To assess the pharmacokinetic monitoring of SDC performed from primary healthcare (PH) in patients with chronic treatment.
MethodsCross-sectional retrospective study of patients with chronic treatment with digoxin belonging to the department of a General University Hospital.
Data were analized: age, sex, diagnosis, number of serum digoxin concentration determinations, date and origin of the request for monitoring, analytical result and pharmacokinetic assessment are collected.
Results624 patients are undergoing chronic treatment with digoxin, 68% women, mean age 78.4 (39-98) years. 308 (49.4%) patients haven’t analytical determination of SDC (Group 1), 183 (29.3%) patients have a SDC occasionally performed with a request from specialist care (Group 2) and 133 (21,3%) patients have CSD performed with a request from primary healthcare doctors, with an average of 2.42 monitoring per patient and year (Group 3). These are those patients who have pharmacokinetic monitoring of chronic treatment with digoxin.
Of the group 2.25 (13.6%) patientes were hospital admission from emergency department for presenting digitalis intoxication with CSD>2 ng/ml, and 39 (21.3%) patients for low dosing with CSD<0.5 ng/ml. Group 3.4 (3%) patients presented digitalis intoxication and 5 (3.8%) for insufficient dosing.
ConclusionsA small proportion of patients undergoing chronic treatment with digoxin are under pharmacokinetic monitoring and a reduction in complications derived from inappropriate CSD compared to those not under pharmacokinetic follow-up is observed.
Evaluar el seguimiento farmacocinético de las CSD que se realiza desde Atención Primaria (AP) en pacientes con tratamiento crónico.
MétodosEstudio trasversal observacional retrospectivo de pacientes en tratamiento crónico con digoxina que pertenecen al departamento de un Hospital General Universitario.
Se recogen datos de edad, sexo, diagnóstico, número de determinaciones séricas de digoxina realizadas, fecha y origen de la solicitud de monitorización, resultado analítico y valoración farmacocinética. (Infradosificación, normodosificación o supradosificación).
Resultados624 pacientes están en tratamiento crónico con digoxina: 68% mujeres, edad media 78,4 (39-98) años. 308 (49,4%) pacientes no tienen realizada ninguna determinación analítica de CSD (Grupo 1), 183 (29,3%) pacientes tienen CSD realizadas de manera esporádica con solicitud tramitada desde Atención Especializada (Grupo 2) y 133 (21,3%) pacientes tienen CSD realizadas de manera periódica con solicitud cursada por médicos de AP, con un promedio de 2,42 monitorizaciones por paciente y año (Grupo 3). Estos son los que tienen un seguimiento farmacocinético del tratamiento crónico con digoxina. Del Grupo 2,25(13,6%) entran por el Servicio de Urgencias por presentar intoxicación digitálica con CSD>2 ng/ml, y 39 (21,3%) pacientes por baja dosificación con CSD<0,5ng/ml. Del Grupo 3,4 (3%) presentan intoxicación digitálica y 5 (3,8%) infradosificación.
ConclusionesUna pequeña parte de los pacientes que se encuentran en tratamiento crónico con digoxina están en seguimiento farmacocinético. Se observa una reducción de las complicaciones derivadas de CSD inapropiadas con respecto a los que no están en seguimiento farmacocinético.
Published bibliography, such as the DIG clinical trial, supports that digoxin must be used while monitoring serum concentration, creatinine levels, and potassium levels, in order to minimize the risk of toxicity. To conduct an adequate pharmacokinetic follow-up of digoxin concentrations in serum in patients under chronic treatment will be useful for an adequate pharmacological management of HF (heart failure) and AF (atrial fibrillation). Serum digoxin concentrations (SDC) are a major factor to predict toxicity, and are associated with mortality rates; therefore, an individualized dosing will be particularly interesting for these patients.
