Advanced therapy medicinal products have emerged In recent years as new pharmacotherapeutic strategies. In this context, hospital pharmacy services have had to adapt to the new challenges posed by the inclusion of advanced therapies in their roster of services against the background of the complex pharmacotherapeutic process patients typically go through.
All the activities carried out in the hospital pharmacy services must abide by the rules established in the Spanish legislation and ensure both the quality of the different drugs they manage and the safety of every single patient.
Advanced therapy medicinal products are associated certain peculiarities, including the need to select and evaluate potential candidates to receive them; recourse to financing mechanisms based on risk sharing; and their extreme fragility, which means that the personnel in charge of handling them must be properly trained to maintain their viability and that special storage conditions, involving temperatures below 180 °C in the case of chimeric antigen receptor T cell therapies, must be maintained.
In addition, use of advanced therapy medicinal products in the clinical setting has made it necessary for scientific societies to produce consensus documents recognizing the pivotal role of hospital pharmacists as indispensable members of the multidisciplinary healthcare team and ensuring the same traceability, conservation, custody and pharmacotherapeutical monitoring standards imposed on other drugs to provide for adequate pharmaceutical care. Scientific societies have also highlighted the importance of intensifying clinical research, an essential requirement for the safe incorporation of new therapeutic targets.
The present document is intended to describe the challenges pharmacists may face when using advanced therapy medicinal products at the different stages or processes in the patient's clinical journey.
Los medicamentos de terapia avanzada han emergido en los últimos años como nuevas estrategias farmacoterapéuticas. En este contexto, los servicios de farmacia hospitalaria nos hemos tenido que adaptar al nuevo reto que ha supuesto su inclusión en nuestra cartera de servicios dentro del complejo proceso farmacoterapéutico en el que están inmersos los pacientes.
Todas las actividades que se desarrollan en los servicios de farmacia hospitalaria cumplen con una base legal establecida en nuestra legislación y garantizan la calidad y seguridad tanto de los pacientes atendidos como de todos y cada uno de los medicamentos que se gestionan.
Los medicamentos de terapia avanzada tienen unas características especiales a considerar que van desde las fases iniciales de selección y evaluación de los pacientes candidatos y su modelo de financiación, basado en riesgo compartido, hasta una fragilidad en su manipulación que requiere de una adecuada y adaptada formación del personal implicado en la logística para mantener su viabilidad, al necesitar unas condiciones de conservación, en ocasiones, a temperaturas de menos 180 °C, en el caso de las células T con receptores quiméricos de antígenos.
Además, la utilización clínica de los medicamentos de terapia avanzada ha necesitado de documentos de consenso de las sociedades científicas que pongan en valor el posicionamiento del farmacéutico hospitalario, como miembro indispensable dentro del equipo multidisciplinar asistencial, y que garanticen, como en cualquier otro medicamento, la trazabilidad, la correcta conservación y custodia y el seguimiento farmacoterapéutico asociado a una adecuada atención farmacéutica de nuestros pacientes, sin olvidar la importancia de la creciente investigación clínica, necesaria e imprescindible para una incorporación segura de nuevas dianas terapéuticas.
Por todo ello, consideramos necesario el presente documento, en donde se ponen de manifiesto los retos o necesidades, desde el punto de vista farmacéutico, en cada una de las etapas o procesos a considerar en la utilización de los medicamentos de terapia avanzada dentro de nuestro amplio arsenal terapéutico.
The recent advent of the new advanced therapy medicinal products (ATMPs) for human use has posed a new challenge for hospital pharmacists, whose expertise in drug safety and quality, and profound understanding of the different stages of the pharmacotherapeutic process (selection, preparation, acquisition, storage, custody, dispensing, clinical follow-up and research), are well known.
ATMPs possess intrinsic characteristics that make them special and different from other chemically structured drugs: they are drugs with a high economic and health impact; they are financed on the basis of a risk sharing model whereby pharmaceutical companies are not paid for medicines if the expected results are not achieved; and they require special storage conditions, ranging from cold storage to storage at extreme temperatures (cryopreservation), which have forced hospital pharmacies to adapt their facilities.
The European Medicines Agency (EMA) has so far approved five ATMPs (excluding vaccines): tisagenlecleucel, axicabtagen ciloleucel, darvadstrocel, voretigen neparvovec and onasemnogen abeparvovec.
