Drug-drug interactions may modify the therapeutic effect or the safety profile of the medicines used in pediatric populations. Although interest on potential drug interactions in these age groups has increased, information on clinically relevant drug-drug interactions is still scarce. The aim of this study was to explore the prevalence and characteristics of potential and clinically relevant drug-drug interactions among pediatric patients hospitalized in two pediatric hospitals of Mexico City.
MethodA cross-sectional study was conducted on patient records in critical, oncological, burns and other non-critical services by a pediatric resident physician at both hospitals. Micromedex® was used as a source of potential drug-drug interactions data. Subsequently, each interaction's prevalence, severity and evidence level were estimated. Additionally, drug-drug interaction causality with regard to diverse clinical outcomes of hospitalized patients was determined through the Drug Interaction Probability Scale. The clinical consequences of each interaction were classified by severity.
ResultsThe observed prevalence of one or more potential drug-drug interactions in hospitalized patients was 61.3% (52.2-70.4%), whilst the prevalence of real drug-drug interactions was 3.6% (0.1-7.1%). Of potential drug-drug interactions, 60.5% were considered major and only 5.1% contraindicated. These were generally more common in intensive care and burn units. The main pharmacological agents involved in potential drug-drug interactions were opioids analgesics and anti-infective and neurologic agents. Four clinically relevant drug-drug interactions required a regimen change and another prompted an extension of the patient's hospital stay.
ConclusionsPotential drug-drug interactions were common in the pediatric patients studied, whereas the frequency of real drug-drug interactions was low. However, some drug-drug interactions required medical actions in addition to routine monitoring. More information is needed on real drug-drug interactions as those related to failed efficacy might be underestimated.
Las interacciones fármaco-fármaco pueden modificar el efecto terapéutico o la seguridad de los medicamentos usados en poblaciones pediátricas. Aunque el interés sobre interacciones potenciales en estos grupos etarios viene incrementando, aún es escasa la información sobre interacciones fármaco-fármaco que se manifiestan clínicamente en el paciente (reales). El propósito de este estudio fue explorar la prevalencia y características de las interacciones fármaco-fármaco potenciales y reales en pacientes ingresados en dos hospitales pediátricos de la Ciudad de México.
MétodoSe llevó a cabo un estudio transversal en expedientes de pacientes atendidos en servicios críticos, oncológicos, de quemados y otros no críticos por un médico residente de pediatría en ambos hospitales. Se usó Micromedex® como fuente de datos de interacciones potenciales, luego se estimó su prevalencia por paciente, gravedad y nivel de evidencia. Adicionalmente, se determinó la causalidad de las interacciones fármaco-fármaco con diversos desenlaces clínicos de los pacientes hospitalizados mediante la Drug Interaction Probability Scale, y finalmente se clasificaron por gravedad.
ResultadosSLa prevalencia observada de pacientes hospitalizados con una o más interacciones fármaco-fármaco potenciales fue del 61,3% (52,5-70,4%), mientras que la prevalencia de interacciones fármaco-fármaco reales fue del 3,6% (0,1-71%). Entre las interacciones potenciales, el 60,5% se consideraron importantes y sólo el 5,1% contraindicadas. En general, las interacciones fármaco-fármaco potenciales fueron más comunes en los servicios de cuidados intensivos y de quemados. Los principales grupos farmacológicos involucrados en interacciones potenciales fueron agentes analgésicos opioides, antibióticos y neurológicos. Cuatro interacciones reales requirieron modificación de la farmacoterapia y una prolongó la estancia hospitalaria.
ConclusionesLas interacciones potenciales fueron comunes en los pacientes pediátricos estudiados, mientras que la frecuencia de interacciones reales fue baja; sin embargo, sus consecuencias requirieron acciones médicas adicionales a la monitorización habitual. Se requiere más información sobre las interacciones reales, aquellas referidas a faltas de eficacia podrían estar subestimadas.
Use of medicines in pediatric patients entails more difficulties than in adults as physiological development brings about significant changes in the bioavailability of the drugs in the patients’ body1,2. As a result, special attention must be paid not only to the dosing required by each particular individual but also to any potential drug-drug interactions (DDIs) that could interfere with the effect of the therapy, negatively impacting its effectiveness and/or safety profile3,4.
When DDIs appear as a clinical manifestation in the patient they are called real DDIs (rDDIs). Such clinical manifestations, regardless of their specific mechanisms, may involve the appearance of adverse drug reactions (ADRs) or a modification of the drug's therapeutic effect. DDIs without clinical manifestations are called potential DDIs (pDDIs)5.