This study shows that patients on chronic treatment with digoxin who have no pharmacokinetic follow-up of serum concentrations by Primary Care will require emergency healthcare with higher frequency. Digoxin lab tests requested from the Emergency Unit shows a significant proportion of patients with SDCs beyond therapeutic range; the majority are above the interval, and present clinical expressions of toxicity. This study reinforces the recommendations stating that pharmacokinetic monitoring of SDCs must be one more parameter to be controlled in the follow-up of patients on digitalis treatment, in order to minimize the risk of toxicity.
IntroductionCardiac glycosides cause a positive inotropic effect, they reduce sympathetic activation, and have electrophysiological properties. These actions are the basis for their use in the treatment of two relevant clinical situations: Congestive Heart Failure due to systolic dysfunction, and supraventricular tachyarrhythmia such as atrial fibrillation. Digitoxin has not been available in many countries since the 80s, and therefore digoxin is the most widely used in clinical practice.
Even though it is no longer first choice treatment, mainly due to the widespread use of beta-blockers, amiodarone and calcium antagonists, the efficacy of digoxin has been supported by numerous clinical trials 1,2 such as PROVED3 or RADIANCE4.
Due to its pharmacokinetic characteristics, digoxin presents a narrow therapeutic range that defines its safety and efficacy: serum digoxin concentration (SDC) must be between 0.8 and 2ng/mL (1-2.5nmol/l)5. Clinical trials have demonstrated that, in patients with normal sinus rhythm, the benefit of digoxin therapy has appeared with mean concentrations of 0.5-1.75 ng/ ml6, and the post-hoc analysis of the Digitalis Investigation Group (DIG) study1,7 has demonstrated an improvement in results in patients with serum digoxin concentrations between 0.5 and 0.8ng/ml. The coverage offered by Primary Care (PC) has resulted in the essential role of GPs in the diagnosis, treatment and follow-up of patients with Congestive Heart Failure (CHF) and Atrial Fibrillation (AF). The most recent recommendations 8,9 are positioned in favour of monitoring serum digoxin concentrations as one more parameter to be controlled in the follow-up of patients on chronic digitalis treatment. The objective is to individualize therapy through the use of pharmacokinetic criteria, in order to find a balance between maximum efficacy and minimum toxicity, orientated by lab tests determining the serum concentration of the drug.
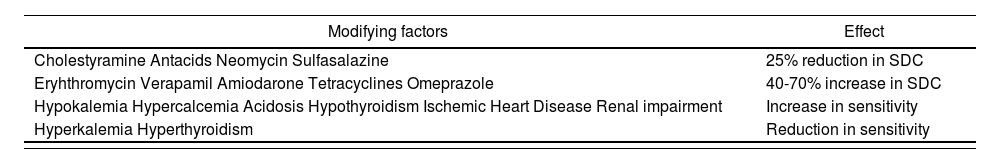
If SDCs go beyond the upper end of the interval, there is a higher risk of presenting clinical expressions of toxicity; on the other hand, concentrations below this range can lead to poor disease control. The most frequent expressions of digitalis toxicity are: nausea, vomiting, diarrhoea, asthenia, dizziness and confusion, together with hydroelectrolytic and electrocardiographic alterations (sinus brachycardia, atrioventricular block and extrasystole). Monitoring is particularly important in persons with chronic renal impairment, because a significant reduction in renal function can lead to a buildup of digoxin, and favour its toxicity. But there are also other factors such as age, thyroid function, or drug interactions, which can have an impact on SDC variations 10.
The objective of this study is to assess the pharmacokinetic follow-up of SDCs conducted from PC in patients on chronic treatment with digoxin within our area.
MethodsAn observational retrospective study of patients on chronic treatment with digoxin. On March, 2015, a transversal cut was made of all patients on treatment with digoxin within the area of a General University Hospital with 6 Health Centres (HCs) affiliated, and a population of referral of approximately 200,000 inhabitants.
Information about patients on chronic treatment with digoxin was obtained from the database provided from PC through the Abucasis® program. This information was compared in the Pharmacy Unit with the database from the Hospital Pharmacokinetic Unit, where there is a record of all lab test results for drugs conducted from 2006 until today.