The aim of this article is to describe the different aspects to be considered during the pharmacotherapeutic management of ATMPs. The paper is divided into the following sections: definitions and legal framework; research, development and subsequent market introduction; patient selection and therapy evaluation; hospital logistics; health outcomes assessment and performance-based payments; and compounding of ATMPs in the hospital pharmacy as a trend for the future.
Definitions and legal frameworkATMPs are a group of drugs approved for specific therapies: gene therapy (gene-based), somatic cell therapy (cell-based) or tissue engineering (tissue-based)1.
- •
Gene therapy products are obtained through a set of manufacturing processes aimed at transferring a gene into human cells, in vivo or ex vivo, for subsequent expression in vivo, and with the use of vectors of viral or non-viral origin.
- •
Somatic cell therapy involves the use in humans of living autologous, allogeneic, or xenogeneic somatic cells whose biological characteristics have been substantially altered to obtain desired effects. Two kinds of cell therapy can be distinguished:
- -
Mesenchymal cell-based therapies employ multipotent stem cells which, after being expended, develop a series of regenerative characteristics that are therapeutically important for the manufacture and repair of damaged tissues.
- -
CAR-T cell-based therapies use patients’ own lymphocytes which, by means of viral vectors, incorporate specific chimeric receptors that identify, attack and destroy malignant tumor cells.
- -
- •
Tissue engineered therapy involves the use of products containing or consisting of engineered cells or tissues. They may also contain other substances, such as cellular products, biomolecules, biomaterials, chemicals, scaffolds or matrices.
In the European Union, use of ATMPs is governed by Regulation (EC) 1394/20071 of the European Parliament and of the Council, which introduced additional provisions to those established in Directive 2001/83/EC. The Regulation establishes that ATMPs must follow the same principles as other medicinal products, i.e., pre-marketing authorization, demonstration of quality, safety and efficacy with a positive risk-benefit ratio; and post-authorization pharmacovigilance2. They must also comply with the regulations on genetically modified pharmaceutical products3,4.
As with any other medication, Good Clinical Practices (GCP) must be observed. This is stated in the Guide to Good Practices for the Preparation of Medications in Hospital Pharmacy Services5, which emphasizes that the fragility of these medications makes them different from traditional pharmacological and biotechnological products6,7.
The current legislation governing the work of HPDs together with the laws regulating the management of pharmaceutical products in the different autonomous regions constitute a cornerstone for the rational use of medicines, guaranteeing and assuming technical responsibility and establishing an efficient and safe distribution system that ensures correct administration, follow-up and monitoring of the efficacy and safety of the medicines administered8,9.
The functions of HPDs include the handling and adaptation of formulations, as stated in Article 7 of Royal Decree 16/2012, of 20th April on urgent measures to guarantee the sustainability of the National Health System and improve the quality safety of its services10. HPDs, under the supervision of the Directorate-General for the Common Portfolio of Services of the National Health and Pharmacy System, are involved in the handling, fractioning, dosing and transformation of medicines.
These handling functions are also contemplated in Article 47.3 of Royal Legislative Decree 1/2015, which contains general criteria and requirements, and in several European regulations regarding authorization, which are applicable to industrially manufactured advanced therapy medicinal products.
Important aspects of traceability must be considered within the pharmacotherapeutic process involving ATMPs, including: acquisition, storage, dispensing of the drugs in the HPDs themselves, patient follow-up and pharmacovigilance.
Finally, in the case of CAR-T drugs, the National Health System's Plan for Approaching Advanced Therapies, centered specifically on CAR-T drugs5, establishes the need for HPDs to participate in the care process. It states that hospital pharmacists must be members of the expert groups in charge of selecting the hospitals where the treatments will be provided HPDs and in those responsible for regulating the use of CAR drugs at health system level.
HPDs have therefore been provide with adequate regulation in the field of ATMPs, which are just one more drug in the broad therapeutic arsenal pharmacists can avail themselves of to discharge their functions.
