During the pandemic caused by the SARS-CoV-2 virus, pharmacy services have had to adapt their service portfolio, and yet ensure efficient, equitable and quality pharmaceutical care. Given the limited scientific evidence available, most drugs have been used off-label or in the context of clinical trials, which should be the preferred option in order to create new evidence.
Among kind different situations we have faced are the increase in workload, the expansion of coverage to new wards and ICUs and shortages, which have caused the use of alternative drugs and even other routes of administration. Given that covid-19 affects elderly population with greater severity and many of them are polymedicated, great effort have been focused on monitoring interactions, both pharmacokinetic and pharmacodynamic (specially prolongation of the QT interval), monitoring correct concentrations of electrolytes, nutritional support, adaptation of chemotherapy treatment protocols and anticoagulant management, among others.
The use of personal protective equipment added difficulty for nursing work and some measures had been taken to minimize the number of entries into the rooms. Eventually, team's split to guarantee care, the challenge of teleworking, remote validation, telemedicine and telepharmacy for communication between professionals and patients, as well as training in this pandemic situation have been a challenge for our profession.
These difficulties have risen up new learning opportunities we hope will be useful to us in the event we have to face similar situations in the future.
La pandemia ocasionada por el virus SARS-CoV-2 ha hecho que los servicios de farmacia hayan tenido que adaptar su cartera de servicios, y sin embargo asegurar una atención farmacéutica eficiente, equitativa y de calidad. Dada la escasa evidencia científica disponible, la mayoría de los medicamentos se han empleado fuera de indicación o en el contexto de ensayos clínicos, que debería ser la opción preferente para generar nueva evidencia.
Entre las diversas situaciones que se han tenido que afrontar se encuentran el incremento de trabajo asistencial, la ampliación de la cobertura a nuevas salas y unidades de cuidados intensivos y los desabastecimientos, que han ocasionado el uso de fármacos alternativos e incluso otras vías de administración. Dado que la COVID-19 afecta con mayor gravedad a población de edad avanzada, muchos de ellos polimedicados, se ha tenido que dedicar un gran esfuerzo al seguimiento de interacciones, tanto farmacocinéticas como farmacodinámicas (en especial, prolongación del intervalo QT), monitorización de concentraciones correctas de electrolitos, soporte nutricional, adaptación de pautas de quimioterapia y manejo de los anticoagulantes, entre otros.
La dificultad adicional para enfermería de la administración de medicamentos con equipos de protección individual ha supuesto la adaptación de formas de administración para minimizar el número de entradas en las habitaciones. Por último, el fraccionamiento del equipo para garantizar la atención, el reto del teletrabajo, la validación en remoto, la telemedicina y la telefarmacia para la comunicación entre profesionales y pacientes, así como la formación en esta situación de pandemia, han supuesto un reto para nuestra profesión.
Estos desafíos han creado nuevas oportunidades de aprendizaje que esperemos nos puedan ser de utilidad en el caso de que tuviéramos que afrontar situaciones semejantes en el futuro.
In the past, pharmaceutical care was focused on medicines, understood as the care provided to a patient to ensure an efficient, safe, and rational use of medicines1. However, a more humanistic approach has been adopted, and pharmaceutical care is now defined as the provision of services related to medications, including indication (understanding), effectiveness (expectations), safety (concerns), and convenience (non-adherence) to prevent or solve a drug-related problem and improve healthcare-related quality of life2.
The outbreak of the pandemic caused by the SARS-CoV-2 virus has forced pharmacy services to adapt their portfolio of services to the new context, while guaranteeing the continuity of quality pharmacy services.
This article describes a series of situations generated by COVID-19 and its impact on hospital pharmacy services.
Strategies developedOff-label medicines (use of off-label drugs)To date, the unavailability of approved drugs for SARS-CoV-2 infection has led to the off-label use of numerous potentially effective drugs3 either for their potential mechanism of action4 or for their modulatory effect on hyperinflammatory immune response5.
In the context of a global pandemic, with its associated health, social and economic effects, the use of experimental therapies seems to be justified. In this sense, the different regulatory agencies have facilitated access to these therapies6. However, this practice has raised ethical concerns, and drug testing should be performed in a more controlled context and be given priority. Inclusion of patients in clinical trials should be a priority7. If it is not possible, oral consent should be kept in electronic medical record and generated data should be used in observational studies8.