Data were collected, including demographic details (age and gender), diagnosis, number of serum digoxin concentration tests conducted, data and source of the application for monitoring (HC and physician assigned per patient), lab test results, and pharmacokinetic assessment (underdosing, normal dosing, or overdosing).
The results from the six HCs in the area were collated, and patients were classified into three groups:
Group 1Patients with non-monitored serum digoxin concentrations; Patients for whom no lab test for digoxin determination has been conducted.
Group 2Patients with known serum digoxin concentrations, but not monitored from PC: Patients with one or more digoxin lab tests which coincide with their visits to ER or admission to the hospitalization ward. Lab test determinations were managed exclusively from Specialized Care (SC), either by the Emergency Unit or the hospitalization units of the hospital.
Group 3Patients with known serum digoxin concentrations that are monitored from PC: These are patients with tests requested by PC physicians, with pharmacokinetic follow-up of chronic treatment with digoxin, including patients with digoxin tests conducted periodically or on specific occasions. There was a comparison between Groups 2 and 3 in order to determine the utility / value of the pharmacokinetic follow-up of SDCs. To this aim, there was a quantification of the number of patients who attend the Emergency Unit with inadequate digoxin levels, requested from the Emergency Unit due to suspected underdosing or poisoning. Besides, there was also an assessment in Group 3 of the annual frequency of lab test determinations.
ResultsIn total, 624 patients are on chronic treatment with digoxin in our area: 68% of them are women, with a mean age of 78.4 (39-98) years; 41 have a HF diagnosis (6.6%); 339 have an AF diagnosis (54.1%); 77 present AF + HF (12.3%), and 169 have other diagnoses (27%).
After comparing databases, the result obtained was that 308 patients (49.4%) on chronic treatment with digoxin have no lab test determination of their serum concentration, and are included in Group 1; while 316 patients (50.6%) have one or more lab test determinations, and belong to Groups 2 and 3.
Regarding the patients classified in Groups 2 and 3 with SDC determinations recorded in the database of our hospital unit, in 183 (57.9%) cases, the request was conducted by SC physicians at specific moments; these patients belong to Group 2. On the other hand, 133 patients (42.1%) have SDC values recorded due to a monitoring request by PC physicians in a periodical way. These patients are classified within Group 3. Of the patients assigned to Group 2, with serum digoxin concentrations not monitored by PC, 25 patients (13.6%) have attended the Emergency Unit in 29 occasions due to digitalis poisoning symptoms, with serum digoxin levels above 2ng/ml; and 39 more patients (21.3%) have attended the Emergency Unit in 46 occasions due to underdosing symptoms, with a SDC below 0.5ng/ml.
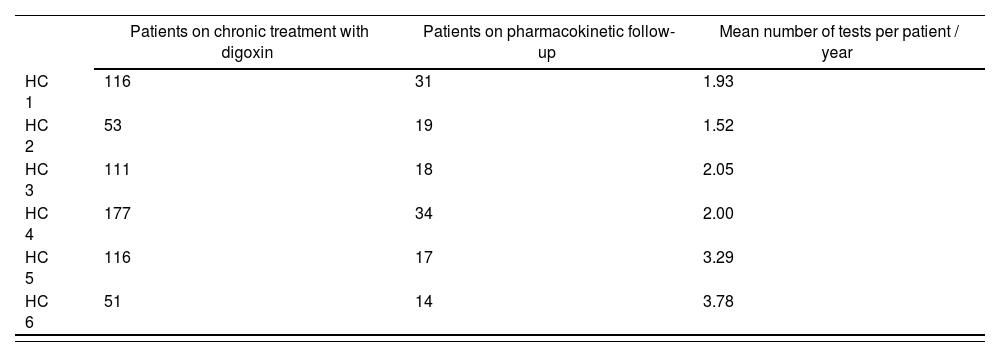
According to the established definition for Patient on Pharmacokinetic Follow-up of SDCs by PC (Group 3), 133 (21.3%) of the 624 patients on chronic treatment with digoxin belong to this group, with an average 2.42 monitorings conducted per patient and year (1.52 monitorings / patient / year from the HC with the lower frequency of pharmacokinetic controls, and 3.78 monitorings / patient / year from the HC that requests lab test determinations with the highest frequency). (Table 1: Results by HC.) On the other hand, 4 (3%) of the patients classified in Group 3 have visited the Emergency Unit due to digitalis poisoning, and 5 (3.8%) due to underdosing.