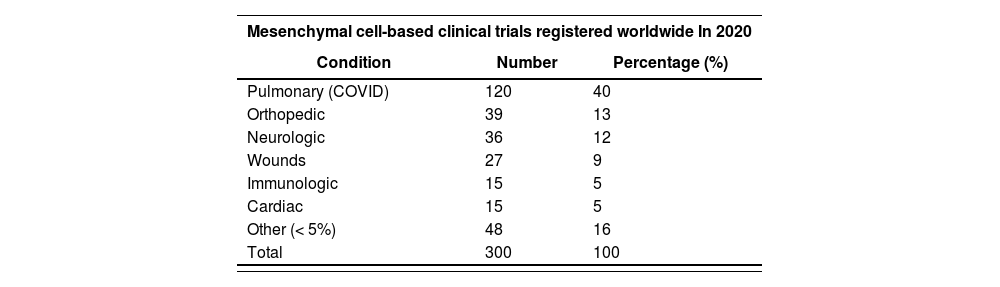
Research, development and subsequent market introductionA systematic review conducted by Hanna E et al. in 2016, identified 939 clinical trials (CTs) on ATMPs11. Since then, a significant growth has taken place in the number of studies on ATMPs, especially in the fields of hemato-oncology and CAR-T therapies. It should be noted that 2020 was a boom year in mesenchymal cell research, with 300 CTs published that year as compared with 156 in 2019. Studies typically looked into the treatment of non-oncological conditions such as post-covid fibrosis, trauma, wounds and cardiovascular and neurological diseases, among others12. Table 1 shows the CTs performed with mesenchymal cells and their associated pathologies during 2020.
Clinical trials on mesenchymal cells conducted in 2020, per condition
| Mesenchymal cell-based clinical trials registered worldwide In 2020 | ||
|---|---|---|
| Condition | Number | Percentage (%) |
| Pulmonary (COVID) | 120 | 40 |
| Orthopedic | 39 | 13 |
| Neurologic | 36 | 12 |
| Wounds | 27 | 9 |
| Immunologic | 15 | 5 |
| Cardiac | 15 | 5 |
| Other (< 5%) | 48 | 16 |
| Total | 300 | 100 |
Following publication of the 2019 consensus report of the Alliance for Regenerative Medicine (https://alliancerm.org) 1,220 CTs on ATMPs were carried out in 2020, including various phases of research and different kinds of advanced therapies13. Of these, 549 studied drugs for hemato-oncological applications (45% of total). A total of 787 of the 1,220 CTs were on cell therapy, 423 on gene therapy and 10 on tissue engineering.
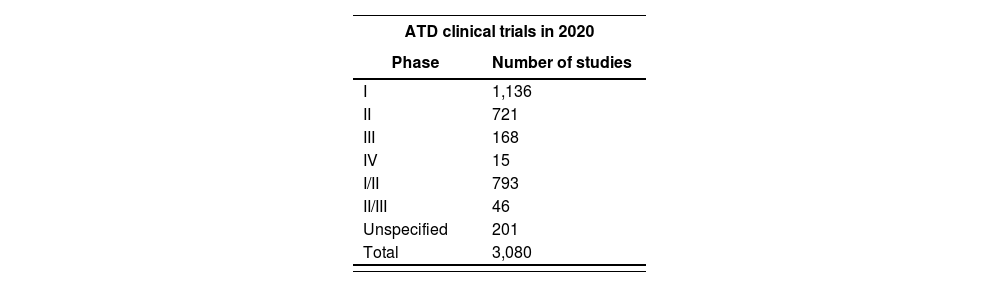
On the other hand, according to the website of the American Society of Gene & Cell therapy, there are currently 3,080 trials underway with ATMPs, more than half of them at phases I or II14. Table 2 shows a breakdown of the ATD RCTs performed in 2020 by stage of development.
Clinical research into drugs is typically carried out by means of robust randomized, controlled and blinded studies. However, studies often present with methodological flaws, including the following:
- -
Follow-up periods tend to be too short to capture long-term efficacy. This means that, for some ATMPs, indirect comparisons and extrapolations may be particularly relevant, particularly when the comparator does not represent the standard of care or when ethical considerations call for a single arm CT15. In addition, two ATMPs (tisagenlecleucel and axicabtagen ciloleucel) have been approved and marketed on the basis of a single-arm CT and historical data, which has made it necessary to plan and initiate controlled trials in the post-approval phase16.
- -
The dose ranges studied are often difficult to identify as classical dosefinding pharmacokinetic analysis is problematic or even impossible in this type of patient given their severity of their condition and their comorbidities.