There are certain factors that are specific to pediatric patients and which could increase the risk of pDDIs. These include sudden changes in hepatic and renal function parameters, in the physiology of the stomach, the patency of the cells, and the ratio of total body water to fat-free body mass, all of which tend to affect the drugs’ pharmacokinetic profile. Such changes usually affect neonates more than other patient groups given that the drug clearance rate in those patients is lower, which increases the medicines’ half-life6. Moreover, other factors such as polypharmacy7-9, especially during adolescence10,11; long hospital stays7,8; certain conditions such as neoplasms, autoimmune diseases, diseases of the central nervous system and congenital diseases7-9; admission to the intensive care unit7; and use of antineoplastic, antiepileptic, gastrointestinal, diuretic or adrenergic drugs, or opioids, antibiotics, or inmunosuppressants8,9,11,12, are also associated with a higher incidence of pDDIs.
Although multiple studies have in the last few years analyzed the occurrence of DDIs in pediatric patients8-15, the information available is still scarce and almost exclusively restricted to pDDIs or their risk factors. The estimated prevalence of pDDIs among pediatric patients on two or more drugs is highly variable, ranging from 1.4%11 to 83.5%14. Such variability reflects a significant methodological diversity and often results from the use of multiple data bases as DDI identification tools, which may differ in terms of predictive value, classification of clinical relevance, access, and ease of management16.
In addition, evidence on the occurrence of DDIs in pediatric patients is still scarce. A study of acute patients who required hospitalization showed a very low prevalence of rDDIs (0.009%)17. However, the study was not specifically geared toward evaluating DDIs bur rather to an analysis of medication-related problems in general, which means that DDIs were not examined or described in great detail.
Another limitation is the lack of information on patients admitted to pediatric burn units. The challenging clinical situation of these patients, which usually requires complex pharmacotherapeutic management and the continuous use of drugs with high DDI potential, would deserve a more specific description18, along the lines of the reports on pediatric patients admitted to critical care units or on those with a hemato-oncologic diagnosis7,9. As a result, study of DDIs in these patient groups is essential, distinguishing the specificities of such interactions for clinical practice and their implications for the patients’ safety. The purpose of this study was therefore to explore the prevalence and characteristics of pDDIs and rDDIs in two pediatric hospitals in Mexico City.
MethodsStudy designThis was a cross-sectional pilot study of acute pediatric patients who were admitted to different hospital departments between May 2017 and May 2019. The study was designed and led by a pharmacist.
Population and settingPatients were treated at either the Federico Gómez Children's Hospital or the Tacubaya Pediatric Hospital, both in Mexico City. The former is a third-level 229-bed teaching hospital, one-quarter of whose admissions are cancer patients. The latter is a second-level 76-bed hospital that provides hospitalization and intensive care services to burnt children.
A resident pediatrician was assigned to rotate through the different departments of both hospitals. From each department, a selection was made of pediatric patients of up to 18 years of age who were admitted for hospitalization and concomitantly received two or more medicines during their stay.
Study variablesThe primary variable in this study was the prevalence of DDIs arising from the drug treatment administered during the patients’ stay in hospital. To explore the phenomenon, both pDDIs and rDDIs were examined. The former were defined as potential interactions between two or more drugs, which could bring about adverse events; the latter were defined as actual interactions between two or more drugs that brought about quantifiable clinical events, taking into consideration the patients’ individual status5.
Sources of clinical informationThe information on drug therapies and on the patients’ clinical evolution during their hospital stay was extracted from the medical records using a template specifically designed to document one single hospitalization period per patient. Medical records in both hospitals are filled in manually onto predesigned computer-based charts which are then printed out in paper in accordance with the Mexican Official Medical Records Standard. Demographic variables recorded included sex, date of birth, weight, and height on admission. Other variables recorded were the patients’ diagnosis on admission [in accordance with the International Classification of Diseases, tenth edition (ICD-10)], length of hospital stay and history of drug allergies. Finally, a list of all the medicines administered concomitantly to patients during their hospital stay was drawn up with information on dose per kg of body weight, route of administration, and date of initiation and termination of administration. Medicines were then grouped in accordance with their anatomic, therapeutic, and chemical classification.