Dealing with the shortage of drugs: Use of alternative routes of administration and drugsTo confront the health crisis generated by COVID-19, the health system had to intensify efforts. This has resulted in the scarcity of drugs such as some forms of heparin and methylprednisolone, among others, or drugs for critical patients (i.e. midalozam). Added to drug shortages, due to this situation, the Spanish Agency of Medicines and Medicinal Products (AEMPS) regulated drug distribution. Additionally clinicians had to seek other drug alternatives i.e. the use of sedatives was prioritized for critical patients, whereas other less frequent drugs were used for other settings such as end-of-life care (levomepromazine, phenobarbital, or clonazepam). Likewise, subcutaneous administration was prioritized9.
Monitoring of drug interactionsThe treatment for COVID-19 used in Spain durig COVID-19 outbreak may cause drug interactions. One of the antivirals used, lopinavir/ritonavir, is a known enzymatic inhibitor of cytochrome CYP3A4 and may cause severe interactions when used in combination with common drugs (simvastatin, clopidogrel, immunosuppressants, and even inhalers like salmeterol / fluticasone).
Other experimental antivirals such as remdesivir are metabolized in the liver. The evidence available on drug interactions is scarce and their use in combination with potent enzyme inducers is not recommended. This situation requires careful revision of the validation process. The use of open access databases has been extremely useful in this regard (https://www.covid19-druginteractions.org/, http://www.interaccionesvih.com/).
Other drugs such as the immunosuppresors used for rheumatologic diseases (tocilizumab, sarilumab, anakinra or baricitinib) have been used to treat the inflammatory process associated with COVID-19 infection.
These drugs are short-term treatments, though some have a long half-life. Although the risk for drug interactions is lower, they may occur. A moderate reduction of liver metabolism has been observed in the presence of inflammation and elevated levels of some cytokines (interleukin [IL] 1 or IL-6, among others).
When these cytokines are blocked, hepatic metabolism returns to normality and the plasma concentrations of some drugs may decrease10. Drugs with a high hepatic metabolism and narrow therapeutic range should be closely monitored. Finally, respiratory support does not show relevant interactions11.
Based on some initial reports, other interactions (i.e. drug-disease) also required close monitoring such as the use of renin-angiotensin axis antagonists (such as angiotensin-converting enzyme inhibitors [IECA] or angiotensin II receptor blockers [ARA-II]). SARS-CoV-2 uses the Spike protein to bind the ACE2 receptor (target of IECA/ARA-II) of human cells12. The initial hypothesis was that these treatments might interact. However, this interaction has been proven to be irrelevant13,14.
In the light that ischemic heart disease and cardiac insufficiency have been associated with poorer outcomes and mortality in COVID-19 patients, these therapies must not be withdrawn in patients with a high cardiovascular risk due to the risk posed by a decompensation of their underlying diseases15.
Monitoring effectiveness and safety. Pharmacological surveillanceSome situations have raised safety concerns in pharmacy services. Thus, the prolongation of the QT intervals and potential occurrence of malignant arrhythmias (torsade de pointes) induced by the use of chloroquine/hydroxychloroquine and azithromycin can worsen with the use of other drugs such as lopinavir/ritonavir16.
In this sense, the recommendations of international medical societies have been implemented17. Close monitoring has been performed of the concentrations of some electrolytes to maintain adequate levels ([K+] ≥ 4.0 mEq/l; [Mg2+] ≥ 2.0 mEq/l; [Ca2+] ≥ 8.5 mEq/l) and supplementation has been considered when necessary (especially with the concomitant use of diuretics).
Alerts on the use of these drugs and recommendations on continuous monitoring of drugs that cause a prolongation of the QT interval have also been useful, especially in high-risk population (long baseline QTc intervals). Some open-access online tools (https://www.crediblemeds.org/; https://medsafetyscan.org/) have also been of help to identify the drugs that cause a prolongation of the QT interval and calculate the risk of occurrence18.
One of the physiopathogenic effects of SARS-CoV-2 infection is the increased risk for thromboembolism19,20. Monitoring anticoagulation was crucial, especially in the context of dose adjustment in patients with comorbidities (i.e. renal insufficiency, obesity, and high or very high risk of thrombosis, among others)21.
Finally, variability between prescribers was reduced by the use of clinical protocols for the standardization of treatments and computer-assisted prescription systems.
Guidelines for the safe administration of medicinesA specific aspect to be considered due to the characteristics of the virus is the safety of the nursing staff. The lower the number of contacts the lower the risk of contagion. This was taken into account for the validation of treatments, and the frequency of administration was adapted to the best schedule.
Pharmacy services have collaborated in the preparation of sterile intravenous solutions in infusion bags to minimize visits to COVID rooms.