- -
Determination of efficacy, particularly in gene therapy studies, depends on three important aspects: the efficiency of gene transfer, the capacity of the vector to reach the target cells and the level of expression of the gene of interest, not to mention the existence of inactive particles that may impact the efficiency of transduction and its potency, and the mutagenesis that is likely to occur by insertion or inadvertent alteration of gene expression. This has been shown to constitute a serious problem by previous gene therapy or cell therapy studies17.
- -
Cohort study populations are typically small due to low disease prevalence or low manufacturing capacity, comparators unsuited to randomization, impossibility of blinding or the presence of ethically unjustifiable placebos.
In short, there are a series of methodological aspects such as the patient's situation and the efficiency of the graft or the transduction process which, although justified in the trial protocol and amply supported by the existing data in the investigator's manual,7 provide a new methodological framework that implies new research challenges that are inexcusably linked to the products’ subsequent market introduction.
Patient selection and therapy evaluationThe emergence of advanced therapies has posed a major challenge for regulatory agencies and drug evaluators. Against this background, in 2009 the EMA established the Committee for Advanced Therapies (CAT) for the initial evaluation of ATMPs. This committee brings together experts in gene therapy, cell therapy, tissue therapy, biotechnology, ethics, pharmacovigilance, risk management, medical devices or surgery17. Surprisingly, however, no expert in drug evaluation sits on this committee.
The key issues for HPDs include the following:
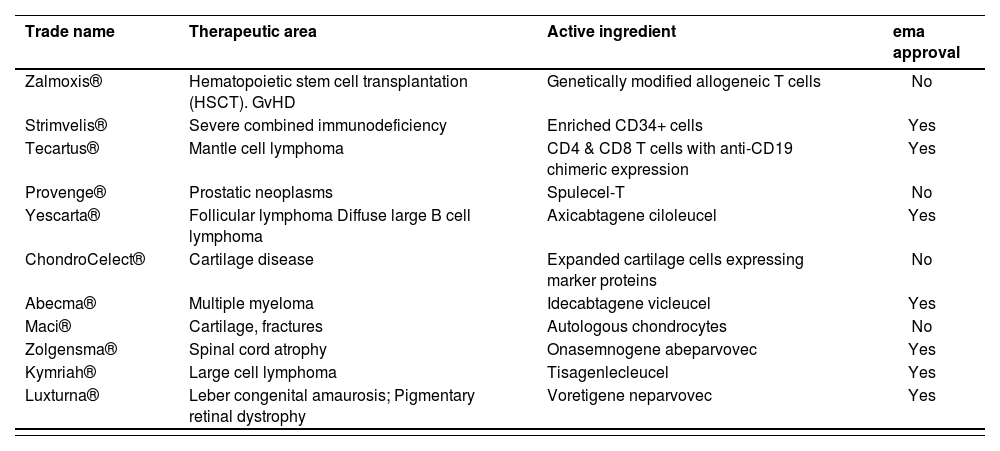
EvidenceAccording to the data available, 11 ATMPs were submitted for approval to the EMA out of which 4 were rejected16. Table 3 shows the drugs with their trade name, therapeutic area, active ingredient and authorization status.
Advanced therapy drugs submitted for approval to the European Medicines Agency up to June 2021, specifying trade name, therapeutic area, active ingredient and approval status
| Trade name | Therapeutic area | Active ingredient | ema approval |
|---|---|---|---|
| Zalmoxis® | Hematopoietic stem cell transplantation (HSCT). GvHD | Genetically modified allogeneic T cells | No |
| Strimvelis® | Severe combined immunodeficiency | Enriched CD34+ cells | Yes |
| Tecartus® | Mantle cell lymphoma | CD4 & CD8 T cells with anti-CD19 chimeric expression | Yes |
| Provenge® | Prostatic neoplasms | Spulecel-T | No |
| Yescarta® | Follicular lymphoma Diffuse large B cell lymphoma | Axicabtagene ciloleucel | Yes |
| ChondroCelect® | Cartilage disease | Expanded cartilage cells expressing marker proteins | No |
| Abecma® | Multiple myeloma | Idecabtagene vicleucel | Yes |
| Maci® | Cartilage, fractures | Autologous chondrocytes | No |
| Zolgensma® | Spinal cord atrophy | Onasemnogene abeparvovec | Yes |
| Kymriah® | Large cell lymphoma | Tisagenlecleucel | Yes |
| Luxturna® | Leber congenital amaurosis; Pigmentary retinal dystrophy | Voretigene neparvovec | Yes |
GvHD: Graft versus host disease.