Classification of drug-drug interactionsA resident pediatrician collected information on each patient's pharmacotherapeutic profile and carried out an evaluation of the data. This first evaluation gave rise to a database with information on the identified pDDIs, the number of pDDIs per patient, pDDI severity, level of evidence, and clinical description. Subsequently, pharmacotherapeutic profiles were evaluated independently by a pharmacist, who verified the work done by the resident pediatrician. Both investigators used Micromedex®19 as it offers a range of attributes wide enough for professional use and, according to the literature20,21, has shown itself reasonably effective in identifying pDDIs. Moreover, the database has been cited in multiple studies analyzing the prevalence of DDIs, either as sole source of data or in combination with others7-10, and is widely known internationally16. DDIs were classified into several categories depending on their severity: “contraindicated”, when concomitant use of certain drug pairs was not recommended; “major”, if the interaction could potentially cause death and/or required medical intervention to minimize or prevent severe adverse events; “moderate”, if the interaction could worsen the patient's situation and/or required a change of therapy; and “minor”, if the interaction could be associated with limited clinical effects but did not require any change in therapy. The level of evidence of each interaction was also captured and rated as excellent, good, or sufficient, according to Micromedex®.
Information on clinical outcomesAll suspected ADRs were documented, including their clinical manifestations, duration, and effects, as well as the relevant lab test results, specialist referrals, and any medical intervention carried out to address such events. An ADRs was defined as a fortuitously harmful response to a drug occurring within the usual therapeutic range in humans22. When the resident pediatrician determined that the clinical description of a pDDI was compatible with the appearance of some adverse event (including ADRs or instances of therapeutic failure), the Drug Interaction Probability Scale (DIPS)23 was applied to determine whether there was a cause/ effect relationship between pDDI and ADR. When the relationship was considered likely (5 to 8 points) or very likely (> 8 points), an rDDI was considered to have occurred. ADRs not related to pDDIs were evaluated using Naranjo's algorithm for proper documentation and reporting into the local pharmacovigilance system. Finally, consequences of rDDIs were classified on the basis of the damage categories of the scale developed by the National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP)24.
Statistical analysisThe prevalence of pDDIs or rDDIs was defined as the proportion of patients experiencing some interaction with respect to the group of subjects on two or three drugs, multiplied by 100.
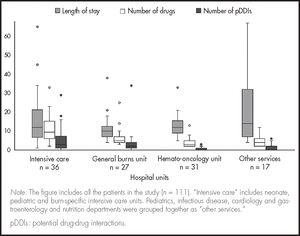
Age was calculated based on the patients’ date of birth and their date of admission to hospitalization. For descriptive purposes, the continuous age variable was transformed into the following categories: < 29 days, neonates (including pre-term neonates); 29 days to 23 months, infants; 24 to 71 months, pre-school age; 72-143 months, school-age; ≥ 144 months, adolescents. Subjects were also classified into four categories according to their body mass index (BMI) z score: normal (-1 ≥ z ≤ +1 SD), malnourished (< -1 SD), overweight (+2 ≥ z > +1 SD), and obese (> +2 SD), using the WHO AnthroPlus software (except for preterm neonates). Finally, the hospitalization unit variable was aggregated into four categories for comparison purposes: “intensive care units” [neonatal and pediatric intensive care units (NICU and PICU, respectively)], “burn units” [basic, intermediate care and intensive care burn units (BBU, IBU and ICBU, respectively)], “oncology units” and “other non-critical units” (cardiology, pediatrics, infectious diseases, gastroenterology, and nutrition). PICU, NICU, IBU and ICBU were all considered critical units. Patients were considered to be on polypharmacy if they were taking 5-9 medications and on excessive polypharmacy if they were on ≥ 10 medications.
Descriptive analysisQualitative variables were expressed as relative frequencies and percentages, including a 95% confidence interval (95% CI) for prevalence indicators. Moreover, distributions of quantitative variables were analyzed using central tendency and dispersion measures. An exploratory bivariate analysis was used to compare sex, age group, BMI z score, number of medications, length of hospital stay, presence of ADRs and type of diagnosis variables between two groups: patients with at least one pDDI and those without pDDIs. The chi-squared test (χ2) or the Exact Fisher test were used (as appropriate) for qualitative variables. In addition, the Mann-Whitney U test was used for quantitative variables without a normal distribution. Associations between continuous variables (number of drugs, length of hospital stay, number of pDDIs) were studied by means of Spearman's rank correlation coefficient (rS). A statistical significance of 95% was accepted for all (two tailed) tests performed (α = 0.05). The statistical analysis was performed using the SPSS v25 software package.