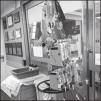
In some hospitals, infusion pumps were installed in the aisle, outside patients’ rooms and connected using a catheter extension set (Figure 1). This way, visits were reduced while individual protection equipment was preserved. In addition, as long as stability and effectiveness are maintained, continuous infusion may save time of line handling and contact of the nursing staff with patients, as compared to the administration of bolus units, which may be especially useful for some antibiotics.
Other actions implemented to ensure a safe administration of drugs was the use of inhaled agents instead of the nebulized form to reduce the generation of aerosols, or the priority use of eye drop unit doses instead of multidose, as SARS-CoV-2 has demonstrated an ocular tropism and the risk of contagion may increase by the accumulation of contaminated material22.
Follow-up of patientsThe reorganization of healthcare services (for example, surgery) and the high rate of infection among hospital staff have forced that numerous specialists (both, physicians and pharmacists) work out of their field of expertise. In this context, pharmaceutical validation and follow-up has become essential to prevent potential medication errors. In this sense, the use of computer-assisted therapeutic decision-making has been extremely useful (vital sign monitoring, laboratory parameters, among others).
Pharmacy services have been particularly aware of some diseases and settings. In relation to nutritional support in critical and non-critical patients, special attention has been paid to the screening and evaluation of patients at a higher risk (such as the elderly) or the optimization of formulas (energy, protein and vitamin composition), since the infection impacts the nutritional status of patients23.
Some interventions have also been performed in cancer patients to reduce the risk of infection. Oral administration has been prioritized over parenteral administration, when indicated (i.e. capecitabine instead of 5-fluorouracil). The regimes requiring fewer visits to the hospital were also prioritized (i.e. administration of taxanes every three weeks instead of weekly). Potential interactions between the drugs used for COVID-19 and oncologic therapies were also considered24,25. In pediatric patients, doses were adapted by the compounding of formulations26.
Finally, as in any discharge process, reconciliation of lifelong and acute treatments was required. Reconciliation after discharge by a phone call was already done before the crisis and has been especially effective in this setting27. Some therapies were adapted after discharge; more specifically, direct-acting oral anticoagulants were prioritized over anti vitamin K28 to reduce visits to the hospital, especially at the start of treatment, as it requires numerous controls visits.
Communication between professionals and patients. Remote validationDirect communication between health professionals has been reduced significantly because of the SARS-CoV-2 crisis. Thus, routine activities such as joint bedside visits of physicians and nurses, shift-to-shift handoffs, multidisciplinary sessions, or education sessions for patients on the use of medication after discharge were seriously restricted to ensure physical distancing and prevent infections. However, some strategies were adopted to overcome this situation.
The traditional mobile phones or beepers were supplemented with digital platforms (for example, Medxat app), which allow the exchange of written messages (chats) or multiple-user videoconferencing. These platforms are required to comply with the Organic Law on Personal Data Protection and Digital Rights Guarantee (Law 3/2018 of December 5th). The requirement of physical distancing and the creation of “mirror or complementary teams” to cover sick leaves resulted in remote validation (teleworking). In some services, teams were created to alternate work at hospital and teleworking, whereas other teams either teleworked or worked at the hospital.
Education and informationThe COVID-19 crisis has originated a multiplicity of publications and work documents generated by different scientific societies, regulatory agencies, webinars, and social networks, to name a few, with the associated risk of “infoxication”, which hinders access to quality information and education.
In this sense, information repositories such as the open-access website of the Spanish Society of Hospital Pharmacy (https://www.sefh.es/covid-19.php) and the European Association of Hospital Pharmacists (EAHP) COVID-19 Resource Centre https://www.eahp.eu/hp-practice/hospital-pharmacy/eahp-covid-19-resource-centre have been extremely useful.
Lessons learned. Future applicability in pharmacy services
The COVID-19 pandemic has posed a challenge to pharmacy services and offered the opportunity to learn some lessons for future health crises. Some lessons learned include understanding the relevance of evaluating scientific evidence to generate knowledge of effective therapies, the importance of ethics (not causing harm), and the need to adapt work practices (telework, videoconferencing, etc).
The impact of the strategies implemented to confront the situation of emergency on pharmacy services could not be evaluated following the traditional procedures (prediction and planning), and continuous re-evaluation after implantation was necessary (experimentation and adaptation).
Therefore, the evaluation and documentation of all the interventions implemented can serve as a starting point for future pandemics. All without forgetting that the ultimate purpose of pharmacy services is to provide the best care possible in relation to the medication of our patients.