Elsallab et al. evaluated the evidence submitted to the EMA in ATMP approval applications and observed that non-randomized, unblinded trials with small patient cohorts were in the majority. In addition, as effect sizes are generally small for primary outcomes, secondary, surrogate or subgroup analyses are often used to demonstrate efficacy16. As a result, some regulatory agencies have urged ATMP manufacturers to generate additional post-approval evidence for urgent and unmet patient needs.
Economic evaluationThe cost of using ATMPs is considered to be high taking into consideration the low number of potential patients benefiting from the treatment, with limitations in the realms of intellectual property, competition and reimbursement18.
In order to carry out the economic evaluation of ATMPs, it is necessary to consider other aspects that clearly differentiate them from other drugs.
- -
Single dosing, with potential lifetime benefits, creates a challenge for payers to sufficiently reward drug manufacturers. Clearly, such lifetime benefits cannot be measured in short-term trials19. This lack of economic data is compensated for by the presumed high efficacy of ATMPs. However, there being no scientific evidence to prove this assumption, estimates of the cost-effectiveness of these therapies are inherently biased20.
- -
There is general agreement that the value of a commodity is determined by the consumers’ willingness to pay for it (demand perspective) or by what they would be willing to give up to acquire it (supply perspective or opportunity cost). In practice, there are tacit or explicit thresholds to help define value, which vary from one organization to another. It has been proposed that this threshold should be calculated on the basis of gross domestic product per capita (2-3 times)2.
The evaluation of ATMPs is generally carried out in a context of uncertainty (scarce or low-quality evidence, lack of understanding of long-term effects, potentially serious adverse effects whose incidence is often unknown). This makes it necessary to consider both the beneficence (maximization of benefits) and non-maleficence (minimization of risks) of the interventions from a “principled’ ethical point of view. Particularly, ATMPs benefiting from hospital exemption, whose approval may be requested by merely submitting a dossier with the trials supporting their therapeutic indication2 and which are not subject to any evaluations, place both patients and clinicians in a difficult situation.
On the other hand, the principle of justice, which is the second of the prima facie ethical principles, requires treating all people equally and must be observed not only by protecting the right of the most disadvantaged or vulnerable (for example, patients suffering from rare diseases) to receive the best treatment, but also by ensuring equity in the allocation of economic resources among the population as a whole.
These principles, which can often conflict with each other, must be taken into account not so much by the evaluators, whose role is to present, assess and summarize the existing evidence, but also by the final (political) decision-makers.
Hospital logisticsHospital pharmacists have technical responsibility for the acquisition, storage and dispensing of drugs: This responsibility is implicit in the logistics of ATMPs as is the need to ensure the quality and viability of treatments, maintain the traceability of the pharmacotherapeutic process and monitor its efficacy21.
In this regard, at the logistic level, HPDs use safe and efficient distribution systems to ensure correct administration of the drugs, as well as active pharmacovigilance systems to manage potential adverse events. Such systems are integrated with other hospital applications and management platforms equipped with consumption allocation capabilities and interoperable with manufacturers’ interfaces. All these aspects add value to the pharmacists’ function as integrated members multidisciplinary healthcare teams.
Given that the intrinsic characteristics of ATMPs depend on their therapeutic function and the need to previously manipulate the cells by modifying and expanding them, specific storage conditions are typically required, which place multiple demands on hospital logistics, ranging from refrigerated conditions (2-8 °C) in the case of mesenchymal cells, to ultra-refrigeration (–80 °C) for gene therapy, and even cryopreservation (–180 °C) for CAR-T therapies.
These special conservation needs, which are indicative of the fragility of ATMPs, must be met to ensure the viability of the drugs. In addition, ATMPs are characterized by short shelf lives (48-72 h for mesenchymal cells and 6-9 months for CAR-T therapies), a far cry from the 5 years established for drugs with chemical structures.
Another important consideration is the fact that ATMPs are manufactured to address a patient's specific clinical requirement at a given time. These situations are often critical, which means that pharma companies must be able to obtain useful products subjected to adequate quality controls even though, on many occasions, the biological material they are based on is not in optimal condition.
