To determine by experimentation whether micafungin and anidulafungin possess physicochemical properties suitable for nebulization.
MethodPH, osmolality, viscosity, density and chloride content were determined by pH monitoring, osmometry, viscometry, densitometry and potentiometry in two samples of different concentrations, 5 and 10 mg/mL each echinocandin.
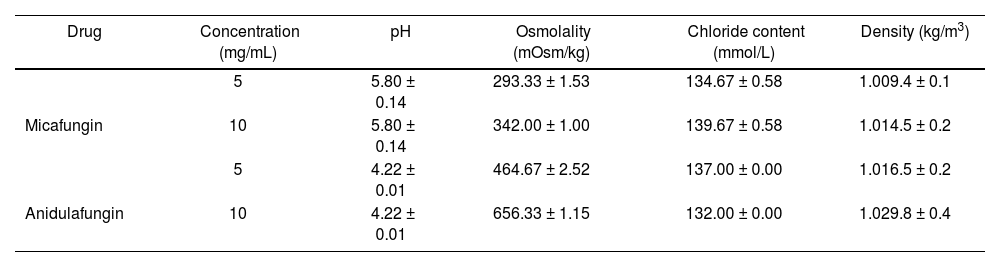
ResultsThe results obtained for micafungin solution were: pH 5.80 (0.14), osmolality 293.33 (1.53) mOsm/kg, chloride content 134.67 (0.58) mmol/L and density 1,009.4 (0,1) kg/m3; while for 10 mg/mL solution: osmolality 342.00 (1.00) mOsm/kg, chloride content 139.67 (0.58) mmol/L and density 1,014.5 (0.2) kg/m3. The results obtained for 5 mg/mL anidulafungin were: pH 4.22 (0.01), osmolality 464.67 (2.52) mOsm/kg, chloride content 137.00 (0.00) mmol/L and density 1,016.5 (0,2) kg/m3; while for 10 mg/mL solution: osmolality 656.33 (1.15) mOsm/kg, chloride content 132.00 (0.00) mmol/L and density 1,029.8 (0.4) kg/m3.
ConclusionsPH, osmolality, chloride content and density values proved to be suitable for proper tolerability by nebulization.
Determinar experimentalmente si micafungina y anidulafungina poseen propiedades fisicoquímicas adecuadas para su nebulización.
MétodoSe determinó el pH, la osmolalidad, la viscosidad, la densidad y el contenido en cloruros mediante pH-metría, osmometría, viscosimetría, densitometría y potenciometría, respectivamente, en dos muestras de diferente concentración, 5 y 10 mg/ml, de cada equinocandina.
ResultadosPara la solución de micafungina 5 mg/ml los resultados obtenidos fueron: pH 5,80 (0,14), osmolalidad 293,33 (1,53) mOsm/kg, contenido en cloruros 134,67 (0,58) mmol/l y densidad 1.009,4 (0,1) kg/m3; y para la solución de 10 mg/ml: osmolalidad 342,00 (1,00) mOsm/kg, contenido en cloruros 139,67 (0,58) mmol/l y densidad 1.014,5 (0,2) kg/m3. Para la solución de anidulafungina 5 mg/ml los resultados obtenidos fueron: pH 4,22 (0,01), osmolalidad 464,67 (2,52) mOsm/kg, contenido en cloruros 137,00 (0,00) mmol/l y densidad 1.016,5 (0,2) kg/m3; y para la solución de 10 mg/ml: osmolalidad 656,33 (1,15) mOsm/kg, contenido en cloruros 132,00 (0,00) mmol/l y densidad 1.029,8 (0,4) kg/m3.
ConclusionesLos valores de pH, osmolalidad, contenido en cloruros y densidad resultaron adecuados para una correcta tolerabilidad mediante nebulización.
In critical or immunosuppressed patients, such as lung transplant patients, systemic fungal infections can carry serious clinical consequences1–4.