Number of patients on digoxin treatment and number of patients on pharmacokinetic treatment per health centre assigned
| Patients on chronic treatment with digoxin | Patients on pharmacokinetic follow-up | Mean number of tests per patient / year | |
|---|---|---|---|
| HC 1 | 116 | 31 | 1.93 |
| HC 2 | 53 | 19 | 1.52 |
| HC 3 | 111 | 18 | 2.05 |
| HC 4 | 177 | 34 | 2.00 |
| HC 5 | 116 | 17 | 3.29 |
| HC 6 | 51 | 14 | 3.78 |
HC: Health Centre.
The outcomes of the study have revealed that there is a significant number of patients in our area on digoxin treatment for two prevalent diagnoses: HF and AF. The mean age of the study population was around 78 years. Monitoring is particularly important in persons with chronic renal impairment or reduced renal clearance, because these present a lower volume of distribution for digoxin, as well as a very important reduction in its elimination, and these circumstances require a dosing adjustment in order to prevent overdosing11 (Table 2. Situations that will modify SDCs.) No lab test determination has been conducted for serum digoxin concentrations in almost half of the study population (Group 1), and therefore it is not known whether these are within therapeutic range. Various published articles state that serum digoxin concentration monitoring must be one more parameter to be controlled within the follow-up of patients on digitalis treatment, in order to minimize the risk of toxicity.8,9,12 In our study, only 20% of the total population on this chronic treatment is being monitored through tests for drug detection in serum (Group 3). Besides, there are clear differences in terms of monitoring frequency among the six HCs within this area.
Agents that modify serum concentration and sensitivity to digoxin
| Modifying factors | Effect |
|---|---|
| Cholestyramine Antacids Neomycin Sulfasalazine | 25% reduction in SDC |
| Eryhthromycin Verapamil Amiodarone Tetracyclines Omeprazole | 40-70% increase in SDC |
| Hypokalemia Hypercalcemia Acidosis Hypothyroidism Ischemic Heart Disease Renal impairment | Increase in sensitivity |
| Hyperkalemia Hyperthyroidism | Reduction in sensitivity |
SDC: Serum Digoxin Concentration.
The most recent recommendations regarding the regularity of SDC monitoring state that a first control must be conducted for HF at 7-10 days after treatment initiation or change of dose; at this time, a stable situation should have been reached.9 Subsequently, this pharmacokinetic control in stable patients should be repeated every 3-6 months, or earlier in case of clinical changes or suspected toxicity.9 When digoxin is used in AF, the frequency of pharmacokinetic monitoring is not as well defined as for HF. Publications suggest that it should be monitored periodically, without mentioning any specific time.8,9,10,12
The data of the study also reveal disparities in the number of visits to the Emergency Unit due to inadequate dosing; these are significantly higher in the group of patients without pharmacokinetic follow-up (Group 2): 10% more patients in this group have attended the Emergency Unit because they presented digitalis poisoning, while 16% more patients in the same group have presented symptoms of underdosing. These results show a better therapeutic control in the group of patients for whom, as well as an assessment of their clinical situation, there is also a periodical pharmacokinetic monitoring of treatment, which allows the individualization of their dosing regimen (Group 3).
The study shows that an adequate pharmacokinetic follow-up of digoxin treatment will present benefits, and that only a low proportion of patients in our area are adequately monitored.
In conclusion, there is no lab test determination of SDC for approximately 50% of patients, and 30% are not tested periodically; therefore, there is no adequate pharmacokinetic follow-up by PC of digoxin treatment in 80% of the patients in our area. There are clear differences in monitoring frequency among the six affiliated HCs, as well as in the number of patient visits to the Emergency Unit due to inadequate dosing.