ATMPs must go through a series of manufacturing phases, each with its specific time frame, before the drugs can be released to the facility from which they can subsequently be distributed. This means that a certain waiting time must be allowed from the manufacturing request to the drugs’ arrival at the HPD, which ranges from 15-20 days for mesenchymal cell-based drugs (which do not usually use the patient's own biological material) to 30 or more days for CAR-T products (which are based on the patient's own lymphocytes). Furthermore, the time elapsing from the arrival of the drug at the HPD to its transfer to the relevant ward and its subsequent administration to the patient can range from 24 hours for mesenchymal cells to 7 days for CAR-T drugs, due to the required re-administration lymphodepletion process.
For all the reasons above, it is vital to involve HPDs in the hospital logistics processes, and train them accordingly, to ensure the viability of the ATMPs from receipt to administration. As already mentioned, ATMPs are produced specifically for individual (critically ill) patients by means of a demanding manufacturing process. The role played by HPDs in managing ATMPs entails a logistic challenge for hospital pharmacists, especially regarding cryopreservation of CAR-T drugs, as stated in the CAR-T drug management procedure, developed by SEFH22.
The need for cryopreservation of CAR-T drugs, together with the strict hospital accreditation criteria imposed by manufacturing laboratories, have resulted in the need to create a new structure within the advanced therapies unit of HPDs consisting of a cryopreservation room where CAR-T drugs can be properly handled from a logistic standpoint. Moreover, specific therapies have been developed to support patients in the postadministration phase23.
The cryopreservation room must be duly appointed and signposted and kept separate from the other HPD facilities. It must comprise differentiated work areas for the handling of CAR-T drugs (reception, therapy transfer and quarantine) and comply with the technical space and safety requirements for handling drugs that must be cryopreserved24. Meeting such requirements is vital to correctly perform the functions of reception, conservation, custody and dispensing of the drugs, and to activate aby contingency plans that may potentially become necessary.
From the infrastructure point of view, HPD premises must possess all the necessary equipment and consumables to ensure correct preservation of CAR-T medicines in liquid nitrogen in gas state25, ensuring at all times that all protection and safety measures regarding the use of personal protective equipment (PPE) are followed and that and all relevant regulations are met26.
In summary, the advent of new therapies such as ATMPs makes it necessary for HPDs to make an efficient contribution to hospital logistics. In addition, in the specific case of CAR-T drugs, specific training in the cryopreservation of drugs in liquid nitrogen in gas state at temperatures of minus 180 °C is necessary with the ultimate goal of involving hospital pharmacists, as the professionals responsible for those drugs, in the broad pharmacotherapeutic process of ATMPs.
Evaluation of health outcomes and performance-based paymentsBecause of their high health and economic impact on health systems, ATMPs are giving rise to the development of new financing and accessibility models based on health outcomes.
The main motivations for this orientation toward health outcomes include a drive for more informed and transparent decision making and higher quality healthcare as well as the need to respond to research challenges and to boost sustainability.
New models for a better access to therapeuticsThe EMA Committee for Advanced Therapies has noted that the main uncertainties around the efficacy of ATMPs are primarily related to the lack of evidence of their real-life and long-term effectiveness, not to mention that administration in clinical practice conditions may differ from administration in the context of a clinical trial.
Studies on the use of CAR-T therapies outside the clinical trial setting have shown that such treatments can have similar safety and efficacy profiles to those shown by pivotal clinical trials27–29. However, in some cases results have been disparate. For example, an English study found overall response and complete response rates of 37% and 21% respectively for axicabtagene ciloleucel and 29% and 17% for tisagenlecleucel30. In a description of the difficulties and challenges involved in the evaluation of these drugs, Jonsson et al. suggest considering financing options different from the usual ones and emphasize the need to obtain long-term results31.
Pay-for-performance schemesCurrently, in most Western countries, flexible access models are being implemented, where benefits and risks are distributed between providers and the healthcare system. Risk-sharing agreements (RSAs) and, among them, payment for performance schemes are the ones likely to provide the best response to the uncertainties around efficacy and cost-effectiveness32.
The experience with these new models is reflected in a study by J⊘rgensen J, et al, which reviews the payment models applied to CAR-T drugs in the last quarter of 2019 in the five largest markets in the European Union: Germany, France, Italy, United Kingdom (England and Scotland) and Spain33 as compared with traditional fixed-price payment models. This research shows that tisagenlecleucel (Kymriah®) and abxicaptagen ciloleucel (Yescarta®) present with a fairly uniform registration price in the five countries evaluated, albeit with certain differences related to the reimbursement and financing schemes negotiated prior to the drugs’ approval in the different countries.