Ethical considerationsThe study protocol was approved by the relevant research ethics committees. All DDI-derived ADRs were reported to the National Regulatory Authority in accordance with the applicable pharmacovigilance standards.
ResultsA total of 111 patients were included in the study. Their characteristics are shown in table 1. The main causes for admission were second degree burns, bacterial pneumonia, and febrile neutropenia, which accounted for 59.4% of admissions. A total of 36 patients (32.4%) were admitted to the critical care unit. The main diagnoses were infection-related and included bacterial pneumonia (14.4%) and septic shock (4.5%). In addition, five patients (4.5%) presented with burns and were admitted to the UQTI. Median hospital stay was 11 days (range: 1-302 days), while the median number of concomitant medications was 5 (range 2-33). Variables such as sex, age group and nutritional status did not show themselves to be associated to the presence of pDDIs (Table 1).
Patients' characteristics
| Variable | Total n = 111 | Without pDDIs n = 43 | With pDDIs n = 68 | p |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 59 (53.2) | 20 (46.5) | 39 (57.4) | |
| Female | 52 (46.8) | 23 (53.5) | 29 (42.6) | 0.265 |
| Age group, n (%) | ||||
| Neonates | 4 (3.6) | 3 (7.0) | 1 (1.5) | |
| Infants | 38 (34.2) | 12 (27.9) | 26 (38.2) | |
| Pre-school | 34 (30.6) | 12 (27.9) | 22 (32.4) | 0.229 |
| School-age | 20 (18.0) | 11 (25.6) | 9 (13.2) | |
| Adolescent | 15 (13.5) | 5 (11.6) | 10 (14.7) | |
| BMI z score, n (%)† | ||||
| Malnourished | 23 (20.7) | 10 (25.0) | 13 (19.4) | |
| Normal | 32 (28.8) | 13 (32.5) | 19 (28.4) | 0.678 |
| Overweight | 19 (17.1) | 5 (12.5) | 14 (20.9) | |
| Obese | 33 (29.7) | 12 (30.0) | 21 (31.3) | |
| Diagnosis on admission (ICD 10), n (%) | ||||
| Second degree burns | 27 (24.3) | 3 (7.0) | 24 (35.3) | |
| Bacterial pneumonia | 22 (19.8) | 3 (7.0) | 19 (27.9) | |
| Febrile neutropenia | 17 (15.3) | 15 (34.9) | 2 (2.9) | <0.001 |
| Septic shock | 12 (10.8) | 7 (16.3) | 5 (7.4) | |
| Neoplasm | 9 (8.1) | 4 (9.3) | 5 (7.4) | |
| Congenital malformations | 6 (5.4) | 2 (4.7) | 4 (5.9) | |
| Other diagnoses | 18 (16.2) | 9 (7.0) | 1 (1.5) | |
| Treating department, n (%) | ||||
| Intensive care | 36 (32.4) | 8 (18.6) | 28 (41.2) | |
| Burn unit | 27 (24.3) | 3 (7.0) | 24 (35.3) | |
| Oncology | 31 (27.9) | 21 (48.8) | 10 (14.7) | <0.001 |
| Other non-critical care units | 17 (15.3) | 11 (25.6) | 6 (8.8) | |
| Length of stay (days), median (IQR) | 11 (7-17) | 11 (7-21) | 11 (7-16) | 0.490 |
| Number of medicines, median (IQR) | 5 (3-9) | 3 (2-4) | 7 (5-13) | <0.001 |
| Polypharmacy (medicines), n (%) | ||||
| 2-4 | 44 (39.6) | 33 (76.7) | 11 (16.2) | |
| 5-9 | 43 (38.7) | 10 (23.3) | 33 (48.5) | <0.001 |
| ≥ 10 | 24 (21.6) | 0 | 24 (35.3) | |
| Occurrence of ADRs, n (%) | ||||
| Yes | 25 (22.5) | 19 (44.2) | 6 (8.8) | |
| No | 86 (77.5) | 24 (55.8) | 62 (91.2) | <0.001 |
ADR: adverse drug reaction; BMI: body mass index; ICD-10: International Classification of Diseases, tenth edition; IQR: Interquartile range; pDDI: potential drug-drug Interaction.
