide.
There are similar initiatives in Spain, such as the establishment of CSURs (Centros, Servicios y Unidades de Referencia del Sistema Nacional de Salud), aimed at providing equitable coverage across Spain through multidisciplinary teams that ensure continuity of healthcare provision23; the multidisciplinary platform ObserveMHe, focused on generating knowledge and information about ODs24; and the Red ÚNICAS, a network of interconnected hospitals aimed at improving care for children with RDs through the implementation of a collaborative model that shortens diagnostic times facilitates research and access to the most advanced therapies.25
In line with the EU strategic objectives for RDs and international experiences, the Strategic Plan has identified trends grouped around 4 main axes for change (see Table 1). In this context, OrPhar-SEFH has developed several initiatives, including the Horizon Scanning Project,7 the application of MCDA to assess the therapeutic value of ODs,8 and the pharmacy services humanisation project regarding the care of patients with RDs.9 Other contributions include teaching activities (e.g. the monograph on hospital pharmacy for patients with RDs10) and research efforts, such as the development of a population pharmacokinetic/pharmacodynamic model to individualise eculizumab therapy in adults with atypical haemolytic uraemic syndrome.26
It is also worth noting that the OrPhar-SEFH Strategic Plan has incorporated patient advocacy in relation to current experiences and expectations regarding the role of HPs in the management of RDs, following the current trends of patient empowerment and increased involvement in therapeutic decision-making.27–29
This is reflected in the SWOT analysis (Table 2), which highlights several weaknesses: limited visibility of HPs among patients and scientific societies; insufficient collaboration and synergy with other scientific societies involved in RDs and among SEFH working groups; lack of shared registries on the use of ODs and RWD in RDs; gaps in RD-specific training; and limited engagement of HPs and the OrPhar-SEFH group with the decision-making bodies involved in OD evaluation and access, as well as with research institutes, universities, public health agencies, patient associations, and scientific societies. Among the strengths identified, we highlight the specialised knowledge and experience of the HPs of the OrPhar-SEFH group in caring for patients with RDs and the management of ODs, along with their extensive network of contacts with healthcare and research professionals, facilitating the development of collaborative projects. Having members from all regions of Spain provides a comprehensive view on the specific needs and challenges of each area, while facilitating the sharing and scaling of best practices across centres.
The main external threats identified include: the lack of presence and visibility on social media; the insufficient adaptation of the Spanish health system to innovative treatments for RDs; inequities in access to treatments and care models across the ACs; and political and social instability, which may affect the availability of key resources, such as timely diagnosis, early treatment, and the growing demand for specialised resources and treatments. The opportunities identified include the growing public awareness of RDs; the availability of digital and training resources provided by the SEFH; and the use of innovation as a driver of change across all levels, such as pharmacogenetics, pharmacokinetics, new funding models, and the integration of disruptive technologies into clinical practice, research, and RWD. Other opportunities include collaboration with the pharmaceutical industry in research and innovation; the intermediary role of HPs across specialised and nonspecialised care levels; and a focus on outcomes, including the use of PROMs and PREMs, which are already implemented in other disease areas and could be adapted to the RD setting.
Based on this analysis, and considering the key success factors identified in Fig. 1, a consensus was reached on 23 lines of action, which aim to foster collaborative work between HPs, professionals involved in the management of RDs, and patients, promote the healthcare role of HPs, guarantee equity in health care, and improve the quality of life of patients with RDs.
In this context, the 6 lines of action outlined under the strategic axis of alliances reflect the commitment of HPs to RDs, as demonstrated by numerous initiatives across healthcare,30 education,24,31 and research.32–34 They also underscore the need to establish and strengthen alliances with external stakeholders—such as regulatory agencies, scientific societies, healthcare professionals, the Ministry of Health, regional authorities, universities, the pharmaceutical industry, research institutes, and patient associations—with the aim of fostering synergies and promoting knowledge exchange and collaboration. It is also worth noting the emergence of new stakeholders, such as start-ups and tech giants, which can join forces with HPs to promote integration and participation in patient monitoring, foster collaboration in research and innovation, and ultimately contribute to a comprehensive and multidisciplinary approach to patients with RDs.35,36
The 3 lines of action defined under the strategic axis of evidence aim to promote critical analysis of existing scientific studies on ODs and encourage participation in protocol development, with the goal of integrating the best available evidence. In parallel, they seek to foster the generation of new evidence and ensure personalised, patient-centred care that takes into account patients' needs and experiences.37–39
The 4 lines of action under the proposed research/innovation axis aim to actively promote scientific research and the use of innovative funding models and evaluation criteria for ODs, going beyond traditional parameters such as efficacy, safety, and cost. They seek to incorporate assessment criteria that capture the therapeutic and social value of ODs from a holistic perspective that considers the needs of patients and the health system.40–47
The fourth axis, optimisation, contains 7 lines of action aimed at ensuring the full participation of HPs in the entire healthcare process, as well as continuous communication and coordination with patients and the professionals involved. These actions also seek to promote the incorporation of new technologies and to support and raise awareness of existing initiatives within OrPhar-SEFH.48–52
Finally, the fifth strategic axis is based on union and integrated, collaborative work in hospital and community settings. It proposes 3 lines of action with the aim of positioning HPs as key players in the treatment of RDs. It aims to increase their visibility to patients and other professionals, and promoting the use of sustainable practices that contribute to environmental care.53–55
The prioritisation of lines of action identifies key initiatives that should be initiated or sustained within each strategic axis, based on their impact and feasibility of implementation. This approach aims to ensure the continuity of ongoing projects and to guide future projects envisaged by the working group.
Finally, the OrPhar-SEFH Strategic Plan, designed for implementation during the period 2024–2027, aims to support HPs in addressing the key challenges in the care of patients with RDs from the perspective of hospital pharmacy. It seeks to improve access to therapeutic innovations that deliver value to both patients and the healthcare system, develop a comprehensive and interdisciplinary care model for patients with RDs, and adopt a holistic and multidimensional approach that facilitates knowledge sharing among professionals. The plan also emphasises the creation of individualised therapeutic and follow-up plans tailored to both the clinical and social health needs of each patient, as well as the involvement of patients and broader society in decision-making processes at various levels.
In summary, the Strategic Plan is intended to provide a roadmap for enhancing the role of hospital pharmacy in the management of RDs, improving access to and evaluation of ODs, and optimising the integrated and interdisciplinary care process to improve health outcomes, quality of life, and patient experience. In parallel, it aims to establish OrPhar-SEFH as a reference body for the health system, healthcare professionals, and patients with RDs.
CRediT authorship contribution statementMónica Climente Martí: Writing – review & editing, Writing – original draft, Validation, Supervision, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Sergio Fernández Espinola: Conceptualization, Investigation, Methodology, Validation, Visualization. Paula Guijarro Martínez: Conceptualization, Investigation, Methodology, Validation, Visualization. Elena Gras Colomer: Conceptualization, Data curation, Methodology, Project administration, Validation, Visualization. Alicia Herrero Ambrosio: Conceptualization, Investigation, Methodology, Supervision, Validation. Silvia Manrique Rodríguez: Conceptualization, Investigation, Methodology, Supervision, Validation. Isabel Martín Herranz: Conceptualization, Investigation, Methodology, Validation, Visualization.
FundingNone declared.
We would like to thank Deloitte Digital for their methodological support in the development of the Strategic Plan. The consulting work provided by Deloitte Digital was funded by Roche Farma.