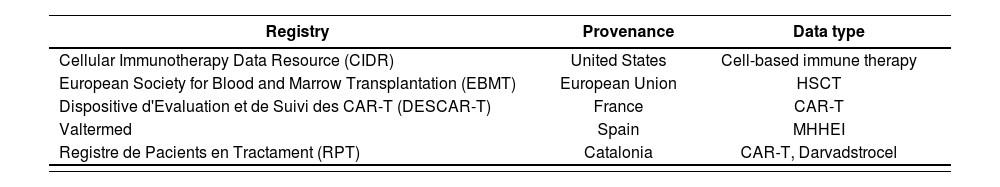
Experience in recording resultsSeveral initiatives have been undertaken to register data on the implementation of ATMPs in clinical practice with the aim of developing data reporting strategies, identifying alternative approaches to share anonymized data, and optimizing observational research based on long-term safety and efficacy data on cell therapies. Table 4 provides an overview of the registries of the use of ATMPs in clinical practice.
Advanced therapy drugs data registries, including their provenance and the type if data collected
| Registry | Provenance | Data type |
|---|---|---|
| Cellular Immunotherapy Data Resource (CIDR) | United States | Cell-based immune therapy |
| European Society for Blood and Marrow Transplantation (EBMT) | European Union | HSCT |
| Dispositive d'Evaluation et de Suivi des CAR-T (DESCAR-T) | France | CAR-T |
| Valtermed | Spain | MHHEI |
| Registre de Pacients en Tractament (RPT) | Catalonia | CAR-T, Darvadstrocel |
HSCT: Hemalopoielic stem cell transplantation; MHHEI: Medicines with high health and economic impact.
- -
Cellular Immunotherapy Data Resource (CIDR), powered in 2016 by the US’ Center for International Blood and Marrow Transplant Research. The register contains data from 159 hospitals, including 4,308 infusions in 4,094 patients (some of them receiving more than one CAR-T therapy, of which 80% were marketed therapies and 20% non-marketed therapies). The data was collected between 2016 and 202134.
- -
European Cellular Therapy Registry of the EBMT, which allows the collection of data on hematopoietic, progenitor or differentiated cells (such as T lymphocytes); non-engineered cell-based treatments (such as donor lymphocyte infusion); in vitro selected and/or expanded and/or genetically engineered cells (such as CAR-T cells); and hematopoietic progenitor cell transplantation. This tool has been valued positively by EMA as a platform for the collection of post-authorization CAR-T safety data. Follow-up data are recorded at day 0 and at 100 days, 6 months and 1 year, and annually thereafter. The activity of this registry has been growing, with more than 2,000 patients registered by June 202135. This increased activity will contribute to improving the infrastructure of CAR-T therapy units36.
- -
The French national registry DESCAR-T (Dispositive d’ Evaluation et de Suivi des CAR-T) includes data up to 12 April 2021 on 647 registered patients from 19 hospitals, 550 of whom were treated (200 with tisa- genlecleucel and 350 with axicabtagene ciloleucel) for diffuse large B-cell lymphoma. Work is underway to connect this registry with the EBMT registry37.
- -
National Valtermed Registry, an information system intended to determine the therapeutic value of medicines with high health and economic Impact on real clinical practice. It was launched in 2019 as an initiative of the Spanish Ministry of Health, Consumer Affairs and Social Welfare through the Directorate-General for the Common Portfolio of Services of the National Health and Pharmacy System aimed at reducing uncertainties associated with the use of this type of therapies in real life in terms of effectiveness and safety. Twenty-four months after the approval of the Spanish NHS’ Advanced Therapies Approach Plan, data were reported on 182 CAR-T administrations (out of 386 treatment requests), with effectiveness outcomes for 96 patients and safety outcomes for 84 patients38.
- -
Registre de Pacients en Tractament (RPT), which has for more than 10 years been collecting data from Catalonian hospitals. The data Is fed into different information systems with a view to improving the quality and efficiency standards of pharmaceutical services, setting health outcome objectives that hospitals can aim for, establishing reimbursement conditions39, and preparing outcome evaluation reports40. This program also records clinical data from patients on CAR-T drugs and darvadstrocel and integrates them into Valtermed.