Possible fungal infections affecting these patients include species of the genus Aspergillus sp. In immediate post-lung transplantation these infections primarily affect respiratory tract and include ulcerative tracheobronchitis and anastomotic infections. Current guidelines prescribe triazole as a first-line treatment and advise the possibility of associating a nebulized antifungal as an adyuvant treatment5. Said association would prove to be beneficial, considering that these patients do not usually reach appropriate drug concentrations in lung with parenteral administration, due to the tissue penetration being low in lung. Most of the patients undergo mechanical ventilation, which produces an alteration in pharmacokinetic parameters of the active substance6.
There is a growing tendency of infections caused by fungal species in addition to Aspergillus sp, whose response to conventional antifungal treatment is very limited, as Scedosporium sp or Scopulariopsis sp, among others. For infections disseminated by Scedosporium sp, voriconazole in monotherapy or either combined with an intravenous echinocandin and/or terbinafine is prescribed7,8. However, the optimal antifungal for Scopulariopsis sp is unknown.
In situations where clinical response to antifungal agents of choice is either ineffective or adverse effects occur, and the source of infection is located in the respiratory tract, antifungal drugs may be considered via nebulization.
Currently, literature on nebulization of anti-infective agents is limited, and most have not authorized this route of administration. There is published literature on nebulization of conventional and liposomal amphotericin B, pentamidine, nystatin, posaconazole, voriconazole, itraconazole, caspofungin and micafungin1,3,4,7,9–12. Published studies on nebulization of micafungin were aimed exclusively at characterizing drug particles released through various nebulizers9,10.
The administration of anti-infective drugs through nebulization, combined with intravenous treatment, may provide an alternative in cases where there is little diffusion across biological membranes as well as a high concentration of drug in the respiratory tract is required to facilitate control on the source of infection6.
In addition, drug nebulization allows reaching local high concentrations with minimal systemic exposure, which generally translates into greater efficiency and fewer systemic side effects1,4,6,7,9–12. Thus, it could be administered via nebulization in monotherapy whenever local action is required or when a patient has adverse effects on intravenous administration2.
Literature suggests that, in order to achieve optimal nebulization, physicochemical properties of active substance, the nebulizer system used and the patient's physical and clinical conditions must be taken into account1,4.
Physicochemical properties such as pH, osmolality, chloride ion concentration, density and particle size of the drug affect the efficacy, as well as the tolerability of nebulization (Table 1). Furthermore, the presence of sodium edetate excipients, benzalkonium chloride, phenols and sulfites can cause poor tolerability4,11.
Studies so far show that extreme pH values, osmolality and lack of chlorides in preparations of nebulization may cause coughing and/or bronchoconstriction4,11,13.
The volume of drug to be administered must be adequate to ensure proper viscosity. Drug dissolution in small volumes lead to a high viscosity, which could make nebulization difficult and could as well cause blockage or damage to the nebulizer, while solution in larger volumes would result in a reduced viscosity that could increase nebulization time6,11.
Particles size, expressed as mass median aerodynamic diameter (MMAD), must lie in the range of 1-5 µm, allowing adequate access to the site of action. Particles exceeding 5 µm MMAD are deposited in the upper airways, while those with less than 1 µm MMAD can be expelled during exhalation4.
The dose to be administered by nebulization of anti-infectives that do not have authorization via nebulization is established empirically in cases where previous pharmacokinetic studies have not been carried out.
The aim of this study focuses on experimentally assessing whether micafungin and anidulafungin antifungals could present adequate tolerability via nebulization, since its data sheet only provides the intravenous route.
MethodsTo perform the analytical determinations, we started from commercial presentations by Mycamine® (micafungin) and Ecalta® (anidulafungin) 100 mg concentrated powder for perfusion solution. They were prepared from those two different concentrations of each echinocandin, 5 mg/mL and 10 mg/mL, using 0.9% of sodium chloride as diluent.
Osmolality determinations and chloride content in triplicate were performed for each of the samples in the hospital's Biochemistry Department. Osmolality was determined through automated osmometer Advanced Instruments INC® A2O, employing the freezing point reduction technique. The chloride content was determined through potentiometry with the Beckmann Coulter® AU5800.
PH determination was conducted in the Pharmacy Service by pH meter Testo 206®, also in triplicate, with only a concentration of 5 mg/mL, as it is an independent concentration variable.