A total of 332 pDDIs (142 different pairs) were identified in 68 patients, which represented an overall prevalence of 61.3% (95% CI 52.2-70.4%). At the same time, five rDDIs were observed in four patients, with a prevalence of 3.6% (95% CI 0.1-7.1%), among children who received two or more drugs while hospitalized.
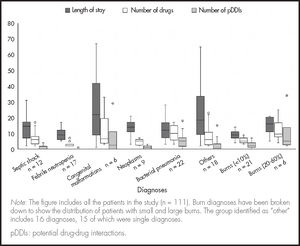
The mean number of pDDIs per patient in this study was 4.8. A positive association was observed between the total number of pDDIs and the number of drugs administered (rS = 0.80; p < 0.001) across all the hospital units under analysis (Figure 1). The length of hospital stay was not correlated with the number of pDDIs or with being on polypharmacy. Some patients admitted to non-critical units with moderate polypharmacy and short hospital stays showed similar pDDI rates. A total of 52.9% of patients presented with neutropenic fever or minor burns affecting 10% of their body surface. Other patients with de novo neoplasms had highly variable hospital stays and a low pDDI rates. On the other hand, 51.2% of patients admitted to critical care units such as PICU or ICBU presented with pneumonia or burns affecting 20-60% of their body surface on admission and displayed highly variable lengths of hospital stay, exposure to polypharmacy and pDDIs (Figure 2).
Of the 332 pDDIs, 17 (5.1%) were classified as contraindicated, 201 (60.5%) as major, 97 (29.2%) as moderate, and 17 (5.1%) as minor. The level of evidence was considered excellent in 20 cases, (6.0%), good in 81 (24.4%), and sufficient in 231 (69.6%). Only one of the five rDDIs observed resulted in an extension of the patients’ hospital stay. The remainder required treatment and closer monitoring than usual (Table 2).
Clinically significant drug-drug interactions
| Interaction (unit) | Objective effect (n) | Clinical description† | Severity‡ | Level of evidence† | DIPS score (causal relationship) |
|---|---|---|---|---|---|
| Salbutamol-furosemide (PICU) | Hypokalemia (n = 2) | Moderate. Concomitant use of salbutamol and potassium-sparing diuretics may cause electrocardiographic changes or hypokalemia. | E: intravenous KCl supplementation was required. | Sufficient | 7 (probable) |
| Digoxin-Norepinephrine (BBU) | Arrythmias (n = 1) | Major. Concomitant use of these drugs may increase the risk of cardiotoxicity (arrythmias). | E: one of the drugs was discontinued and greaterthan-usual monitoring was required. | Sufficient | 6 (probable) |
| L-asparaginase-vincristine (oncology) | Pancreatitis (n = 1) | Major. Concomitant use of these drugs may increase the risk of toxicity. | F: length of hospitalization was extended and greater-than-usual monitoring was required. | Sufficient | 5 (probable) |
| Paracetamol-Cholestyramine (oncology) | Decreased efficacy of paracetamol (n = 1) | Minor. Concomitant use of these drugs may decrease the effectiveness of paracetamol. | E: A new analgesic regimen was required. | Good | 6 (probable) |
BBU: basic burns unit; DIPS: Drug Interaction Probability Scale; KCl: Potassium chloride; PICU: pediatric intensive care unit.
Antibiotics were the most usually prescribed drugs (23.4%), followed by analgesics (19.3%), electrolytes (6.5%) and systemic corticosteroids (5.6%). The drugs most frequently involved in pDDIs were analgesics (22.6%, opioids 18.2%), sedative hypnotics (13.3%), antibiotics (13.1%), diuretics (8.6%), histamine type-2 receptor antagonists (6.8%) and systemic corticosteroids (5.1%).
DiscussionThis is the first study to estimate the prevalence of pDDIs and rDDIs in pediatric patients with burns, cancer, and other medical conditions. A pDDI prevalence of 61.3% was found among patients on two or more drugs; 60.5% of those pDDIs were considered major. Moreover, a prevalence of 3.6% was observed in rDDIs that caused temporary damage and required a medical intervention or resulted in an extension of the patients’ stay in hospital.