Producing ATMPs involves adapting HPDs to endow them with the infrastructure required to meet increasingly complex demands. As with any medicine, good manufacturing practice (GMPs or GMPs) standards have to be met. In November 2017, the European Commission adopted specific GMP guidelines for ATMPs affecting both industrial and galenic preparations41.
ATMPs are complex products whose risks differ greatly depending on the type of drug, the characteristics of the starting materials and the manufacturing process used. This is why GMPs for ATMPs are founded on a risk- based approach, with the manufacturer being responsible for establishing the necessary organizational, technical and structural measures to ensure the quality of the final product. In addition, the viral vectors used in the development of gene therapy drugs must be manipulated in vertical laminar flow hoods following the same procedures as for cytotoxics. The cleaning products, however, are not the same as in the case of ATMPs virucides must be used that are capable of eliminating the vectors used and a certain period time must be allowed before and after the preparation process. Cross-contamination must be avoided at all costs42.
GMPs for ATMPs require the introduction of a risk management protocol to ensure that the product's quality. The system must ensure the following aspects5:
- -
Highly qualified personnel and clear delimitation of responsibilities.
- -
Appropriate facilities and equipment to avoid cross-contamination, as well as proper maintenance. Clean rooms should conform to ISO 14644-1 and be re-evaluated annually. In addition, a cleaning/ sanitization system should be in place for contamination control purposes.
- -
An exhaustive documentation system, with specifications of materials, intermediate, bulk or finished products. This documentation system should allow control, monitoring and recording of all activities that could directly or indirectly affect the quality of ATMPs.
- -
An adequate manufacturing process to ensure consistent production, quality control and compliance with specifications.
- -
The quality control system should be operationally independent of production.
- -
Provisions should be in place for prospective evaluation of planned changes and their approval prior to implementation.
- -
Ability to identify quality defects and process deviations; a system must be in place to investigate causes and take corrective or preventive action.
- -
Full traceability of the final product and of the initial and critical raw materials.
- -
Simulation tests of the aseptic process (media fill) must be carried out with a sterile microbiological growth medium and/or a placebo.
Extemporaneous formulations have always been linked to the HPD. The Spanish NHS’ Advanced Therapies Approach Plan for CAR-T drugs considers hospital pharmacists to be key actors in the management of ATMPs. In addition, Royal Decree 477/2014, which regulates the authorization of non-industrially manufactured ATMPs (hospital exemption)43, establishes the possibility that these drugs may be compounded in hospitals and lays down traceability and pharmacovigilance requirements to that effect. Certain hospitals prepare ATMPs such as CAR-T drugs, on an occasional basis under the sole responsibility of a registered physician and for the purpose of filling an individual prescription for a product intended specifically for a particular patient. These non-industrially compounded ATMPs are subject to a specific authorization procedure adapted to their special compounding and application characteristics, which ensures that all quality, safety, efficacy, identification and information requirements are met.
There are examples of non-industrial ATMPs being granted approval by the Spanish Medicines and Health Products Agency (AEMPS), which demonstrates the responsiveness of the Spanish National Health System. Within this framework, there are examples of hospital pharmacists who have assumed technical responsibility for the compounding of ATMPs44.
The Advanced Therapies Unit (ATU) of the Hospital Politècnic i Universitari La Fe was created with this purpose in mind following an application to the AEMPS. The idea was to conduct pre-clinical and clinical studies and research programs on advanced therapies. On 11 May 2020, Dr. José Luis Poveda was appointed Technical Director of the ATU, which marked a milestone in the involvement of hospital pharmacists in the development of ATMPs.
This unit was authorized by AEMPS to develop four types of advanced therapies for the treatment of viral reactivations in hemato-oncological patients based on allogeneic T lymphocytes against: cytomegalovirus, adenovirus, the Epstein-Barr virus and the BK virus.
Although the development of ATMPs is associated with technical challenges that are not without risk, HPDs have the experience and capacity to address them ensuring treatment quality and patient safety.
FundingNo funding.
AcknowledgementsThe authors would like to thank Farmacia Hospitalaria for inviting them to submit the present manuscript.
Conflict of interestNo conflict of interests.
Early Access date (01/26/2022).