Viscosity and density were determined by the Drug Development Service in the School of Pharmacy and Food Sciences of the University associated with the hospital through the Brookfield CAP 2000® viscometer and Anton Paar® densitometer respectively, and in triplicate for each of the concentrations of both echinocandins.
ResultsThe results obtained, expressed as mean ± standard deviation for samples of micafungin 5 mg/mL and 10 mg/mL and anidulafungin 5 mg/mL and 10 mg/mL are shown in table 2.
Results obtained in echinocandins samples
| Drug | Concentration (mg/mL) | pH | Osmolality (mOsm/kg) | Chloride content (mmol/L) | Density (kg/m3) |
|---|---|---|---|---|---|
| 5 | 5.80 ± 0.14 | 293.33 ± 1.53 | 134.67 ± 0.58 | 1.009.4 ± 0.1 | |
| Micafungin | 10 | 5.80 ± 0.14 | 342.00 ± 1.00 | 139.67 ± 0.58 | 1.014.5 ± 0.2 |
| 5 | 4.22 ± 0.01 | 464.67 ± 2.52 | 137.00 ± 0.00 | 1.016.5 ± 0.2 | |
| Anidulafungin | 10 | 4.22 ± 0.01 | 656.33 ± 1.15 | 132.00 ± 0.00 | 1.029.8 ± 0.4 |
The viscosity of the samples could not be determined as they were aqueous solutions with a very similar viscosity as the water's.
DiscussionThe pH and osmolality values obtained in micafungin and anidulafungin samples in the studied concentrations, as well as the chloride content, are within the ranges accepted for a correct tolerability via nebulization1,4,7,13. The density values obtained are similar to the value of water density (1,000 kg/m3). Thus, aerosolization in the nebulizer would be suitable1. Therefore, the studied physicochemical characteristics indicate that its distribution through nebulization may be suitable. Reconstitution of micafungin and anidulafungin was performed using 0.9% sodium chloride, since according to the data sheet, both were stable and, thus, chloride ions were added to the solution.
In addition, the data sheet indicates that the employed presentations do not contain any excipient (sodium edetate, benzalkonium chloride, phenols and sulfides) in connection with the production of cough and/or bronchoconstriction.
This is the first reported study that determines micafungin physicochemical properties: pH, osmolality, chloride content and density for nebulization. In addition, this is the first study on anidulafungin physicochemical characterization to administer through nebulization. Density values and chloride content obtained for both echinocandins are similar to the results published in Wong-Beringer et al. study on characterization of caspofungin, while the difference in pH and osmolality is higher, but are within the recommended ranges1.
The studied concentrations would allow administration of appropriate volumes for nebulization of a 50 mg dose, used in Shi et al. and Alexander et al.'s studies9,10. In said studies where the release of micafungin solution at a concentration of 10 mg/mL was characterized, MMAD were obtained within the established values. Therefore, a significant proportion of the drug would reach deep airways. These results improve the chances of using micafungin via nebulization.
Unlike amphotericin B deoxycholate or liposomal, currently there are no published studies evaluating the efficacy, safety and tolerability of micafungin and anidulafungin through nebulization. One example is the study of Monforte et al., which showed a local distribution in the lungs of liposomal amphotericin B and an adequate tolerability after a 25 mg administration14. It was also noted that suitable drug levels are maintained for at least 14 days after administration14.
As for the study's limitations, it should be highlighted that results would only be valid for the used trademarks. To ensure effective dissemination of the drug in the lung, it would also be necessary to determine other physical factors, such as particle size, type of nebulizer as well as the patient's physical and clinical characteristics.
The micafungin and anidulafungin solutions described in this study would be suitable for nebulization and they can be used in events of complex respiratory fungal infections caused by susceptible species. They could be administered, either in conjunction with intravenous therapy to intensify the treatment or in monotherapy when the intravenous route is not possible or adequate.
FundingNo funding.
Conflict of interestsNo conflict of interests.
Presentation in CongressesPresentation in poster format at the 63rd National Congress of the Spanish Society of Hospital Pharmacy (SEFH). Palma de Mallorca. November 8 to 10, 2018.
Contribution to scientific literatureThis is the first study to physicochemically characterize micafungin and anidulafungin for nebulization. Nebulization would treat respiratory infections caused by susceptible microorganisms.