The observed prevalence of pDDIs is aligned with the findings of other studies of hospitalized pediatric patients, which reported rates between 41.7% and 67.1%8-10,12,25. Direct comparisons between the estimated pDDI prevalence rates should be avoided given the diversity of sources of information, the different sampling methods used, the wide range of medical specialties involved, and the heterogeneity of age groups in the pediatric patient universe. Moreover, prescription patterns could vary across different countries. For example, the proportion of patients with pDDIs reported in studies including pediatric cancer patients ranges from 56.7%9 to 95%26 in inpatients and 83.5%14 in outpatients whereas in this study the proportion was 32.8%. This could be attributable to the non-probabilistic sampling method used, the low proportion of cancer patients on polypharmacy, and to the fact that only three patients with neoplasms were admitted to critical care.
This is also one of the few studies to look into the occurrence of rDDIs. The previously reported prevalence of rDDIs in pediatric patients requiring hospitalization was 0.009%17, which is significantly lower than the rate found in this analysis. This could be due to the selection bias arising from the sampling method used, whereby patients requiring critical care, especially children with burns who required more drugs to be properly managed and stabilized7,27, were overrepresented18.
Furthermore, rDDIs between paracetamol and cholestyramine, considered minor on the Micromedex® severity scale, required the analgesic regimen to be modified as a result of an objective reduction in effectiveness. In a large-scale population-based study, the majority of pDDIs observed involved reductions in therapeutic effectiveness13. Although this suggests that theoretically minor pDDIs should not be underestimated in clinical practice, it must be borne in mind that the severity categories on the Micromedex® scale were not compared with those of other databases, which could lead to an under- or overestimation of the available evidence on pDDIs.
Like other studies, this analysis demonstrated the existence of a correlation between degree of polypharmacy and number of pDDIs3,5, but not between degree of polypharmacy and length of hospital stay. The small sample size and the wide length-of-stay variability in patients with the same diagnosis made it impossible to establish such a correlation, which had however been found by other authors7,8. Diagnoses showing the widest length-of-stay and length-of-drug-exposure variability were unspecific bacterial pneumonia, de novo neoplasms, septic shock, and congenital malformations. Some of them, such as pneumonia and burns involving over 20% of body surface, were more common in critical units. However, the overrepresentation and grouping together of several single diagnoses led to a wider dispersion between drug exposure and length of hospital stay. These diagnoses included optical neuritis, pancreatitis and intraventricular hemorrhage, whose considerable severity and clinical complexity diluted the correlation between length of hospital stay and pDDI.
Another aspect found to be relevant for the type of pDDI experienced by patients was the observation in this study and in those of other authors that opioid analgesics, antibiotics and CNS drugs were among the three groups of drugs most commonly involved in pDDIs7,10,11,26,28. These groups of drugs are widely used to manage patients in the critical care setting7, including children with large burns (> 20% of their body surface), where opioids, benzodiazepines and sedative hypnotics are used for the management of pain, and antibiotics are used to treat complex infections18,29.
Given its exploratory nature, the present pilot study includes certain methodological aspects, such as the small sample size, the non-probabilistic process used for patient selection, and the cross-sectional design of the analysis, which cannot exclude potential confounding and selection biases, thereby restricting the external validity of the results obtained. Another important limitation was a failure to compare the pDDI results with a second source of information, as is usually done in other studies9,14,30. Although the Micromedex® database is considered a very comprehensive, reliable, and user-friendly source of information21, use of an additional data base to compare the results would have allowed higher levels of sensitivity and accuracy in the identification of pDDIs2, allowing for more robust prevalence estimations. For these reasons, the results of the study must be taken with caution and be set within the context of an exploratory study, i.e., the results are not intended to establish a definitive parameter but to serve as a starting point for further research into this area. Although the participation of a pharmacist was important in this study, hospital pharmacy as a profession is in a teething phase in Mexico and faces many obstacles to effective implementation, which on many occasions could involve risks for the patients’ health.
In a nutshell, the prevalence of pDDIs as identified by means of the Micromedex® database by a resident physician was 61.3% among acute pediatric patients who received two or more drugs during their hospital stay. Only 3.6% of patients at risk presented with clinical manifestations resulting from rDDIs that caused temporary damage. However, up to 65.6% of pDDIs identified were considered severe or contraindicated, which suggests the need to place a greater emphasis on research into preventing risks to patient safety.
FundingNo funding.
Conflict of interestNo conflict of interests.
Contribution to the scientific literature
This is one of the few studies looking into drug-drug interactions resulting in clinical manifestations that offers an estimation of their prevalence in pediatric patients admitted to a wide range of hospital units, including burn units. The findings of this analysis could be used as a basis for future research aimed at establishing priorities to enhance patient safety.
Early Access date (07/09/2021).